Phosphorylation of cPLA2α at Ser505 Is Necessary for Its Translocation to PtdInsP2-Enriched Membranes
Abstract
:1. Introduction
2. Results
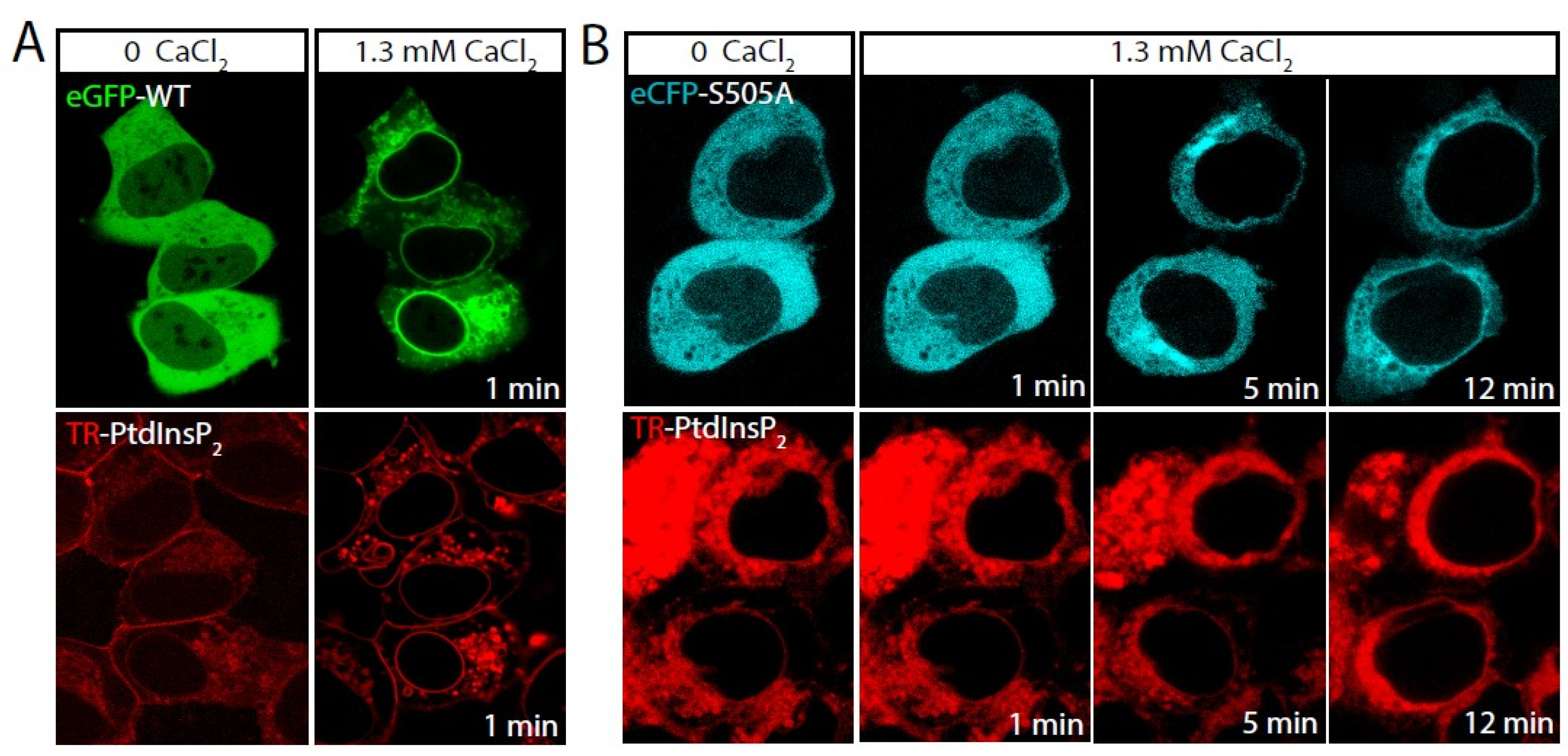
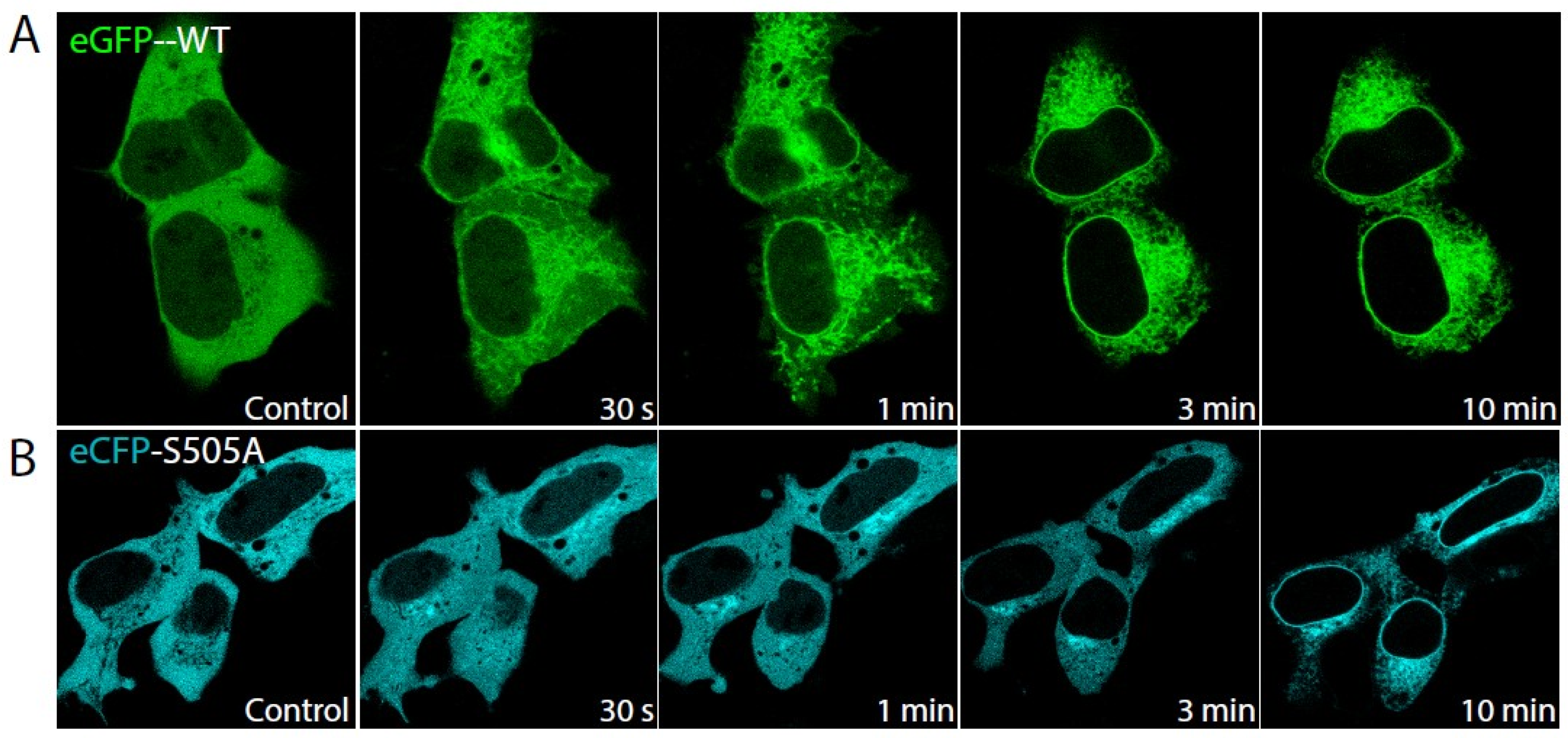
2.1. Role of cPLA2α Phosphorylation in Membrane Translocation in Response to Increases in PtdInsP2 and Calcium
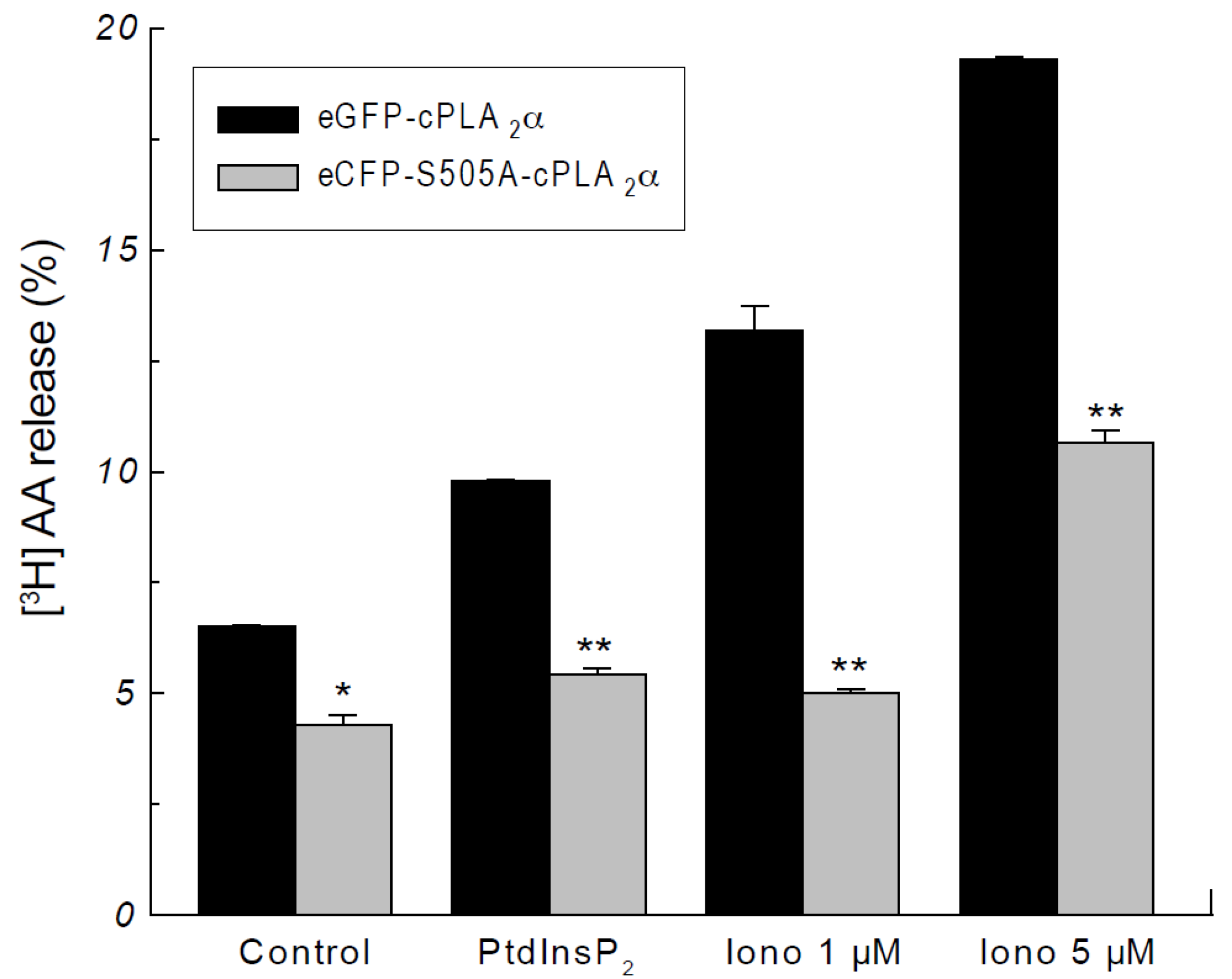
2.2. Phosphorylation of cPLA2α Is Necessary for AA Release in Response to PtdInsP2
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Cells
4.3. Lipid Preparation
4.4. Confocal Microscopy
4.5. AA Release
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dennis, E.A.; Norris, P.C. Eicosanoid storm in infection and inflammation. Nat. Rev. Immunol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Selectivity of phospholipid hydrolysis by phospholipase A2 enzymes in activated cells leading to polyunsaturated fatty acid mobilization. Biochim. Biophys. Acta 2019, 1864, 772–783. [Google Scholar] [CrossRef] [PubMed]
- Gil-de-Gómez, L.; Astudillo, A.M.; Guijas, C.; Magrioti, V.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cytosolic group IVA and calcium-independent group VIA phospholipase A2s act on distinct phospholipid pools in zymosan-stimulated mouse peritoneal macrophages. J. Immunol. 2014, 192, 752–762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubio, J.M.; Astudillo, A.M.; Casas, J.; Balboa, M.A.; Balsinde, J. Regulation of phagocytosis in macrophages by membrane ethanolamine plasmalogens. Front. Immunol. 2018, 9, 1723. [Google Scholar] [CrossRef] [PubMed]
- Lebrero, P.; Astudillo, A.M.; Rubio, J.M.; Fernández-Caballero, J.; Kokotos, G.; Balboa, M.A.; Balsinde, J. Cellular plasmalogen content does not influence arachidonic acid levels or distribution in macrophages: A role for cytosolic phospholipase A2γ in phospholipid remodeling. Cells 2019, 8, 799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M. Novel functions of phospholipase A2s: Overview. Biochim. Biophys. Acta 2019, 1864, 763–765. [Google Scholar] [CrossRef]
