Impact of Barrel Toasting on Ellagitannin Composition of Aged Cognac Eaux-de-Vie
Abstract
:1. Introduction
2. Results and Discussion
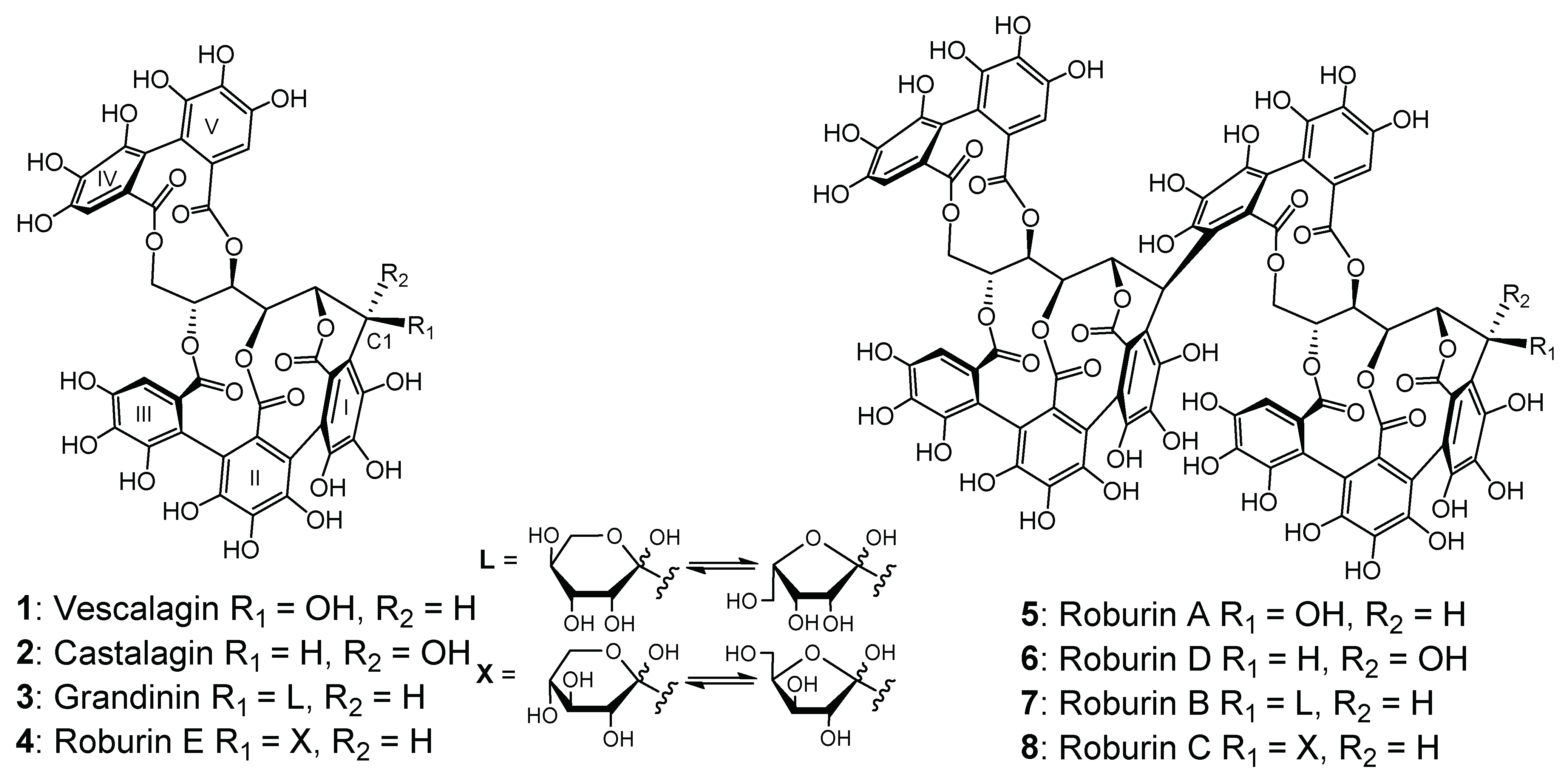
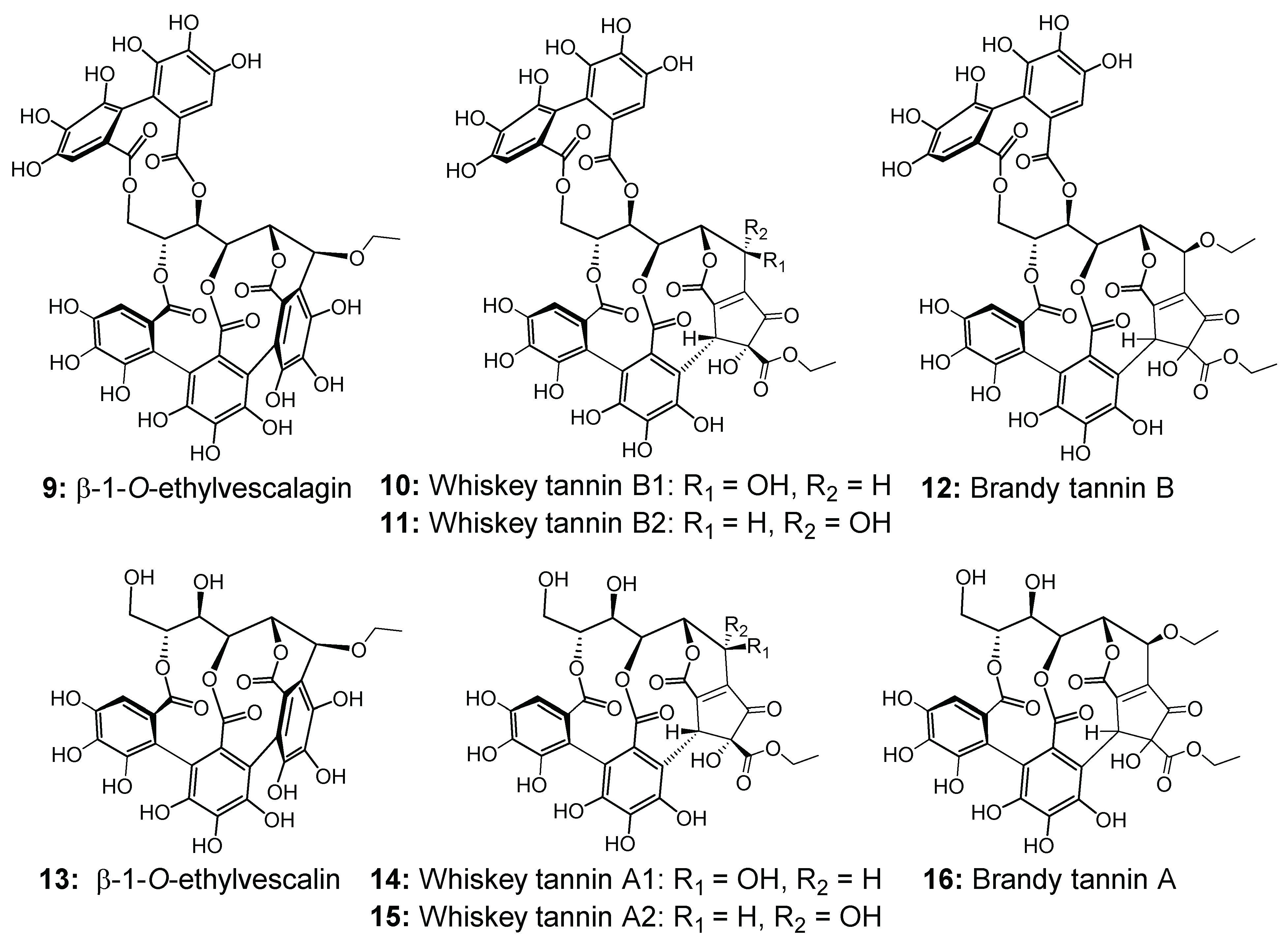
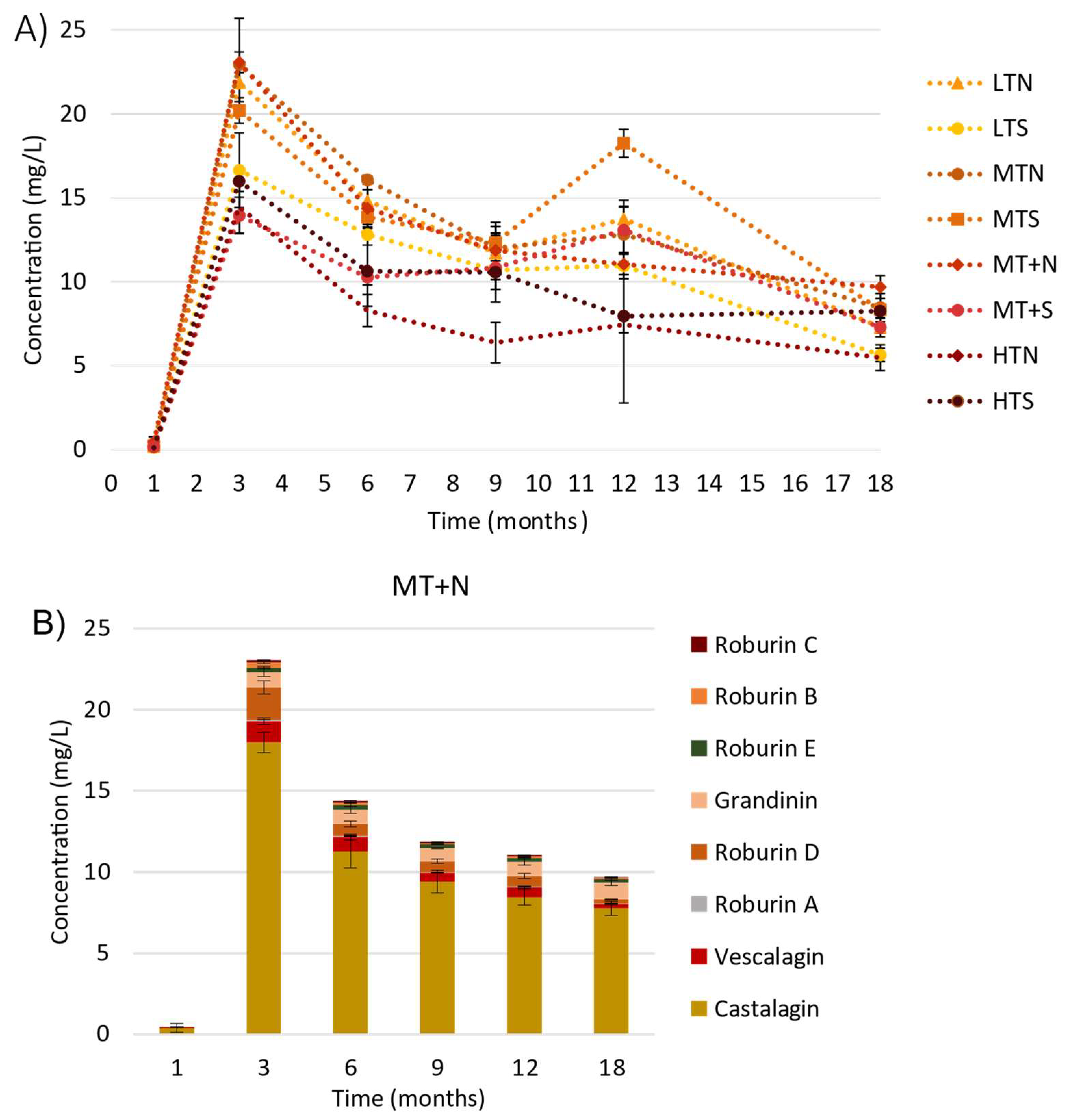
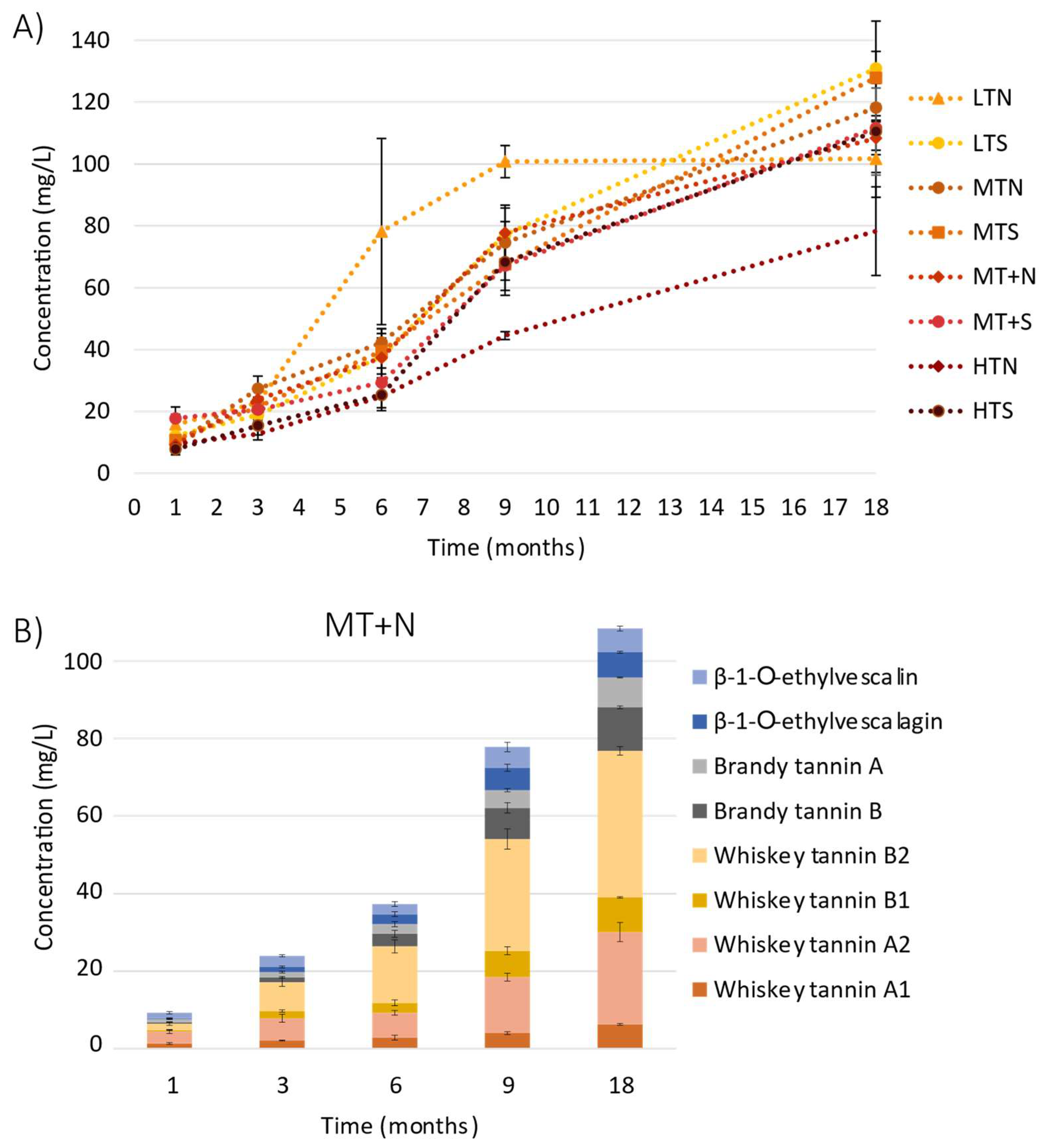
2.1. Evolution of C-Glucosidic Ellagitannins and Ellagitannin-Derived Spirit Compounds in Cognac Eaux-de-Vie during Aging
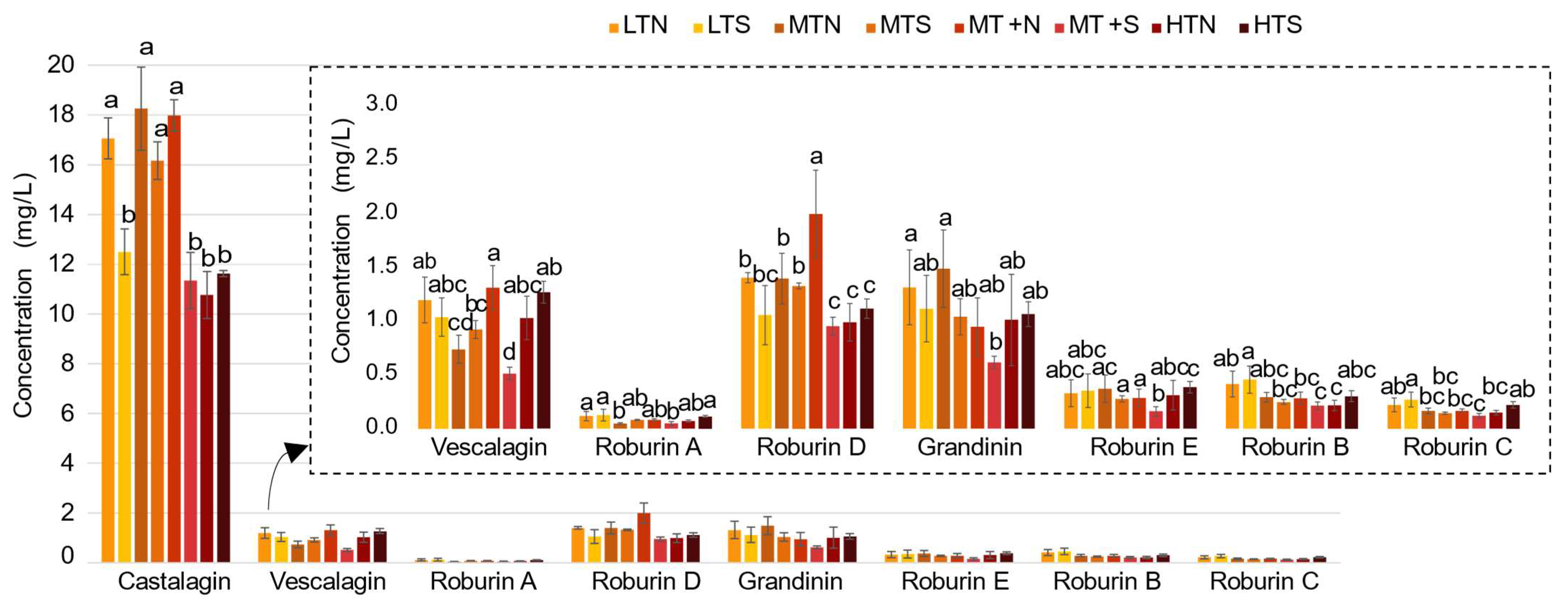
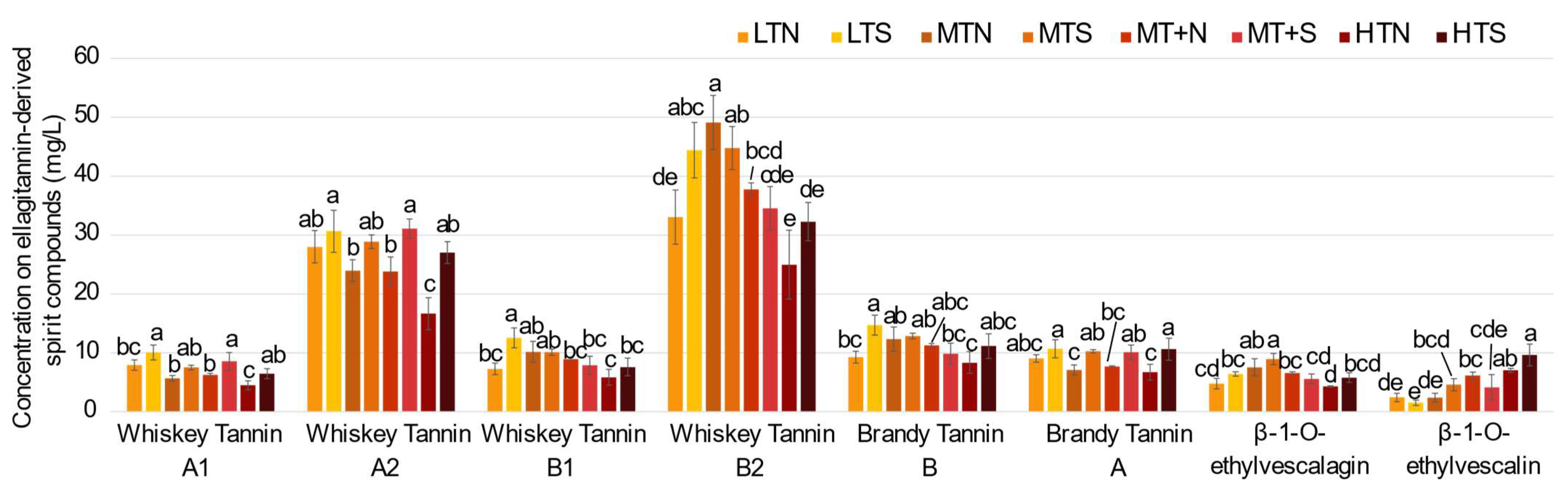
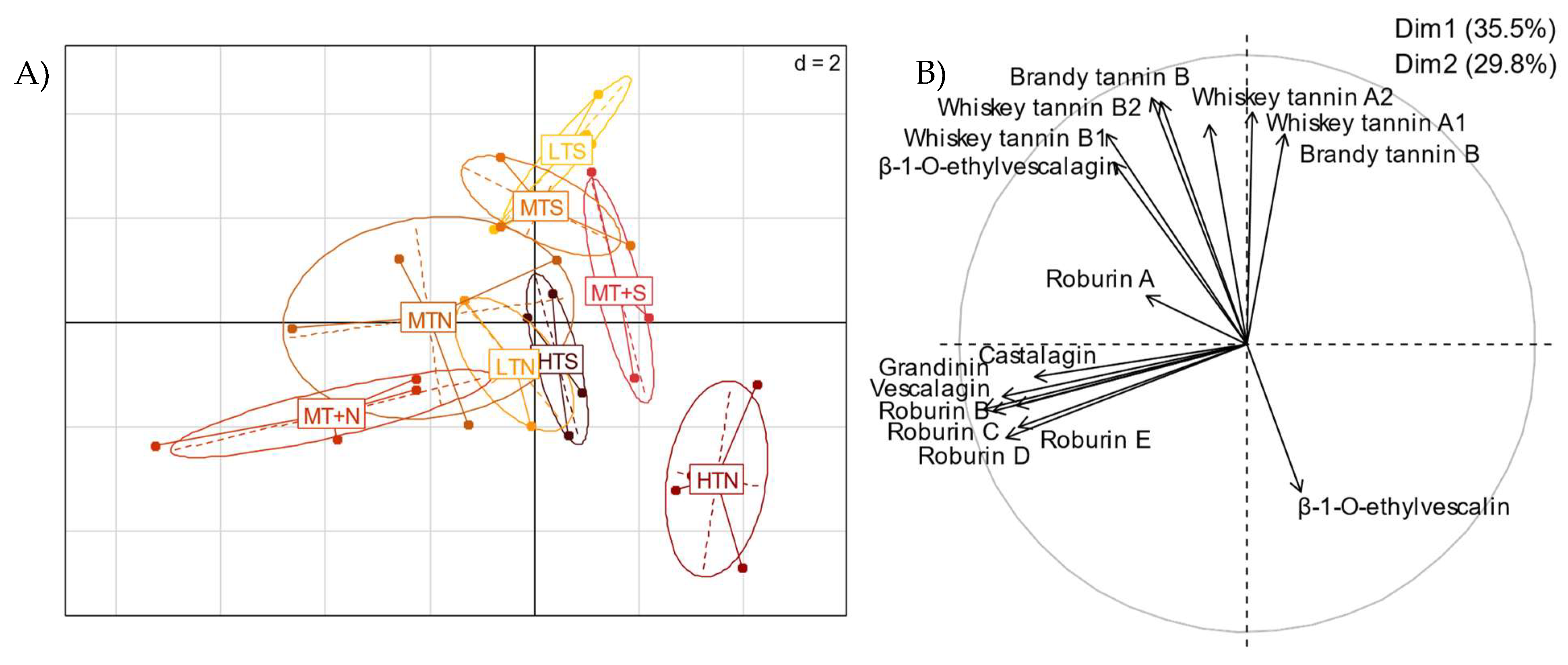
2.2. Impact of the Barrel Toasting Level on Concentrations of the C-Glucosidic Ellagitannins and Ellagitannin-Derived Spirit Compounds during Oak Wood Aging
3. Materials and Methods
3.1. Chemicals
3.2. Cognac Eaux-de-Vie and Sample Preparation
3.3. HPLC-UV-QQQ Analysis
3.4. Calibration Curves
3.5. Statistical Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Scalbert, A.; Monties, B.; Favre, J.-M. Polyphenols of Quercus Robur: Adult Tree and In Vitro Grown Calli and Shoots. Phytochemistry 1988, 27, 3483–3488. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Hatano, T. Classification of Oligomeric Hydrolysable Tannins and Specificity of Their Occurrence in Plants. Phytochemistry 1993, 32, 507–521. [Google Scholar] [CrossRef]
- Mayer, W.; Gabler, W.; Riester, A.; Korger, H. Über Die Gerbstoffe Aus Dem Holz Der Edelkastanie Und Der Eiche, II. Die Isolierung von Castalagin, Vescalagin, Castalin Und Vescalin. Justus Liebigs Ann. Chem. 1967, 707, 177–181. [Google Scholar] [CrossRef]
