ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum
Abstract
1. Introduction
2. Materials and Methods
2.1. Green Synthesis and Characterization of Zinc Oxide Nanoparticles (ZnO NP)
2.2. Characterization of ZnO NPs
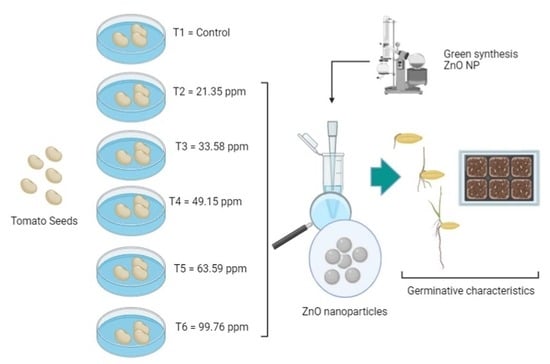
2.3. Germination of Seeds of L. esculentum
2.4. Inoculation of ZnO NPs in Seeds of L. esculentum
2.5. Characterization by Atomic Absorption of Seedlings of L. esculentum
2.6. Statistical Analysis
3. Results
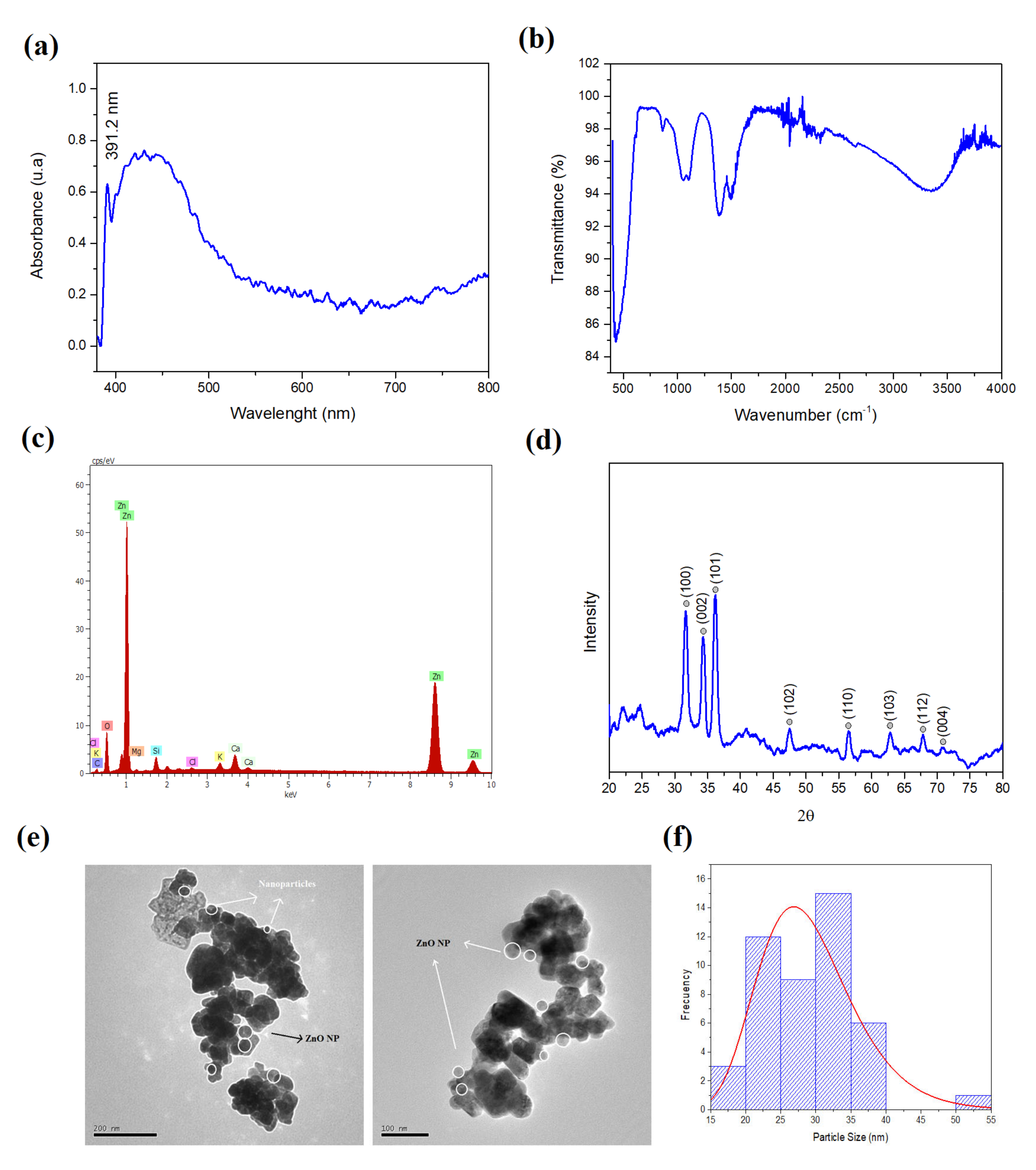
3.1. Characterization of ZnO NPs Mediated by Green Synthesis Using C. sativum Extract
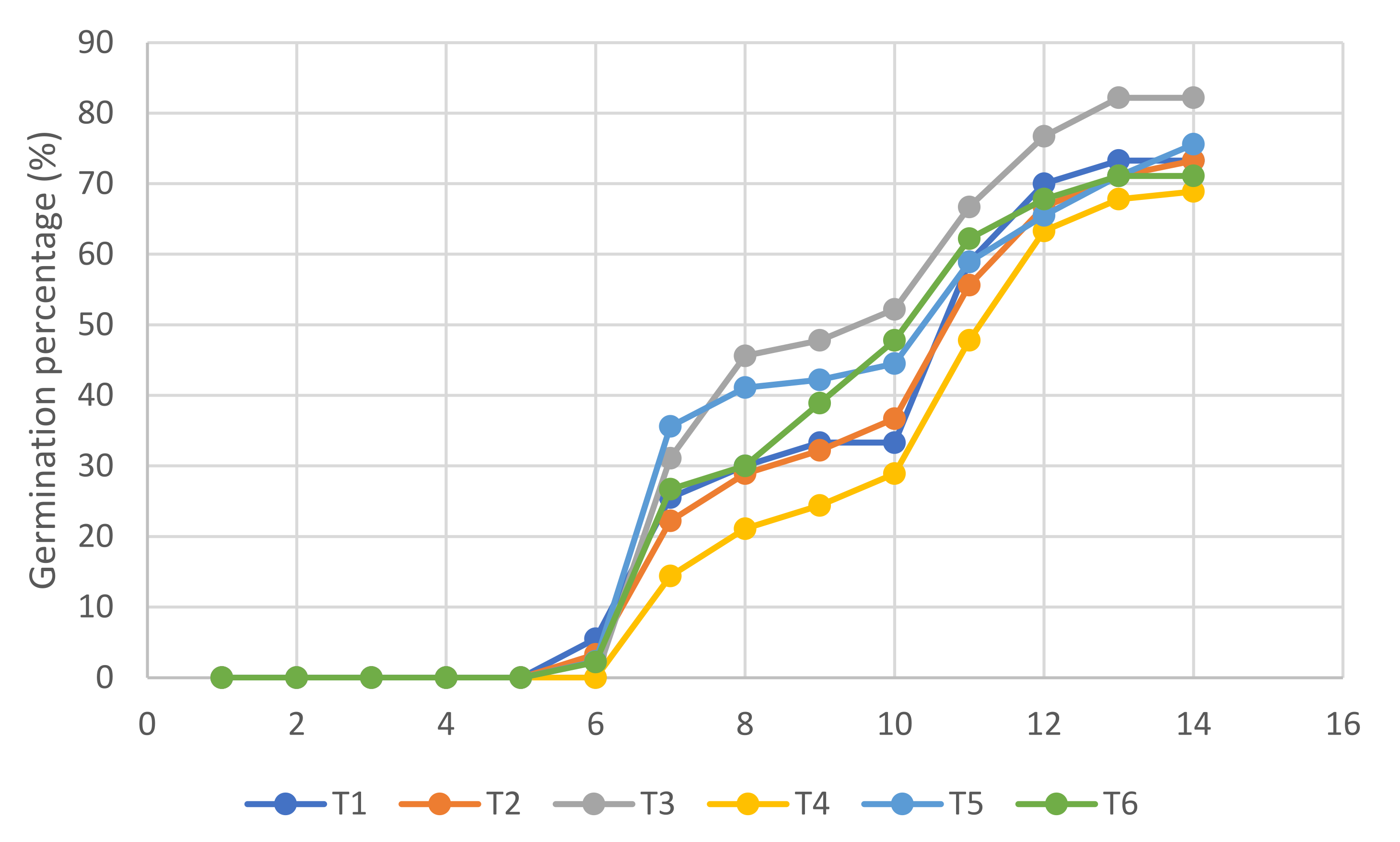
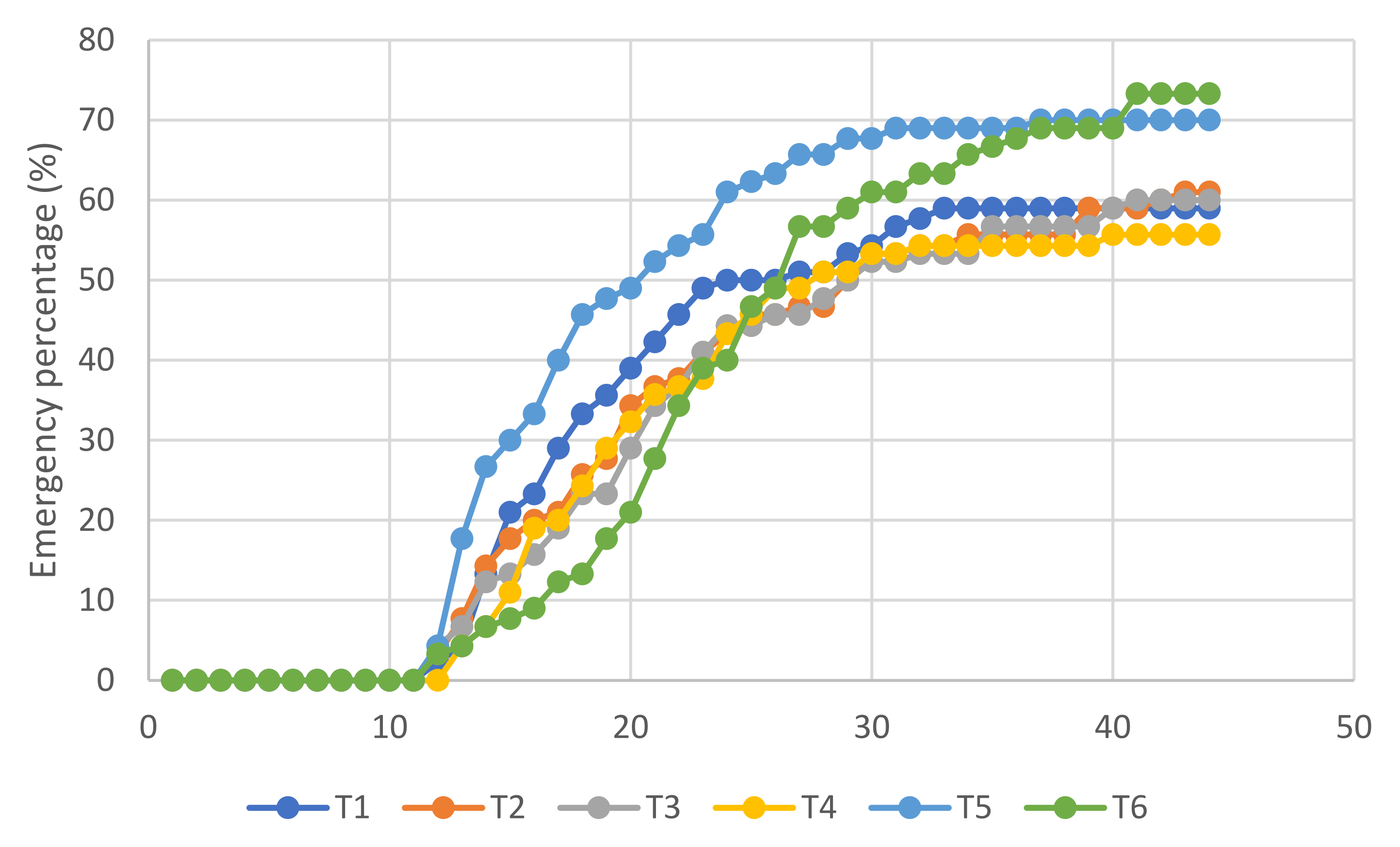
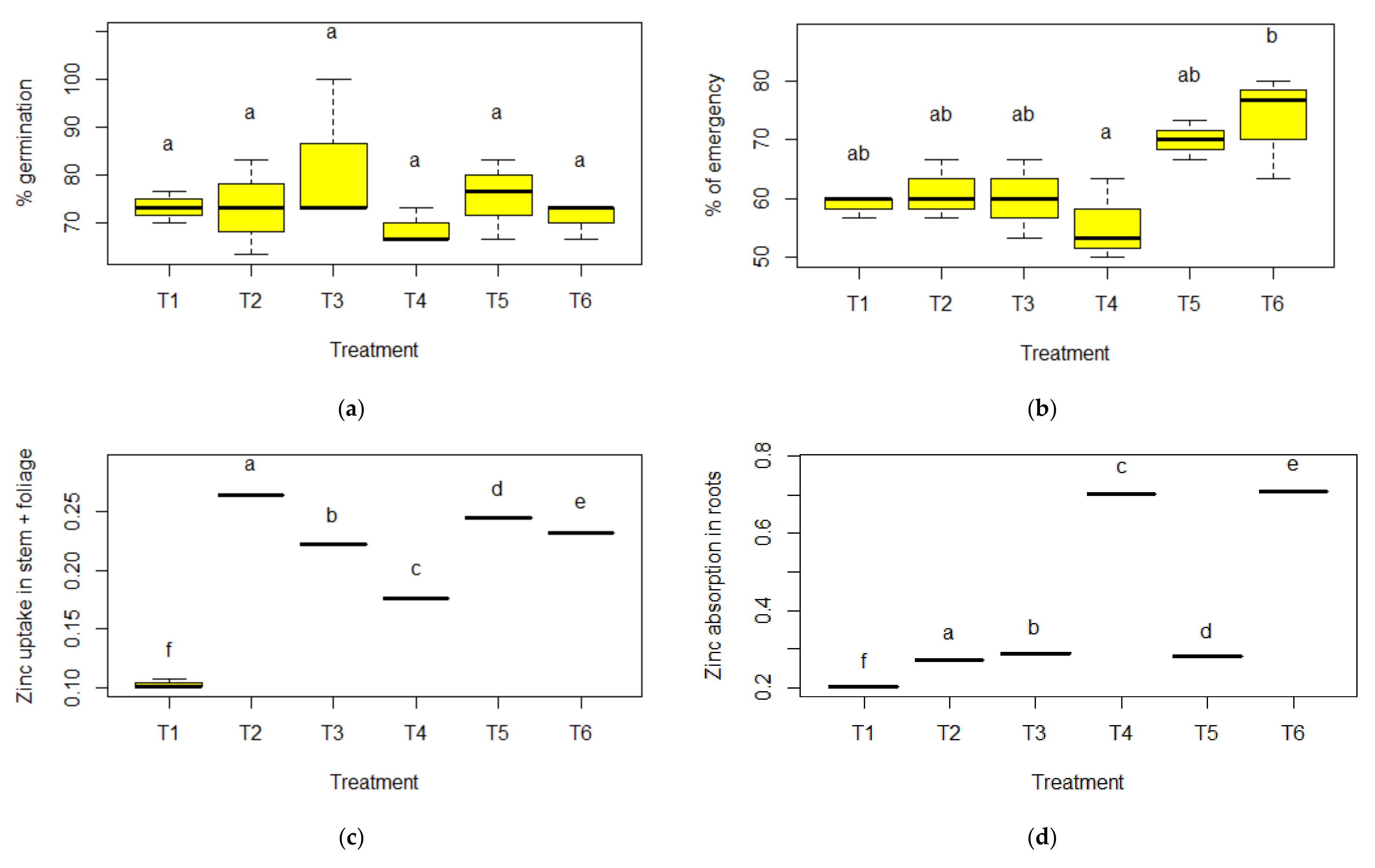
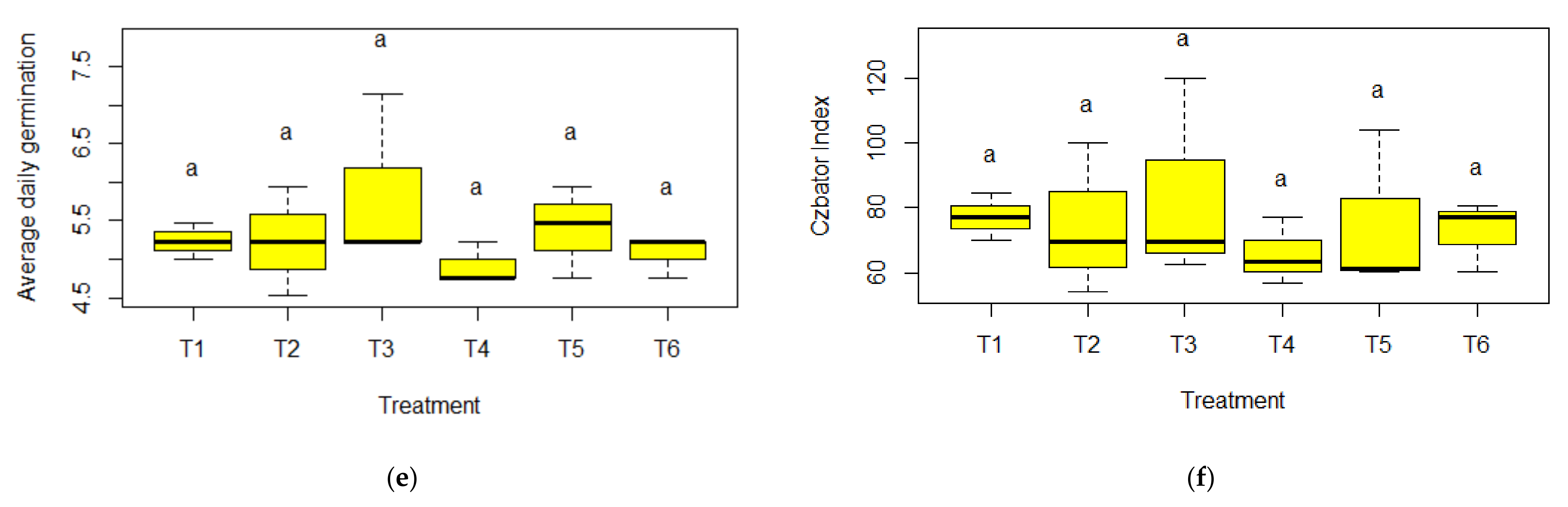
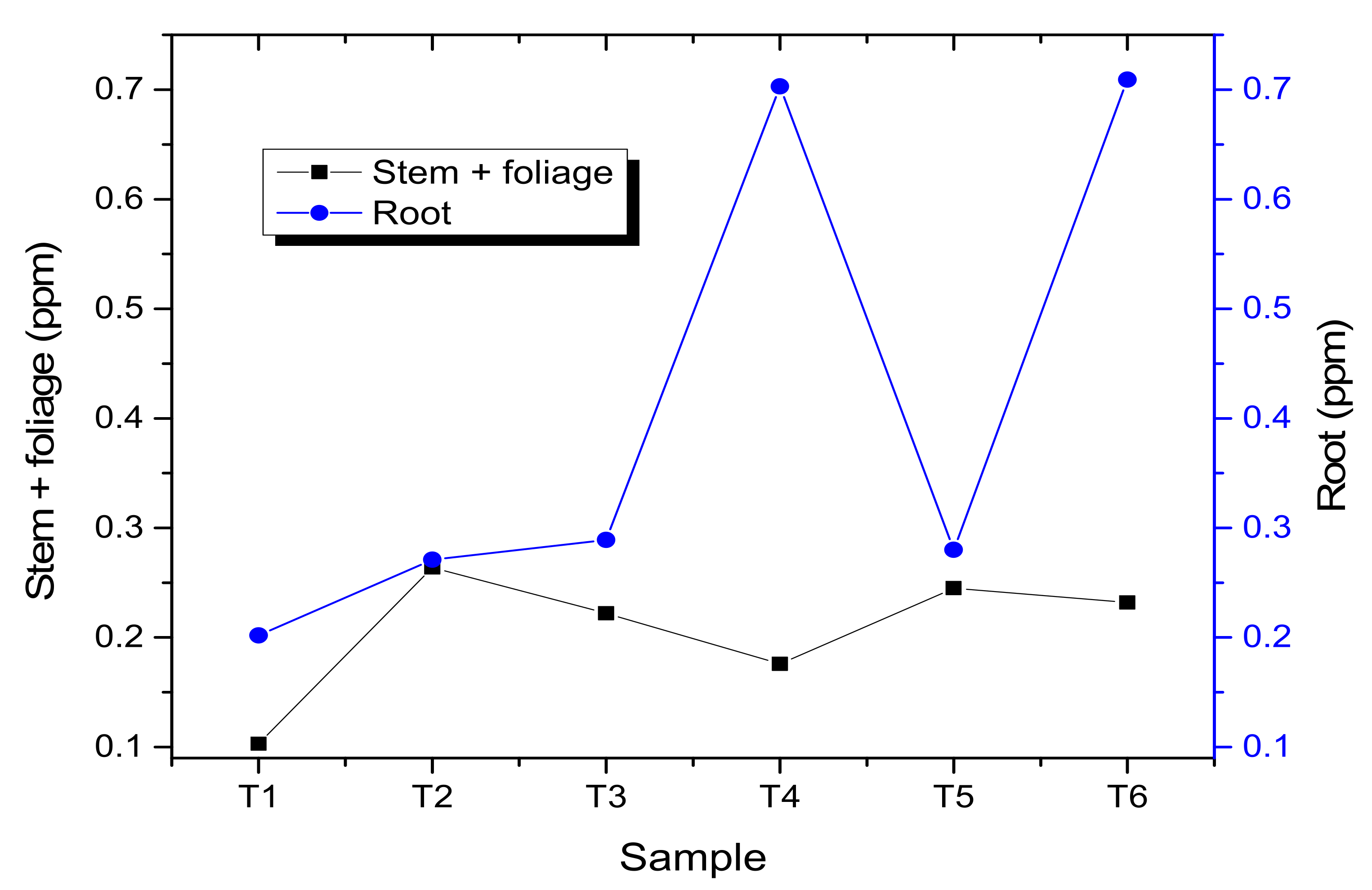
3.2. Germination and Emergence
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Torres, A. Manual de Cultivo del Tomate Bajo Invernadero; Fundación Universidad de Bogotá Jorge Tadeo Lozano: Bogotá, Colombia, 2017. [Google Scholar]