- Mouchlis, V.D.; Dennis, E.A. Phospholipase A2 catalysis and lipid mediator lipidomics. Biochim. Biophys. Acta 2019, 1864, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Jarc, E.; Petan, T. A twist of FATe: Lipid droplets and inflammatory lipid mediators. Biochimie 2020, 169, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Chang, Y.; Fan, J.; Ji, W.; Su, C. Phospholipase A2 superfamily in cancer. Cancer Lett. 2021, 497, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Shindou, H.; Shimizu, T. Cytosolic phospholipase A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta 2019, 1864, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Leslie, C.C. Cytosolic phospholipase A2: Physiological function and role in disease. J. Lipid Res. 2015, 56, 1386–1402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dessen, A. Structure and mechanism of human cytosolic phospholipase A2. Biochim. Biophys. Acta 2000, 488, 40–47. [Google Scholar] [CrossRef]
- Hirano, Y.; Gao, Y.G.; Stephenson, D.J.; Vu, N.T.; Malinina, L.; Simanshu, D.K.; Chalfant, C.E.; Patel, D.J.; Brown, R.E. Structural basis of phosphatidylcholine recognition by the C2–domain of cytosolic phospholipase A2α. eLife 2019, 8, e44760. [Google Scholar] [CrossRef]
- Ward, K.E.; Sengupta, R.; Ropa, J.P.; Amiar, S.; Stahelin, R.V. The cytosolic phospholipase A2α N-terminal C2 domain binds and oligomerizes on membranes with positive curvature. Biomolecules 2020, 10, 647. [Google Scholar] [CrossRef]
- Ward, K.E.; Bhardwaj, N.; Vora, M.; Chalfant, C.E.; Lu, H.; Stahelin, R.V. The molecular basis of ceramide-1-phosphate recognition by C2 domains. J. Lipid Res. 2013, 54, 636–648. [Google Scholar] [CrossRef] [Green Version]