- Mayer, W.; Seitz, H.; Jochims, J.C. Über Die Gerbstoffe Aus Dem Holz Der Edelkastanie Und Der Eiche, IV1 Die Struktur Des Castalagins. Justus Liebigs Ann. Chem. 1969, 721, 186–193. [Google Scholar] [CrossRef]
- Mayer, W.; Seitz, H.; Jochims, J.C.; Schauerte, K.; Schilling, G. Über Die Gerbstoffe Aus Dem Holz Der Edelkastanie Und Eiche, VI. Struktur Des Vescalagins. Justus Liebigs Ann. Chem. 1971, 751, 60–68. [Google Scholar] [CrossRef]
- Zhang, B.; Cai, J.; Duan, C.-Q.; Reeves, M.J.; He, F. A Review of Polyphenolics in Oak Woods. Int. J. Mol. Sci. 2015, 16, 6978–7014. [Google Scholar] [CrossRef]
- Du Penhoat, C.L.H.; Michon, V.M.; Ohassan, A.; Peng, S.; Scalbert, A.; Gage, D. Roburin A, a Dimeric Ellagitannin from Heartwood of Quercus Robur. Phytochemistry 1991, 30, 329–332. [Google Scholar] [CrossRef]
- Penhoat, C.L.H.; Veronique, M. Structural Elucidation of New Dimeric Ellagitannins from Quercus robur L. Roburins A–E. J. Chem. Soc. Perkin 1 1991, 7, 1653–1660. [Google Scholar] [CrossRef]
- Fernandez de Simon, B.; Cadahia, E.; Conde, E.; Garcia Vallejo, M.C. Ellagitannins in Woods of Spanish Oaks. J. Sci. Tech. Tonnelerie Fr. 1998, 83–97. Available online: https://agris.fao.org/agris-search/search.do?recordID=FR1999000361 (accessed on 8 March 2022).
- García-Estévez, I.; Alcalde-Eon, C.; Le Grottaglie, L.; Rivas-Gonzalo, J.C.; Escribano-Bailón, M.T. Understanding the Ellagitannin Extraction Process from Oak Wood. Tetrahedron 2015, 71, 3089–3094. [Google Scholar] [CrossRef] [Green Version]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Effect of the Type of Oak Barrels Employed during Ageing on the Ellagitannin Profile of Wines. Aust. J. Grape Wine Res. 2017, 23, 334–341. [Google Scholar] [CrossRef] [Green Version]
