A New Cationic Fluorescent Probe for HSO3− Based on Bisulfite Induced Aggregation Self-Assembly
Abstract
:1. Introduction
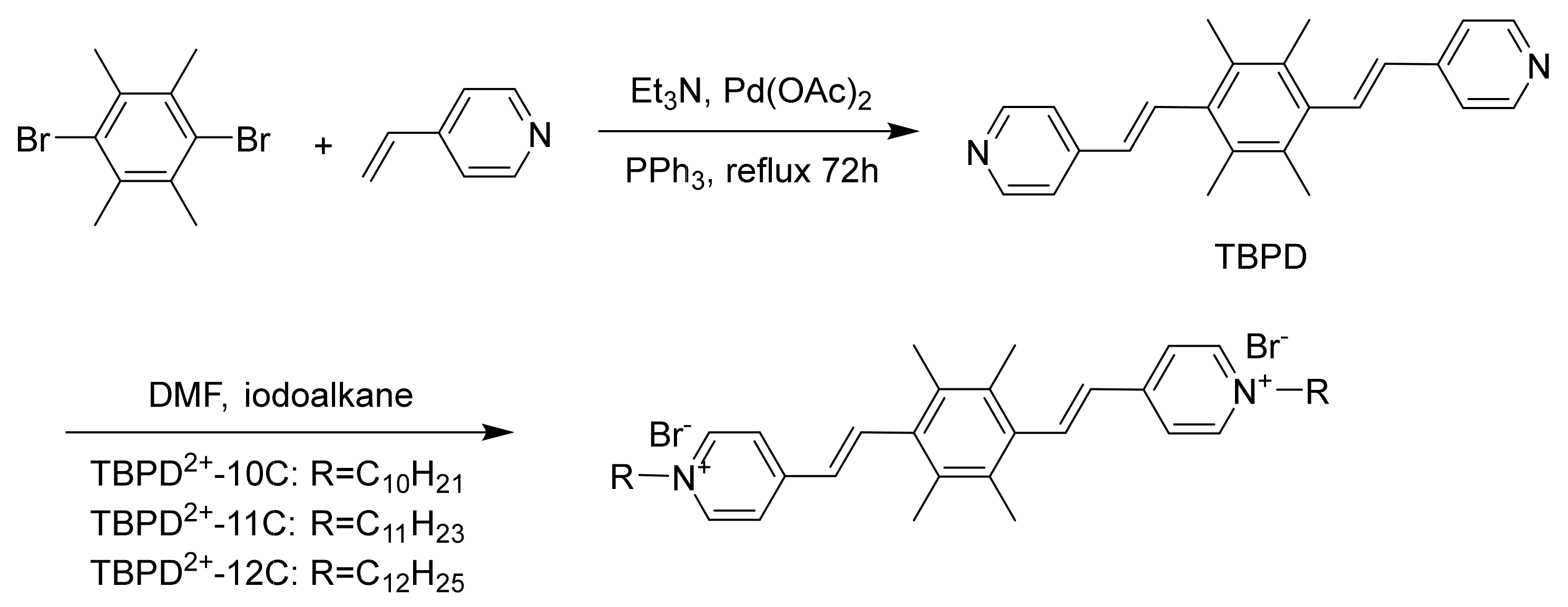
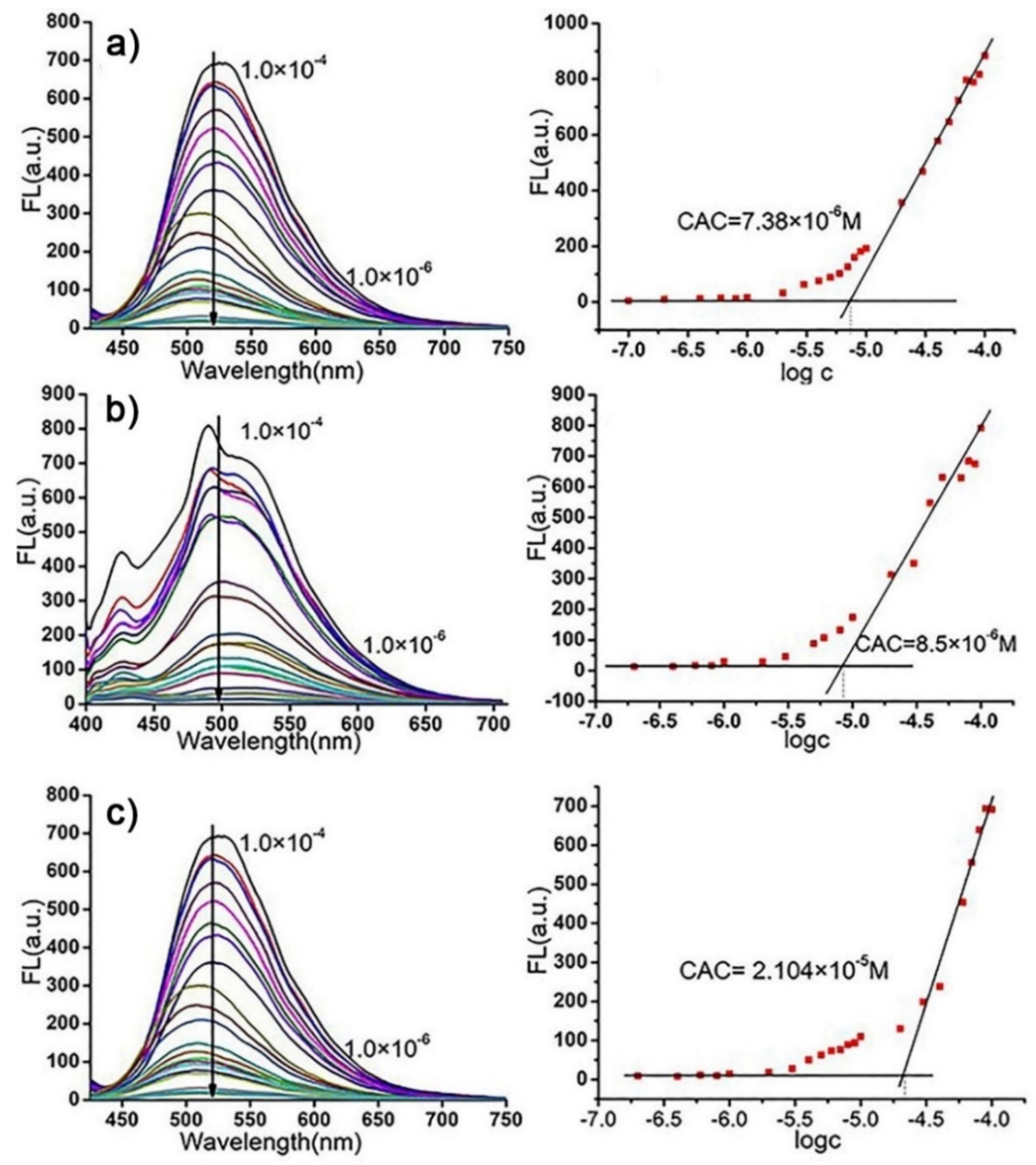
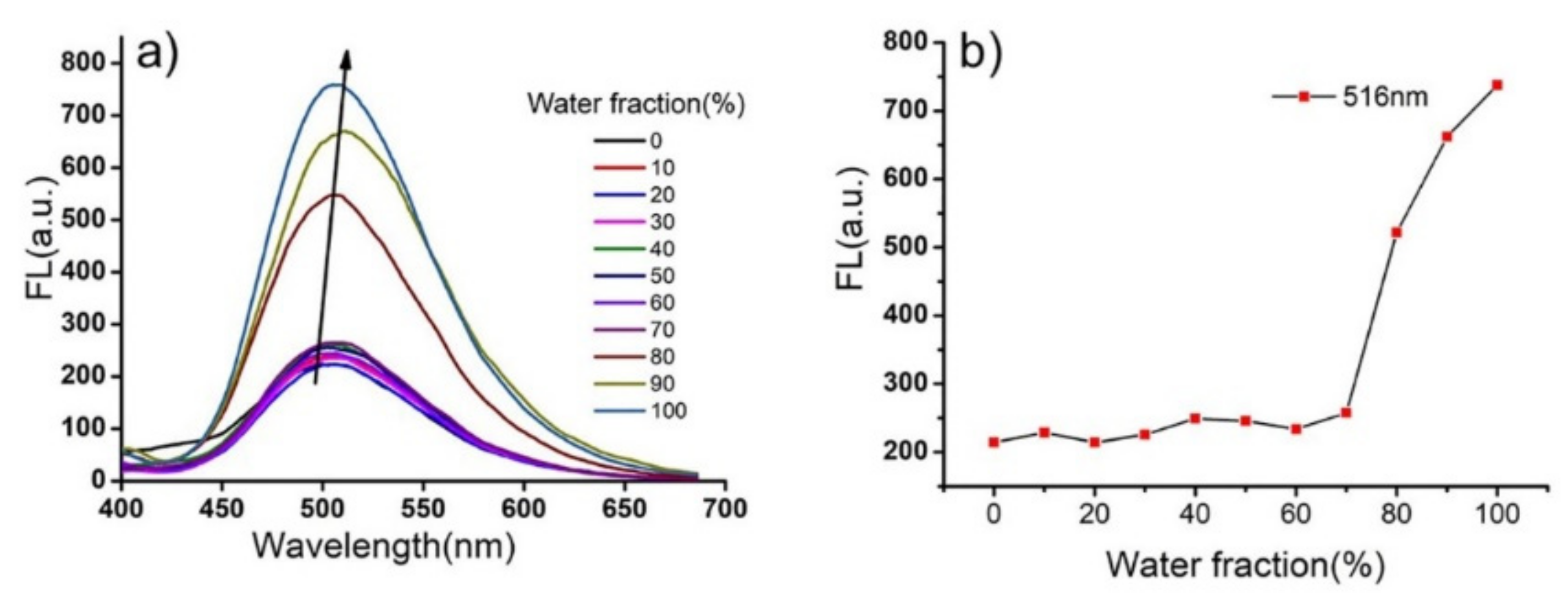
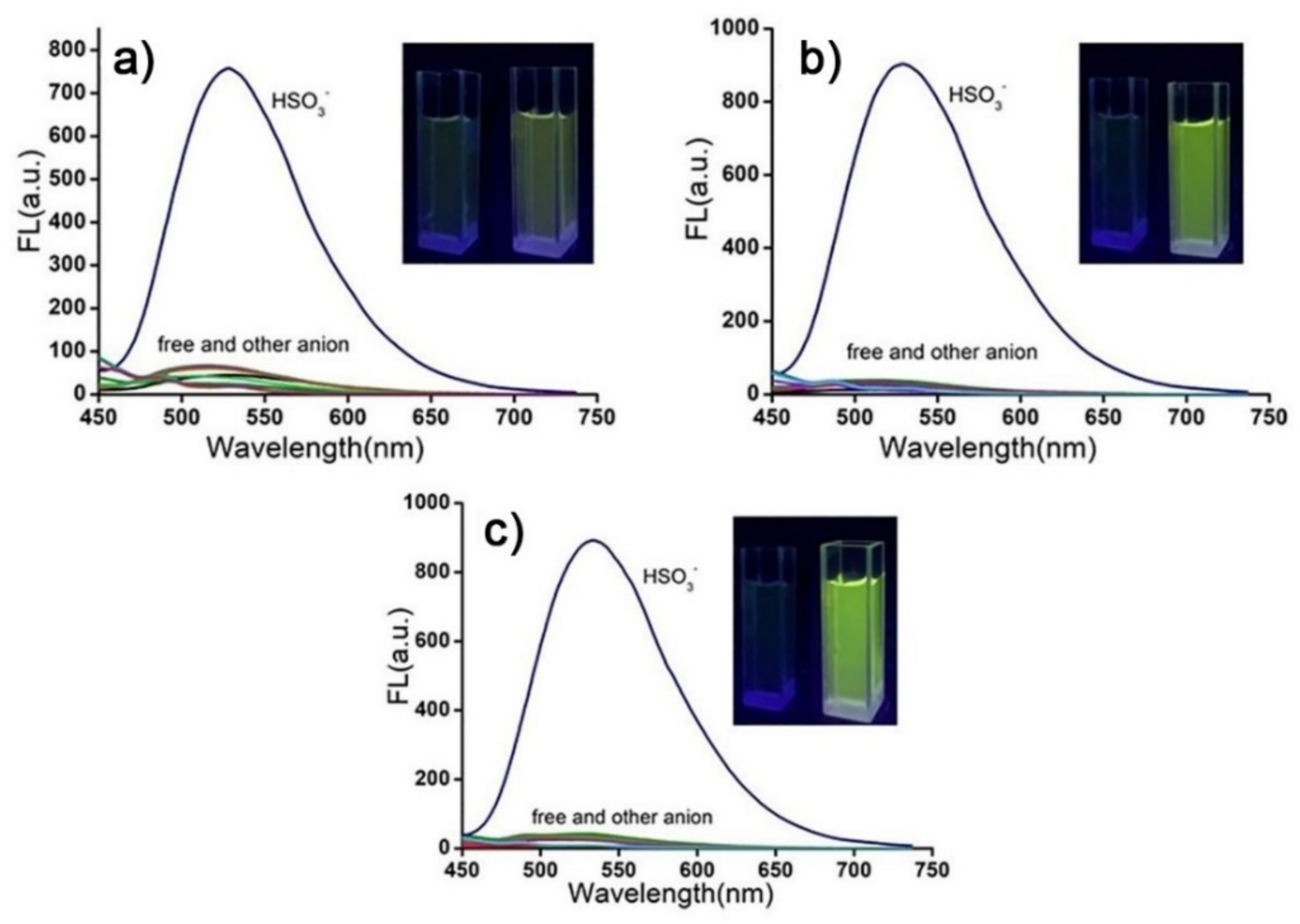
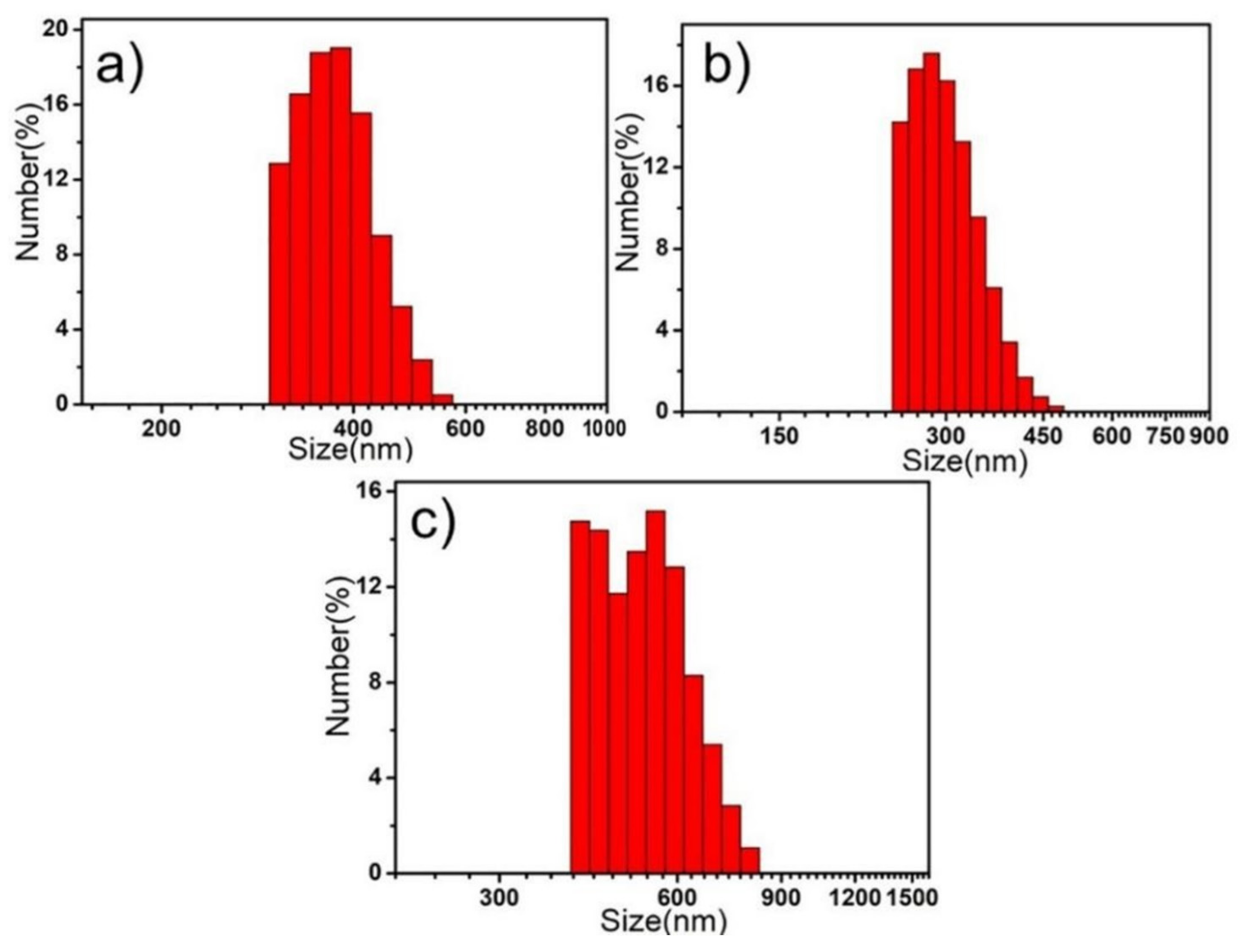
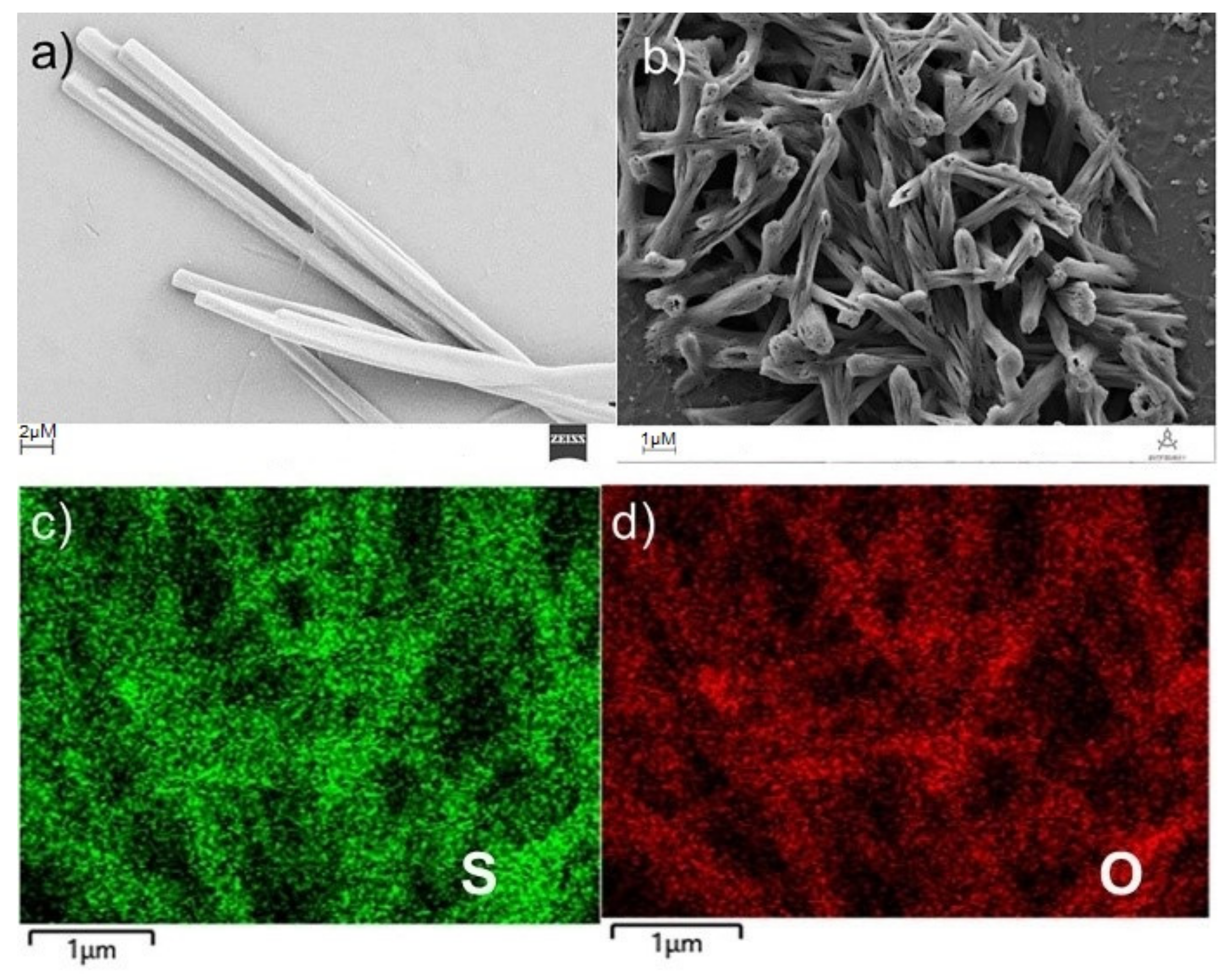
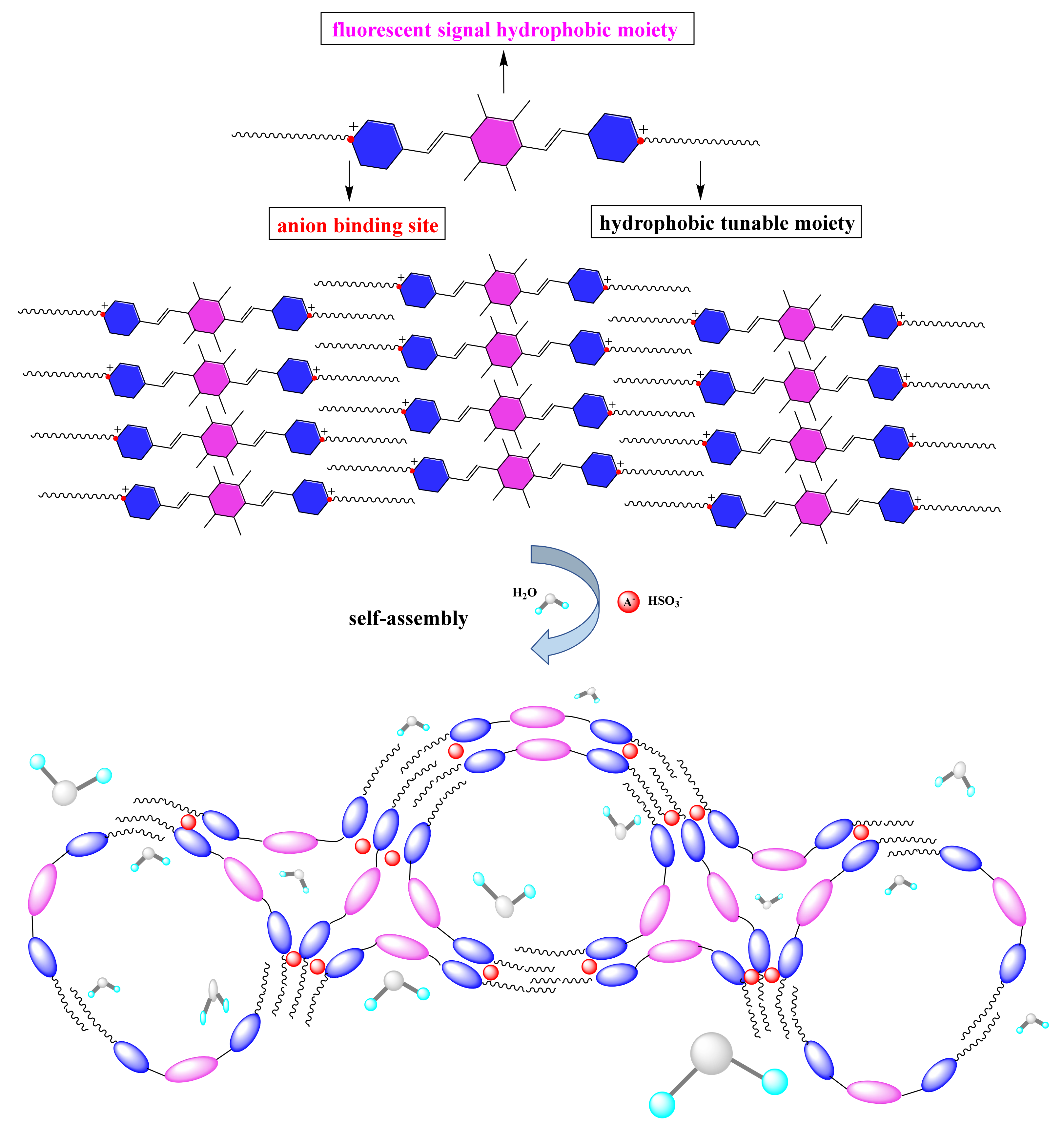
2. Results and Discussion
3. Experimental Section
3.1. Apparatus and Reagents
3.2. Synthesis and Characteristics of TBPD2+-10C, TBPD2+-11C and TBPD2+-12C
3.3. General Methods for Instrument Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ghozikali, M.G.; Heibati, B.; Naddafi, K.; Kloog, I.; Conti, G.O.; Polosa, R.; Ferrante, M. Evaluation of chronic obstructive pulmonary disease (COPD) attributed to atmospheric O3, NO2, and SO2 using Air Q Model (2011–2012 year). Environ. Res. 2016, 144, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, T.; Yin, G.; Niu, T.; Yin, P.; Li, H.; Zhang, Y.; Chen, H.; Zeng, Y.; Yao, S. A