- Stahelin, R.V.; Subramanian, P.; Vora, M.; Cho, W.; Chalfant, C.E. Ceramide-1-phosphate binds group IVA cytosolic phospholipase A2 via a novel site in the C2 domain. J. Biol. Chem. 2007, 282, 20467–20474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosior, M.; Six, D.A.; Dennis, E.A. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J. Biol. Chem. 1998, 273, 2184–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Cho, W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. J. Biol. Chem. 2002, 277, 23838–23846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, J.; Gijón, M.A.; Vigo, A.G.; Crespo, M.S.; Balsinde, J.; Balboa, M.A. Phosphatidylinositol 4,5-bisphosphate anchors cytosolic group IVA phospholipase A2 to perinuclear membranes and decreases its calcium requirement for translocation in live cells. Mol. Biol. Cell 2006, 17, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, J.; Valdearcos, M.; Pindado, J.; Balsinde, J.; Balboa, M.A. The cationic cluster of group IVA phospholipase A2 (Lys488/Lys541/Lys543/Lys544) is involved in translocation of the enzyme to phagosomes in human macrophages. J. Lipid Res. 2010, 51, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Chacón, G.; Astudillo, A.M.; Balgoma, D.; Balboa, M.A.; Balsinde, J. Control of free arachidonic acid levels by phospholipases A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta 2009, 1791, 1103–1113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, B.; Lee, H.; Jayaraja, S.; Suram, S.; Murphy, R.C.; Leslie, C.C. Prostaglandins from cytosolic phospholipase A2α/cyclooxygenase-1 pathway and mitogen-activated protein kinases regulate gene expression in Candida albicans-infected macrophages. J. Biol. Chem. 2016, 291, 7070–7086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruipérez, V.; Astudillo, M.A.; Balboa, M.A.; Balsinde, J. Coordinate regulation of TLR-mediated arachidonic acid mobilization in macrophages by group IVA and group V phospholipase A2s. J. Immunol. 