- Masson, G.; Moutounet, M.; Puech, J.L. Ellagitannin Content of Oak Wood as a Function of Species and of Sampling Position in the Tree. Am. J. Enol. Vitic. 1995, 46, 262–268. [Google Scholar]
- Fernandez de Simon, B.; Cadahía, E.; Conde, E.; García-Vallejo, M.C. Ellagitannins in Woods of Spanish, French and American Oaks. Holzforschung 1999, 53, 147–150. [Google Scholar] [CrossRef]
- Canas, S. Phenolic Composition and Related Properties of Aged Wine Spirits: Influence of Barrel Characteristics. A Review. Beverages 2017, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Cadahía, E.; Varea, S.; Muñoz, L.; Fernández de Simón, B.; García-Vallejo, M.C. Evolution of Ellagitannins in Spanish, French, and American Oak Woods during Natural Seasoning and Toasting. J. Agric. Food Chem. 2001, 49, 3677–3684. [Google Scholar] [CrossRef]
- Cadahía, E.; Fernández de Simón, B.; Jalocha, J. Volatile Compounds in Spanish, French, and American Oak Woods after Natural Seasoning and Toasting. J. Agric. Food Chem. 2003, 51, 5923–5932. [Google Scholar] [CrossRef]
- Chira, K.; Anguellu, L.; Da Costa, G.; Richard, T.; Pedrot, E.; Jourdes, M.; Teissedre, P.-L. New C-Glycosidic Ellagitannins Formed upon Oak Wood Toasting, Identification and Sensory Evaluation. Foods 2020, 9, 1477. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O.; Adams, A.; Demyttenaere, J.; Verhé, R.; De Kimpe, N. Volatile Composition Analysis by Solid-Phase Microextraction Applied to Oak Wood Used in Cooperage (Quercus pyrenaica and Quercus petraea): Effect of Botanical Species and Toasting Process. J. Wood Sci. 2006, 52, 514–521. [Google Scholar] [CrossRef]
