GSH Protects the Escherichia coli Cells from High Concentrations of Thymoquinone
Abstract
1. Introduction
2. Results
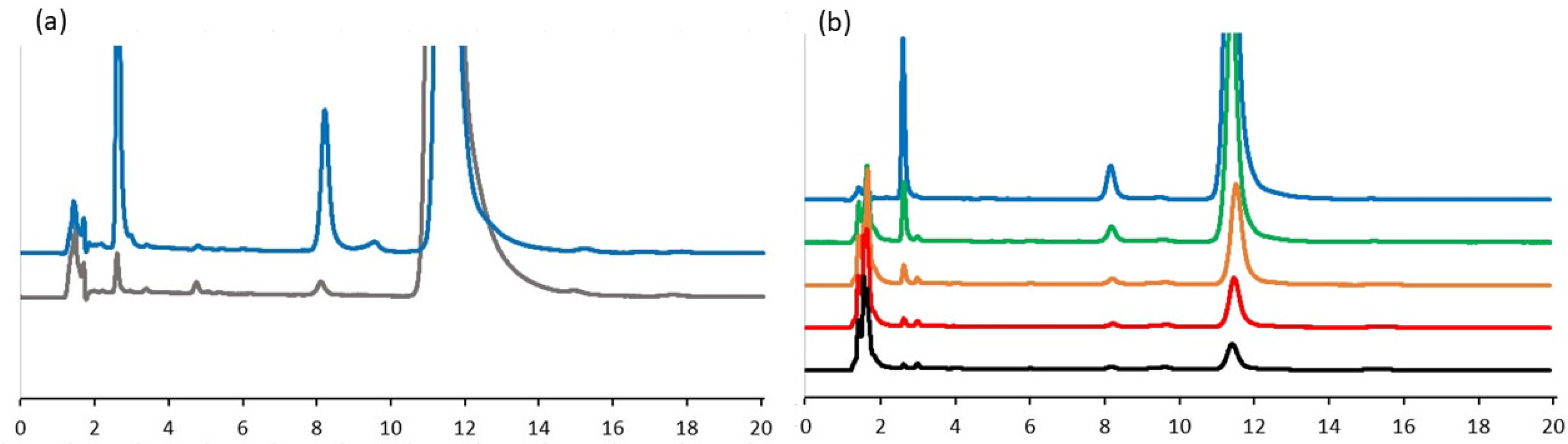
2.1. Interaction of TQ with Organosulfur Compounds
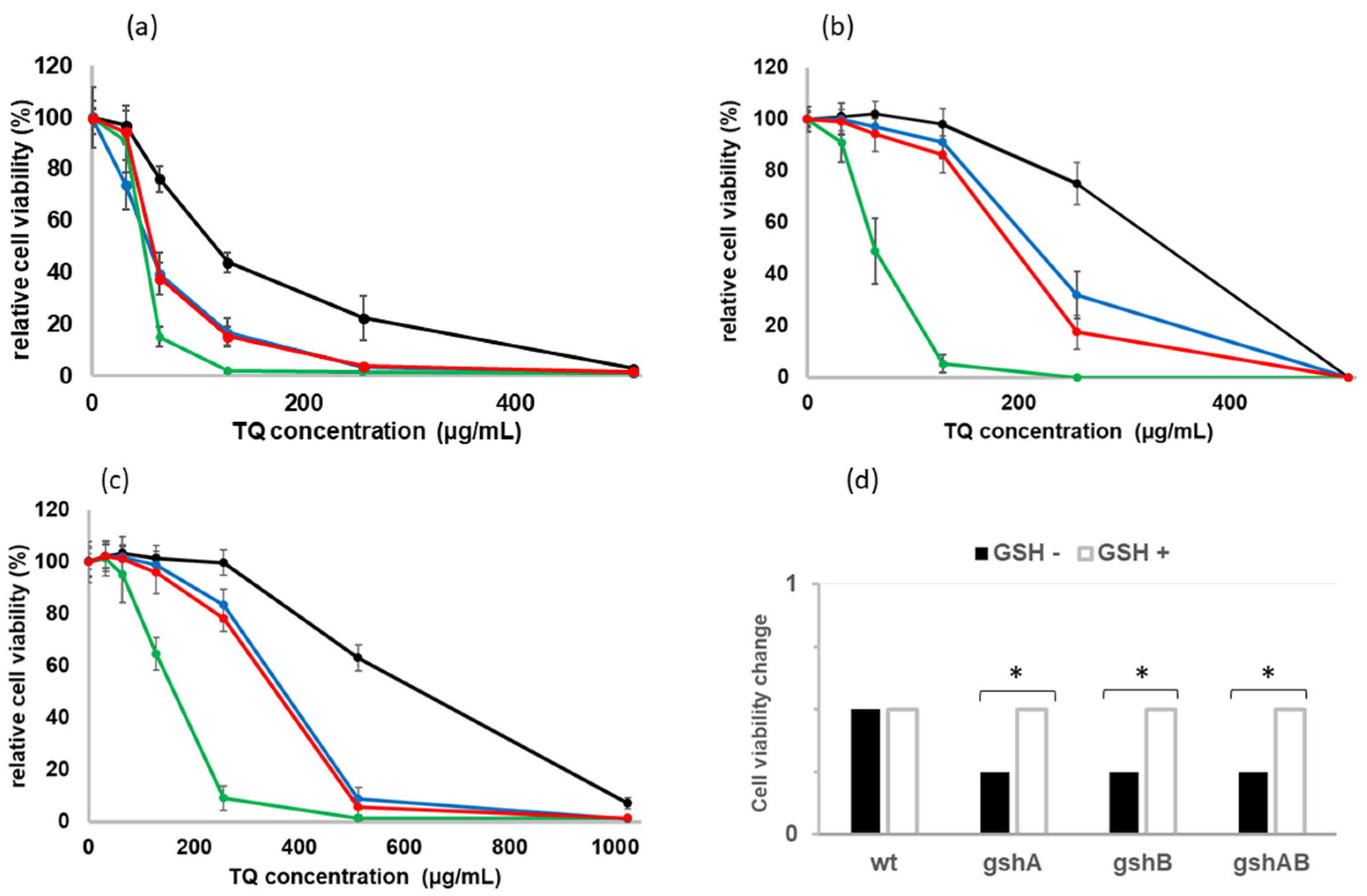
2.2. Sensitivity of GSH-Deficient Strains to TQ
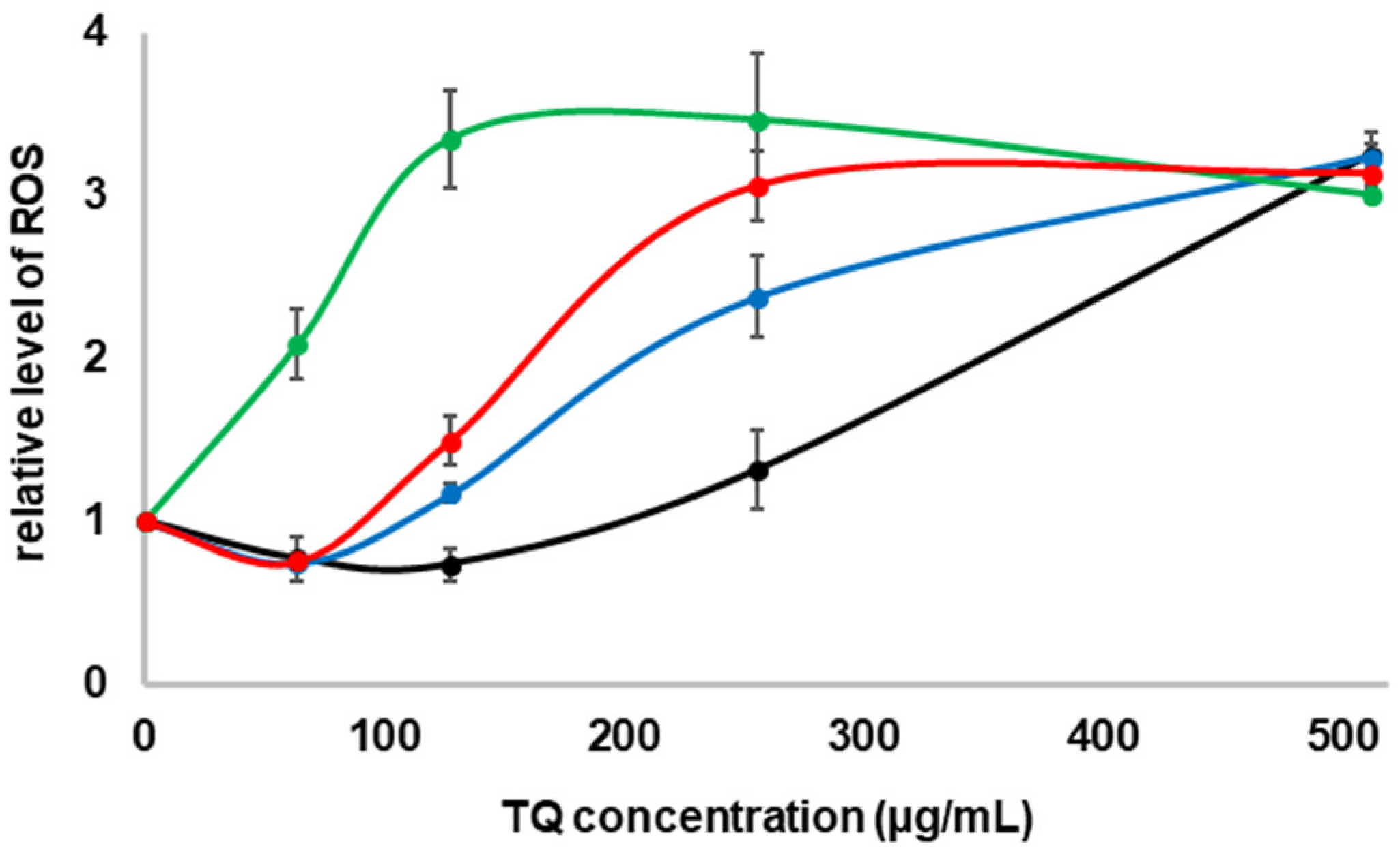
2.3. Formation of ROS in the Presence of TQ in the Culture
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Bacterial Strains
4.3. The Reaction of TQ with Thiol Group
4.4. Minimum Inhibitory Concentration Determination
4.5. Analysis of Interaction of GSH with TQ Derivatives by HPLC Chromatography
4.6. ROS Analysis in the Cultures Treated by TQ
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Loi, V.V.; Rossius, M.; Antelmann, H. Redox regulation by reversible protein S-thiolation in bacteria. Front. Microbiol. 2015, 6, 187. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999, 27, 916–921. [Google Scholar] [CrossRef]
- Griffith, O.W. Biologic and pharmacologic regulation of mammalian glutathione synthesis. Free Radic. Biol. Med. 1999, 27, 922–925. [Google Scholar] [CrossRef]
- Khalife, K.H.; Lupidi, G. Nonenzymatic reduction of thymoquinone in physiological conditions. Free Radic. Res. 2007, 41, 153–161. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Muzyka, N.G.; Oktyabrsky, O.N. Effects of cystine and hydrogen peroxide on glutathione status and expression of antioxidant genes in Escherichia coli. Biochemistry 2005, 70, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, G.; Muzyka, N.; Oktyabrsky, O. Transmembrane glutathione cycling in growing Escherichia coli cells. Microbiol. Res. 2012, 167, 166–172. [Google Scholar] [CrossRef]
- Smirnova, G.V.; Tyulenev, A.V.; Muzyka, N.G.; Oktyabrsky, O.N. Study of the relationship between extracellular superoxide and glutathione production in batch cultures of Escherichia coli. Res. Microbiol. 2020, 171, 301–310. [Google Scholar] [CrossRef]