2009, 182, 3877–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balboa, M.A.; Balsinde, J.; Dillon, D.A.; Carman, G.M.; Dennis, E.A. Proinflammatory macrophage-activating properties of the novel phospholipid diacylglycerol pyrophosphate. J. Biol. Chem. 1999, 274, 522–526. [Google Scholar] [CrossRef] [Green Version]
- Guijas, C.; Pérez-Chacón, G.; Astudillo, A.M.; Rubio, J.M.; Gil-de-Gómez, L.; Balboa, M.A.; Balsinde, J. Simultaneous activation of p38 and JNK by arachidonic acid stimulates the cytosolic phospholipase A2-dependent synthesis of lipid droplets in human monocytes. J. Lipid Res. 2012, 53, 2343–2354. [Google Scholar] [CrossRef] [Green Version]
- Casas, J.; Meana, C.; Esquinas, E.; Valdearcos, M.; Pindado, J.; Balsinde, J.; Balboa, M.A. Requirement of JNK-mediated phosphorylation for translocation of group IVA phospholipase A2 to phagosomes in human macrophages. J. Immunol. 2009, 183, 2767–2774. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Chen, Z.; Ciszewski, C.; Setty, M.; Solus, J.; Tretiakova, M.; Ebert, E.; Han, J.; Lin, A.; Guandalini, S.; et al. Cytosolic PLA2 is required for CTL-mediated immunopathology of celiac disease via NKG2D and IL-15. J. Exp. Med. 2009, 206, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Rafter, J.D.; Kim, K.P.; Gygi, S.P.; Cho, W. Mechanism of group IVA cytosolic phospholipase A2 activation by phosphorylation. J. Biol. Chem. 2003, 278, 41431–41442. [Google Scholar] [CrossRef] [Green Version]
- Ozaki, S.; DeWald, D.B.; Shope, J.C.; Chen, J.; Prestwich, G.D. Intracellular delivery of phosphoinositides and inositol phosphates using polyamine carriers. Proc. Natl. Acad. Sci. USA 2000, 97, 11286–11291. [Google Scholar] [CrossRef] [Green Version]