- Mosedale, J.R.; Puech, J.-L. Wood Maturation of Distilled Beverages. Trends Food Sci. Technol. 1998, 9, 95–101. [Google Scholar] [CrossRef]
- Snakkers, G.; Boulesteix, J.-M.; Estréguil, S.; Gaschet, J.; Lablanquie, O.; Faure, A.; Cantagrel, R. Effect of Oak Wood Heating on Cognac Spirit Matured in New Barrel: A Pilot Study. OENO ONE 2003, 37, 243–255. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P.-L. Chemical and Sensory Evaluation of Wine Matured in Oak Barrel: Effect of Oak Species Involved and Toasting Process. Eur. Food Res. Technol. 2015, 240, 533–547. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Santis, D.D.; Ceccarelli, A. Influence of Oak Woods of Different Geographical Origins on Quality of Wines Aged in Barriques and Using Oak Chips. Food Chem. 2007, 103, 46–54. [Google Scholar] [CrossRef]
- Jourdes, M.; Michel, J.; Saucier, C.; Quideau, S.; Teissedre, P.-L. Identification, Amounts, and Kinetics of Extraction of C-Glucosidic Ellagitannins during Wine Aging in Oak Barrels or in Stainless Steel Tanks with Oak Chips. Anal. Bioanal. Chem. 2011, 401, 1531. [Google Scholar] [CrossRef] [PubMed]