- Martí, R.; Roselló, S.; Cebolla-Cornejo, J. Tomato as a Source of Carotenoids and Polyphenols Targeted to Cancer Prevention. Cancers 2016, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Colmán Martínez, L.M. Efecto del Licopeno y los Carotenoides del Tomate en Marcadores Inflamatorios de la Aterosclerosis en Pacientes de Riesgo Cardiovascular; Universitat de Barcelona: Barcelona, Spain, 2016. [Google Scholar]
- Navarro-González, I.; Periago, M.J. El tomate, ¿alimento saludable y/o funcional? Rev. Esp. Nutr. Hum. Diet. 2016, 20, 323–335. [Google Scholar] [CrossRef][Green Version]
- Tedeschi, P.; Coïsson, J.D.; Maietti, A.; Cereti, E.; Stagno, C.; Travaglia, F.; Arlorio, M.; Brandolini, V. Chemotype and genotype combined analysis applied to tomato (Lycopersicon esculentum Mill.) analytical traceability. J. Food Compos. Anal. 2011, 24, 131–139. [Google Scholar] [CrossRef]
- Torres, C.A.; Andrews, P.K. Developmental changes in antioxidant metabolites, enzymes, and pigments in fruit exocarp of four tomato (Lycopersicon esculentum Mill.) genotypes: β-carotene, high pigment-1, ripening inhibitor, and ‘Rutgers’. Plant Physiol. Biochem. 2006, 44, 806–818. [Google Scholar] [CrossRef]