novel colorimetric and fluorescent probe for simultaneous detection of SO32−/HSO3− and HSO4− by different emission channels and its bioimaging in living cells. Talanta 2018, 176, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, R. Environmental management of sulfur trioxide emission: Impact of SO3 on human health. Environ. Manag. 2001, 27, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Zhong, K.; Chen, L.; Yan, X.; Tang, Y.; Hou, S.; Li, X.; Tang, L. Dual-functional multi-application probe: Rapid detection of H2S and colorimetric recognition of HSO3− in food and cell. Dye. Pigm. 2020, 182, 108656. [Google Scholar] [CrossRef]
- Rajapaksha, A.A.; Fu, Y.-X.; Guo, W.Y.; Liu, S.-Y.; Li, Z.-W.; Xiong, C.-Q.; Yang, W.-C.; Yang, G.-F. Review on the recent progress in the development of fluorescent probes targeting enzymes. Methods Appl. Fluoresc. 2021, 9, 032001. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Yu, Y.; Zhao, Q.; Tang, C.; Zhang, J. A review of bioselenol-specific fluorescent probes: Synthesis, properties, and imaging applications. Anal. Chim. Acta 2020, 1110, 141–150. [Google Scholar] [CrossRef]
- Chowdhury, S.; Rooj, B.; Dutta, A.; Mandal, U. Review on recent advances in metal ions sensing using different fluorescent probes. J. Fluoresc. 2018, 28, 999–1021. [Google Scholar] [CrossRef]
- Dai, J.; Ma, C.; Zhang, P.; Fu, Y.; Shen, B. Recent progress in the development of fluorescent probes for detection of biothiols. Dye. Pigm. 2020, 177, 108321. [Google Scholar] [CrossRef]
- Fu, Y.; Finney, N.S. Small-molecule fluorescent probes and their design. RSC Adv. 2018, 8, 29051–29061. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Huang, Y.; Tian, H. Progress and trends in AIE-based bioprobes: A brief overview. ACS Appl. Mater. Interfaces 2017, 10, 12217–12261. [Google Scholar] [CrossRef]
- Hu, R.; Qin, A.; Tang, B.Z. AIE polymers: Synthesis and applications. Prog. Polym. Sci. 2020, 100, 101176. [Google Scholar] [CrossRef]