- García-Estévez, I.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C.; Alcalde-Eon, C. Validation of a Mass Spectrometry Method To Quantify Oak Ellagitannins in Wine Samples. J. Agric. Food Chem. 2012, 60, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Chira, K.; Teissedre, P.-L. Relation between Volatile Composition, Ellagitannin Content and Sensory Perception of Oak Wood Chips Representing Different Toasting Processes. Eur. Food Res. Technol. 2013, 236, 735–746. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Chira, K.; Teissedre, P.-L. Ellagitannin Content, Volatile Composition and Sensory Profile of Wines from Different Countries Matured in Oak Barrels Subjected to Different Toasting Methods. Food Chem. 2016, 210, 500–511. [Google Scholar] [CrossRef]
- Hofmann, T.; Glabasnia, A.; Schwarz, B.; Wisman, K.N.; Gangwer, K.A.; Hagerman, A.E. Protein Binding and Astringent Taste of a Polymeric Procyanidin, 1,2,3,4,6-Penta-O-Galloyl-β-D-Glucopyranose, Castalagin, and Grandinin. J. Agric. Food Chem. 2006, 54, 9503–9509. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, M.; Rodríguez, M.; Martínez, C.; Bosquet, V.; Guillén, D.; Barroso, C.G. Antioxidant Activity of Brandy de Jerez and Other Aged Distillates, and Correlation with Their Polyphenolic Content. Food Chem. 2009, 116, 29–33. [Google Scholar] [CrossRef]
- Vivas, N.; Vivas de Gaulejac, N.; Bourden-Nonier, M.-F.; Mouche, C.; Rossy, C. Extraction of Phenolics from New Oak Casks during Spirit Maturation: Impact on Spirit Colour. J. Inst. Brew. 2020, 126, 83–89. [Google Scholar] [CrossRef]
- Canas, S.; Casanova, V.; Pedro Belchior, A. Antioxidant Activity and Phenolic Content of Portuguese Wine Aged Brandies. J. Food Compos. Anal. 2008, 21, 626–633. [Google Scholar] [CrossRef]