- Lahoz García, I.; Santos, A.; Malumbres Montorio, Á.; Bozal Yanguas, J.M.; Mauleón Burgos, J.; Arechalde Recio, A.; Calvillo Ruiz, S. Tomate de industria. Campaña 2018. Navarra Agrar. 2019, 11–20. [Google Scholar]
- Eloy, S.; Medina, L.; Efraín, A.; Rivero, G. Características germinativas de semillas de Theobroma cacao L. (Malvaceae) “cacao”. Arnaldoa 2017, 24, 609–618. [Google Scholar] [CrossRef]
- Kazmi, R.H.; Willems, L.A.J.; Joosen, R.V.L.; Khan, N.; Ligterink, W.; Hilhorst, H.W.M. Metabolomic analysis of tomato seed germination. Metabolomics 2017, 13, 145. [Google Scholar] [CrossRef]
- López Medina, E.; López Zavaleta, A.; de la Cruz Castillo, A. Efecto del ácido giberélico en la propagación in vitro de Stevia rebaudiana (Bertoni) Bertoni, “estevia”. Arnaldoa 2017, 24, 599–608. [Google Scholar] [CrossRef][Green Version]
- Iliger, K.S.; Sofi, T.A.; Bhat, N.A.; Ahanger, F.A.; Sekhar, J.C.; Elhendi, A.Z.; Al-Huqail, A.A.; Khan, F. Copper nanoparticles: Green synthesis and managing fruit rot disease of chilli caused by Colletotrichum capsici. Saudi J. Biol. Sci. 2021, 28, 1477–1486. [Google Scholar] [CrossRef]