- Gao, M.; Tang, B.Z. AIE-based cancer theranostics. Coord. Chem. Rev. 2020, 402, 213076. [Google Scholar] [CrossRef]
- Liu, S.; Feng, G.; Tang, B.Z.; Liu, B. Recent advances of AIE light-up probes for photodynamic therapy. Chem. Sci. 2021, 12, 6488. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, S.; Ni, X.-L. Anion recognition triggered nanoribbon-like self-assembly: A fluorescent chemosensor for nitrate in acidic aqueous solution and living cells. Anal. Chem. 2015, 87, 7461–7466. [Google Scholar] [CrossRef]
- Fueyo-González, F.; González-Vera, J.A.; Alkorta, I.; Infantes, L.; Jimeno, M.L.; Fernández-Gutiérrez, M.; González-García, M.C.; Orte, A.; Herranz, R. Fluorescence mechanism switching from ICT to PET by substituent chemical manipulation: Macrophage cytoplasm imaging probes. Dye. Pigm. 2020, 175, 108172. [Google Scholar] [CrossRef]
- Würthner, F. Aggregation-induced emission (AIE): A historical perspective. Angew. Chem. Int. Ed. 2020, 59, 14192–14196. [Google Scholar] [CrossRef]
- Su, S.; Zheng, Z.; Zhang, Q.-L.; Ni, X.-L. Fatty-chain-switched cationic fluorescent probe for SCN−, PF6−, and HSO3− recognition in water: Study of anion selectivity and sensitivity. Dye. Pigm. 2021, 185, 108903. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, L.-Y.; Zhang, Q.-L.; Yang, X.-J.; Huang, Y.-L.; Redshaw, C.; Xu, H. A simple turn-off Schiff base fluorescent sensor for copper (II) ion and its application in water analysis. Molecules 2021, 26, 1233. [Google Scholar] [CrossRef]
- Karakuş, E.; Gunduz, S.; Liv, L.; Ozturk, T. Fluorescent and electrochemical detection of Cu (II) ions in aqueous environment by a novel, simple and readily available AIE probe. J. Photochem. Photobiol. A Chem. 2020, 400, 112702. [Google Scholar] [CrossRef]
- Wang, P.; Cao, S.; Yin, T.; Ni, X.-L. Unprecedented tunable hydrophobic effect and anion recognition triggered by AIE with Hofmeister series in water. Chin. Chem. Lett. 2021, 32, 1679–1682. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.Y.; Li, Y.; Zhuang, J.B.; Cui, R.R.; Gong, Q.; Zhao, N.; Tang, B.Z. Fine tuning of emission behavior, self-assembly, anion sensing, and mitochondria targeting of pyridinium-functionalized tetraphenylethene by alkyl chain engineering. ACS Appl. Mater. Interfaces 2018, 10, 24249–24257. [Google Scholar] [CrossRef]
- Chen, S.; Ni, X.-L. Development of an AIE based fluorescent probe for the detection of nitrate anions in aqueous solution over a wide pH range. RSC Adv. 2016, 6, 6997–7001. [Google Scholar] [CrossRef]
- Cai, Z.-F.; Chen, T.; Gu, J.-Y.; Wang, D.; Wan, L.-J. Ionic interaction-induced assemblies of bimolecular “chessboard” structures. Chem. Commun. 2017, 53, 9129–9132. [Google Scholar] [CrossRef] [PubMed]
- D’Alfonso, A.; Freitag, B.; Klenov, D.; Allen, L. Atomic-resolution chemical mapping using energy-dispersive x-ray spectroscopy. Phys. Rev. B 2010, 81, 100101. [Google Scholar] [CrossRef]
- Gong, X.; Zhang, Q.-L.; Zhang, S.; Xia, Y.; Bai, Q.; Chen, S.; Ni, X.-L. A cationic fluorescent probe for BF4− and PF6− anions by aggregation self-assembly: An efficient approach to recognition of anions in aqueous solution. Dye. Pigm. 2019, 163, 502–508. [Google Scholar] [CrossRef]
- Shen, J.; Xin, X.; Liu, G.; Pang, J.; Song, Z.; Xu, G.; Yuan, S. Fabrication of smart pH-responsive fluorescent solid-like giant vesicles by ionic self-assembly strategy. J. Phys. Chem. C 2016, 120, 27533–27540. [Google Scholar] [CrossRef]
- Chen, H.; Huang, C.; Deng, Y.; Sun, Q.; Zhang, Q.-L.; Zhu, B.-X.; Ni, X.-L. Solvent-switched schiff-base macrocycles: Self-sorting and self-assembly-dependent unconventional organic particles. ACS Nano 2019, 13, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bonizzoni, M. A supramolecular sensing array for qualitative and quantitative analysis of organophosphates in water. J. Am. Chem. Soc. 2014, 136, 14223–14229. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Su, S.-Y.; Chen, X.-T.; Shen, L.-Y.; Zhang, Q.-L.; Ni, X.-L.; Xu, H.; Wang, Z.-Y.; Redshaw, C. A New Cationic Fluorescent Probe for HSO3− Based on Bisulfite Induced Aggregation Self-Assembly. Molecules 2022, 27, 2378. https://doi.org/10.3390/molecules27082378
Zhang X, Su S-Y, Chen X-T, Shen L-Y, Zhang Q-L, Ni X-L, Xu H, Wang Z-Y, Redshaw C. A New Cationic Fluorescent Probe for HSO3− Based on Bisulfite Induced Aggregation Self-Assembly. Molecules. 2022; 27(8):2378. https://doi.org/10.3390/molecules27082378
Chicago/Turabian StyleZhang, Xing, Shao-Yuan Su, Xuan-Ting Chen, Ling-Yi Shen, Qi-Long Zhang, Xin-Long Ni, Hong Xu, Zhi-Yong Wang, and Carl Redshaw. 2022. "A New Cationic Fluorescent Probe for HSO3− Based on Bisulfite Induced Aggregation Self-Assembly" Molecules 27, no. 8: 2378. https://doi.org/10.3390/molecules27082378
APA StyleZhang, X., Su, S.-Y., Chen, X.-T., Shen, L.-Y., Zhang, Q.-L., Ni, X.-L., Xu, H., Wang, Z.-Y., & Redshaw, C. (2022). A New Cationic Fluorescent Probe for HSO3− Based on Bisulfite Induced Aggregation Self-Assembly. Molecules, 27(8), 2378. https://doi.org/10.3390/molecules27082378