- Gadrat, M.; Lavergne, J.; Emo, C.; Teissedre, P.-L.; Chira, K. Validation of a Mass Spectrometry Method to Identify and Quantify Ellagitannins in Oak Wood and Cognac during Aging in Oak Barrels. Food Chem. 2021, 342, 128223. [Google Scholar] [CrossRef]
- Saucier, C.; Jourdes, M.; Glories, Y.; Quideau, S. Extraction, Detection, and Quantification of Flavano-Ellagitannins and Ethylvescalagin in a Bordeaux Red Wine Aged in Oak Barrels. J. Agric. Food Chem. 2006, 54, 7349–7354. [Google Scholar] [CrossRef] [PubMed]
- Jourdes, M. Réactivité, Synthèse, Couleur et Activité Biologique D’ellagitannins C-Glycosidiques et Flavano-Ellagitannins. Ph.D. Dissertation, Université de Bordeaux, Bordeaux, France, 2003. [Google Scholar]
- Fujieda, M.; Tanaka, T.; Suwa, Y.; Koshimizu, S.; Kouno, I. Isolation and Structure of Whiskey Polyphenols Produced by Oxidation of Oak Wood Ellagitannins. J. Agric. Food Chem. 2008, 56, 7305–7310. [Google Scholar] [CrossRef] [PubMed]
- Gadrat, M.; Capello, Y.; Emo, C.; Lavergne, J.; Quideau, S.; Jourdes, M.; Teissèdre, P.-L.; Chira, K. Identification, Quantitation and Sensory Contribution of New C-Glucosidic Ellagitannin-Derived Spirit Compounds. Food Chem. 2022, 384, 132307. [Google Scholar] [CrossRef] [PubMed]
- Winstel, D.; Gautier, E.; Marchal, A. Role of Oak Coumarins in the Taste of Wines and Spirits: Identification, Quantitation, and Sensory Contribution through Perceptive Interactions. J. Agric. Food Chem. 2020, 68, 7434–7443. [Google Scholar] [CrossRef]