- Gallo Ramírez, J.P.; Ossa Orozco, C.P.; Gallo Ramírez, J.P.; Ossa Orozco, C.P. Fabricación y caracterización de nanopartículas de plata con potencial uso en el tratamiento del cáncer de piel. Ing. Desarro. 2019, 37, 88–104. [Google Scholar] [CrossRef]
- Méndez-Argüello, B.; Vera-Reyes, I.; Mendoza-Mendoza, E.; García-Cerda, L.A.; Puente-Urbina, B.A.; Lira-Saldívar, R.H. Growth promotion of Capsicum annuum plants by zinc oxide nanoparticles. Nova Sci. 2016, 8, 140–156. [Google Scholar] [CrossRef]
- Oroz, M.M. Nanopartículas de plata: Métodos de síntesis en disolución y propiedades bactericidas. An. R. Soc. Esp. Quím. 2009, 1, 33–41. [Google Scholar]
- Noman, M.; Shahid, M.; Ahmed, T.; Tahir, M.; Naqqash, T.; Muhammad, S.; Song, F.; Abid, H.M.A.; Aslam, Z. Green copper nanoparticles from a native Klebsiella pneumoniae strain alleviated oxidative stress impairment of wheat plants by reducing the chromium bioavailability and increasing the growth. Ecotoxicol. Environ. Saf. 2020, 192, 110303. [Google Scholar] [CrossRef] [PubMed]
- Altuwirqi, R.M.; Albakri, A.S.; Al-Jawhari, H.; Ganash, E.A. Green synthesis of copper oxide nanoparticles by pulsed laser ablation in spinach leaves extract. Optik 2020, 219, 165280. [Google Scholar] [CrossRef]