- Gil-de-Gómez, L.; Astudillo, A.M.; Meana, C.; Rubio, J.M.; Guijas, C.; Balboa, M.A.; Balsinde, J. A phosphatidylinositol species acutely generated by activated macrophages regulates innate immune responses. J. Immunol. 2013, 190, 5169–5177. [Google Scholar] [CrossRef] [Green Version]
- Six, D.A.; Dennis, E.A. Essential Ca2+-independent role of the group IVA cytosolic phospholipase A2 C2 domain for interfacial activity. J. Biol. Chem. 2003, 278, 23842–23850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casas, J.; Gijón, M.A.; Vigo, A.G.; Crespo, M.S.; Balsinde, J.; Balboa, M.A. Overexpression of cytosolic group IVA phospholipase A2 protects cells from Ca2+-dependent death. J. Biol. Chem. 2006, 281, 6106–6116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balboa, M.A.; Balsinde, J.; Dennis, E.A. Phosphorylation of cytosolic group IV phospholipase A2 is necessary but not sufficient for arachidonic acid release in P388D1 macrophages. Biochem. Biophys. Res. Commun. 2000, 267, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Dennis, E.A.; Cao, J.; Hsu, Y.H.; Magrioti, V.; Kokotos, G. Phospholipase A2 enzymes: Physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem. Rev. 2011, 111, 6130–6185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsinde, J.; Balboa, M.A.; Li, W.H.; Llopis, J.; Dennis, E.A. Cellular regulation of cytosolic group IV phospholipase A2 by phosphatidylinositol 4,5-bisphosphate. J. Immunol. 2000, 164, 5398–5402. [Google Scholar] [CrossRef] [Green Version]
- Börsch-Haubold, A.G.; Bartoli, F.; Asselin, J.; Dudler, T.; Kramer, R.M.; Apitz-Castro, R.; Watson, S.P.; Gelb, M.H. Identification of the phosphorylation sites of cytosolic phospholipase A2 in agonist-stimulated human platelets and HeLa cells. J. Biol. Chem. 1998, 273, 4449–4458. [Google Scholar] [CrossRef] [Green Version]