- Rasines-Perea; Jacquet; Jourdes; Quideau; Teissedre Ellagitannins and Flavano-Ellagitannins: Red Wines Tendency in Different Areas, Barrel Origin and Ageing Time in Barrel and Bottle. Biomolecules 2019, 9, 316. [CrossRef] [Green Version]
- Winstel, D.; Capello, Y.; Quideau, S.; Marchal, A. Isolation of a New Taste-Active Brandy Tannin A: Structural Elucidation, Quantitation and Sensory Assessment. Food Chem. 2022, 377, 131963. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Ellagitannins from Portuguese Oak Wood (Quercus pyrenaica Willd.) Used in Cooperage: Influence of Geographical Origin, Coarseness of the Grain and Toasting Level. Holzforschung 2007, 61, 155–160. [Google Scholar] [CrossRef]
- Quideau, S.; Varadinova, T.; Karagiozova, D.; Jourdes, M.; Pardon, P.; Baudry, C.; Genova, P.; Diakov, T.; Petrova, R. Main Structural and Stereochemical Aspects of the Antiherpetic Activity of Nonahydroxyterphenoyl-Containing C-Glycosidic Ellagitannins. Chem. Biodivers. 2004, 1, 247–258. [Google Scholar] [CrossRef]
| Toast | Notation | Sum °C.min * |
|---|---|---|
| Light toast normal | LTN | 3560 |
| Light toast slow | LTS | 5036 |
| Medium toast normal | MTN | 4566 |
| Medium toast slow | MTS | 5134 |
| Medium toast plus normal | MT + N | 5088 |
| Medium toast plus slow | MT + S | 6015 |
| High toast normal | HTN | 6892 |
| High toast slow | HTS | 7320 |
| Compounds | Retention Time (min) | m/z |
|---|---|---|
| Vescalagin (1) | 8.0 | 933 a, 915, 613, 301 |
| Castalagin (2) | 13.5 | 933 a, 915, 613, 301 |
| Grandinin (3) | 5.6 | 1065 a, 915, 613, 301 |
| Roburin E (4) | 7.1 | 1065 a, 915, 613, 301 |
| Roburin A (5) | 3.9 | 1849, 933, 924 a, 915, 301 |