- Helbig, K.; Bleuel, C.; Krauss, G.J.; Nies, D.H. Glutathione and transition-metal homeostasis in Escherichia coli. J. Bacteriol. 2008, 190, 5431–5438. [Google Scholar] [CrossRef]
- Gawron, G.; Krzyczkowski, W.; Łyżeń, R.; Kadziński, L.; Banecki, B. Influence of Supercritical Carbon Dioxide Extraction Conditions on Extraction Yield and Composition of Nigella sativa L. Seed Oil-Modelling, Optimization and Extraction Kinetics regarding Fatty Acid and Thymoquinone Content. Molecules 2021, 26, 6419. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.M.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black Cumin (Nigella sativa L.): A Comprehensive Review on Phytochemistry, Health Benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar]
- Malik, S.; Singh, A.; Negi, P.; Kapoor, V.K. Thymoquinone: A small molecule from nature with high therapeutic potential. Drug Discov. Today 2021, 26, 2716–2725. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Molecular mechanisms of quinone cytotoxicity. Chem. Biol. Interact. 1991, 80, 1–41. [Google Scholar] [CrossRef]
- Kruk, I.; Michalska, T.; Lichszteld, K.; Kladna, A.; Aboul-Enein, H.Y. The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere 2000, 41, 1059–1064. [Google Scholar] [CrossRef]
- Bolton, J.L.; Trush, M.A.; Penning, T.M.; Dryhurst, G.; Monks, T.J. Role of quinones in toxicology. Chem. Res. Toxicol. 2000, 13, 135–160. [Google Scholar] [CrossRef]
- Armutcu, F.; Akyol, S.; Akyol, O. The interaction of glutathione and thymoquinone and their antioxidant properties. Electron. J. Gen. Med. 2018, 15, em59. [Google Scholar] [CrossRef]
- Hariharan, P.; Paul-Satyaseela, M.; Gnanamani, A. In vitro profiling of antimethicillin-resistant Staphylococcus aureus activity of thymoquinone against selected type and clinical strains. Lett. Appl. Microbiol. 2016, 62, 283–289. [Google Scholar] [CrossRef]