- Guijas, C.; Rodríguez, J.P.; Rubio, J.M.; Balboa, M.A.; Balsinde, J. Phospholipase A2 regulation of lipid droplet formation. Biochim. Biophys. Acta 2014, 1841, 1661–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balboa, M.A.; Pérez, R.; Balsinde, J. Amplification mechanisms of inflammation: Paracrine stimulation of arachidonic acid mobilization by secreted phospholipase A2 is regulated by cytosolic phospholipase A2-derived hydroperoxyeicosatetraenoic acid. J. Immunol. 2003, 171, 989–994. [Google Scholar] [CrossRef] [Green Version]
- Pérez, R.; Matabosch, X.; Llebaria, A.; Balboa, M.A.; Balsinde, J. Blockade of arachidonic acid incorporation into phospholipids induces apoptosis in U937 promonocytic cells. J. Lipid Res. 2006, 47, 484–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Diez, E.; Balsinde, J.; Aracil, M.; Schüller, A. Ethanol induces release of arachidonic acid but not synthesis of eicosanoids in mouse peritoneal macrophages. Biochim. Biophys. Acta 1987, 921, 82–89. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casas, J.; Balsinde, J.; Balboa, M.A. Phosphorylation of cPLA2α at Ser505 Is Necessary for Its Translocation to PtdInsP2-Enriched Membranes. Molecules 2022, 27, 2347. https://doi.org/10.3390/molecules27072347
Casas J, Balsinde J, Balboa MA. Phosphorylation of cPLA2α at Ser505 Is Necessary for Its Translocation to PtdInsP2-Enriched Membranes. Molecules. 2022; 27(7):2347. https://doi.org/10.3390/molecules27072347
Chicago/Turabian StyleCasas, Javier, Jesús Balsinde, and María A. Balboa. 2022. "Phosphorylation of cPLA2α at Ser505 Is Necessary for Its Translocation to PtdInsP2-Enriched Membranes" Molecules 27, no. 7: 2347. https://doi.org/10.3390/molecules27072347
APA StyleCasas, J., Balsinde, J., & Balboa, M. A. (2022). Phosphorylation of cPLA2α at Ser505 Is Necessary for Its Translocation to PtdInsP2-Enriched Membranes. Molecules, 27(7), 2347. https://doi.org/10.3390/molecules27072347