| Roburin D (6) | 5.8 | 1849, 933, 924 a, 915, 301 |
| Roburin B (7) | 3.9 | 1981, 1065, 990 a, 915, 301 |
| Roburin C (8) | 4.2 | 1981, 1065, 990 a, 915, 301 |
| β-1-O-ethylvescalagin (9) | 21.2 | 961 a, 915, 480, 301 |
| Whiskey tannin B1 (10) | 24.0 and 26.4 | 977 a, 933, 675, 631, 301 |
| Whiskey tannin B2 (11) | 24.0 and 26.4 | 977 a, 933, 675, 631, 301 |
| Brandy tannin B (12) | 32.5 | 1005 a, 703, 301 |
| β-1-O-ethylvescalin (13) | 21.4 | 659 a |
| Whiskey tannin A1 (14) | 4.9 and 8.6 | 675 a |
| Whiskey tannin A2 (15) | 4.9 and 8.6 | 675 a |
| Brandy tannin A (16) | 27.7 | 703 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadrat, M.; Emo, C.; Lavergne, J.; Teissèdre, P.-L.; Chira, K. Impact of Barrel Toasting on Ellagitannin Composition of Aged Cognac Eaux-de-Vie. Molecules 2022, 27, 2531. https://doi.org/10.3390/molecules27082531
Gadrat M, Emo C, Lavergne J, Teissèdre P-L, Chira K. Impact of Barrel Toasting on Ellagitannin Composition of Aged Cognac Eaux-de-Vie. Molecules. 2022; 27(8):2531. https://doi.org/10.3390/molecules27082531
Chicago/Turabian StyleGadrat, Mathilde, Catherine Emo, Joël Lavergne, Pierre-Louis Teissèdre, and Kléopatra Chira. 2022. "Impact of Barrel Toasting on Ellagitannin Composition of Aged Cognac Eaux-de-Vie" Molecules 27, no. 8: 2531. https://doi.org/10.3390/molecules27082531
APA StyleGadrat, M., Emo, C., Lavergne, J., Teissèdre, P.-L., & Chira, K. (2022). Impact of Barrel Toasting on Ellagitannin Composition of Aged Cognac Eaux-de-Vie. Molecules, 27(8), 2531. https://doi.org/10.3390/molecules27082531