- Asmat-Campos, D.; Abreu, A.C.; Romero-Cano, M.S.; Urquiaga-Zavaleta, J.; Contreras-Cáceres, R.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Nazario-Naveda, R.; Rengifo-Penadillos, R.; Fernández, I. Unraveling the Active Biomolecules Responsible for the Sustainable Synthesis of Nanoscale Silver Particles through Nuclear Magnetic Resonance Metabolomics. ACS Sustain. Chem. Eng. 2020, 8, 17816–17827. [Google Scholar] [CrossRef]
- Mohamed, E.A. Green synthesis of copper & copper oxide nanoparticles using the extract of seedless dates. Heliyon 2020, 6, e03123. [Google Scholar] [CrossRef]
- Siddiqui, V.U.; Ansari, A.; Chauhan, R.; Siddiqi, W.A. Green synthesis of copper oxide (CuO) nanoparticles by Punica granatum peel extract. Mater. Today Proc. 2021, 36, 751–755. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef]
- Phongtongpasuk, S.; Poadang, S.; Yongvanich, N. Environmental-friendly Method for Synthesis of Silver Nanoparticles from Dragon Fruit Peel Extract and their Antibacterial Activities. Energy Procedia 2016, 89, 239–247. [Google Scholar] [CrossRef]
- Luminita, D.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanoparticles using European black elderberry fruits extract. Colloids Surf. B. Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef]
- Odeniyi, M.A.; Okumah, V.C.; Adebayo-Tayo, B.C.; Odeniyi, O.A. Green synthesis and cream formulations of silver nanoparticles of Nauclea latifolia (African peach) fruit extracts and evaluation of antimicrobial and antioxidant activities. Sustain. Chem. Pharm. 2020, 15, 100197. [Google Scholar] [CrossRef]
- Abdulhameed, M.F.; Taha, A.A.; Ismail, R.A. Improvement of cabbage growth and yield by nanofertilizers and nanoparticles. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100437. [Google Scholar] [CrossRef]
- Imada, K.; Sakai, S.; Kajihara, H.; Tanaka, S.; Ito, S. Magnesium oxide nanoparticles induce systemic resistance in tomato against bacterial wilt disease. Plant Pathol. 2016, 65, 551–560. [Google Scholar] [CrossRef]
- Rico, C.M.; Lee, S.C.; Rubenecia, R.; Mukherjee, A.; Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Cerium oxide nanoparticles impact yield and modify nutritional parameters in wheat (triticum aestivum L.). J. Agric. Food Chem. 2014, 62, 9669–9675. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Kumar, S.; Alok, A.; Upadhyay, S.K.; Rawat, M.; Tsang, D.C.W.; Bolan, N.; Kim, K.H. The potential of green synthesized zinc oxide nanoparticles as nutrient source for plant growth. J. Clean. Prod. 2019, 214, 1061–1070. [Google Scholar] [CrossRef]
- Arumugam, M.; Manikandan, D.B.; Dhandapani, E.; Sridhar, A.; Balakrishnan, K.; Markandan, M.; Ramasamy, T. Green synthesis of zinc oxide nanoparticles (ZnO NPs) using Syzygium cumini: Potential multifaceted applications on antioxidants, cytotoxic and as nanonutrient for the growth of Sesamum indicum. Environ. Technol. Innov. 2021, 23, 101653. [Google Scholar] [CrossRef]
- Urbina, J.E. Influencia de las Nanopartículas de Óxido de Zinc Sobre la Calidad Fisiológica y Sanitaria de la Semilla de Maíz Nativo (Zea Mays L.); Universidad Autónoma de Guerrero: Iguala de la Independencia, Mexico, 2018. [Google Scholar]
- Rivero, A.G. Características germinativas de semillas del algodón nativo, Gossypium sp., de fibra verde, lila y marrón. Rebiol 2016, 35, 39–46. [Google Scholar]