- Goel, S. Mishra Thymoquinone inhibits biofilm formation and has selective antibacterial activity due to ROS generation. Appl. Microbiol. Biotechnol. 2018, 102, 1955–1967. [Google Scholar] [CrossRef]
- Salmani, J.M.; Asghar, S.; Lv, H.; Zhou, J. Aqueous solubility and degradation kinetics of the phytochemical anticancer thymoquinone; probing the effects of solvents. pH and light. Molecules 2014, 19, 5925–5939. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar] [CrossRef]
- Zubair, H.; Khan, H.Y.; Sohail, A.; Azim, S.; Ullah, M.F.; Ahmad, A.; Sarkar, F.H.; Hadi, S.M. Redox cycling of endogenous copper by thymoquinone leads to ROS-mediated DNA breakage and consequent cell death: Putative anticancer mechanism of antioxidants. Cell Death Dis. 2013, 4, e660. [Google Scholar] [CrossRef]
- Newton, G.L.; Fahey, R.C.; Rawat, M. Detoxification of toxins by bacillithiol in Staphylococcus aureus. Microbiology 2012, 158 Pt 4, 1117–1126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Di Monte, D.; Ross, D.; Bellomo, G.; Eklow, L.; Orrenius, S. Alterations in intracellular thiol homeostasis during the metabolism of menadione by isolated rat hepatocytes. Arch. Biochem. Biophys. 1984, 235, 334–342. [Google Scholar] [CrossRef]
- Goswami, M.; Mangoli, S.H.; Jawali, N. Involvement of reactive oxygen species in the action of ciprofloxacin against Escherichia coli. Antimicrob. Agents Chemother. 2006, 50, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A.; Taha, R.A.; Gamal El-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2013, 26, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Di Monte, D.; Bellomo, G.; Thor, H.; Nicotera, P.; Orrenius, S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch. Biochem. Biophys. 1984, 235, 343–350. [Google Scholar] [CrossRef]
- Ahmad, Z.; Laughlin, T.F.; Kady, I.O. Thymoquinone Inhibits Escherichia coli ATP Synthase and Cell Growth. PLoS ONE 2015, 10, e0127802. [Google Scholar]
- Suzuki, H.; Koyanagi, T.; Izuka, S.; Onishi, A.; Kumagai, H. The yliA, -B, -C, and -D genes of Escherichia coli K-12 encode a novel glutathione importer with an ATP-binding cassette. J. Bacteriol. 2005, 187, 5861–5867. [Google Scholar] [CrossRef]
- Gawron, G.; Krzyczkowski, W.; Lemke, K.; Oldak, A.; Kadzinski, L.; Banecki, B. Nigella sativa seed extract applicability in preparations against methicillin-resistant Staphylococcus aureus and effects on human dermal fibroblasts viability. J. Ethnopharmacol. 2019, 244, 112135. [Google Scholar] [CrossRef]
- Reed, P.; Atilano, M.L.; Alves, R.; Hoiczyk, E.; Sher, X.; Reichmann, N.T.; Pereira, P.M.; Roemer, T.; Filipe, S.R.; Pereira-Leal, J.B.; et al. Staphylococcus aureus Survives with a Minimal Peptidoglycan Synthesis Machine but Sacrifices Virulence and Antibiotic Resistance. PLoS Pathog. 2015, 11, e1004891. [Google Scholar] [CrossRef]
- Seo, Y.; Park, K.; Hong, Y.; Lee, E.S.; Kim, S.S.; Jung, Y.T.; Park, H.; Kwon, C.; Cho, Y.S.; Huh, Y.D. Reactive-oxygen-species-mediated mechanism for photoinduced antibacterial and antiviral activities of Ag3PO4. J. Anal. Sci. Technol. 2020, 11, 21. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łyżeń, R.; Gawron, G.; Kadziński, L.; Banecki, B. GSH Protects the Escherichia coli Cells from High Concentrations of Thymoquinone. Molecules 2022, 27, 2546. https://doi.org/10.3390/molecules27082546
Łyżeń R, Gawron G, Kadziński L, Banecki B. GSH Protects the Escherichia coli Cells from High Concentrations of Thymoquinone. Molecules. 2022; 27(8):2546. https://doi.org/10.3390/molecules27082546
Chicago/Turabian StyleŁyżeń, Robert, Grzegorz Gawron, Leszek Kadziński, and Bogdan Banecki. 2022. "GSH Protects the Escherichia coli Cells from High Concentrations of Thymoquinone" Molecules 27, no. 8: 2546. https://doi.org/10.3390/molecules27082546
APA StyleŁyżeń, R., Gawron, G., Kadziński, L., & Banecki, B. (2022). GSH Protects the Escherichia coli Cells from High Concentrations of Thymoquinone. Molecules, 27(8), 2546. https://doi.org/10.3390/molecules27082546