- Rabieh, S.; Bagheri, M.; Heydari, M.; Badiei, E. Microwave assisted synthesis of ZnO nanoparticles in ionic liquid [Bmim]cl and their photocatalytic investigation. Mater. Sci. Semicond. Process. 2014, 26, 244–250. [Google Scholar] [CrossRef]
- Rajapriya, M.; Sharmili, S.A.; Baskar, R.; Balaji, R.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Alanzi, K.F.; Vaseeharan, B. Synthesis and Characterization of Zinc Oxide Nanoparticles Using Cynara scolymus Leaves: Enhanced Hemolytic, Antimicrobial, Antiproliferative, and Photocatalytic Activity. J. Clust. Sci. 2019, 31, 791–801. [Google Scholar] [CrossRef]
- Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M. Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 2016, 84, 1213–1222. [Google Scholar] [CrossRef]
- Handore, K.; Bhavsar, S.; Horne, A.; Chhattise, P.; Mohite, K.; Ambekar, J.; Pande, N.; Chabukswar, V. Novel Green Route of Synthesis of ZnO Nanoparticles by Using Natural Biodegradable Polymer and Its Application as a Catalyst for Oxidation of Aldehydes. J. Macromol. Sci. Part A 2014, 51, 941–947. [Google Scholar] [CrossRef]
- Das, R.K.; Gogoi, N.; Bora, U. Green synthesis of gold nanoparticles using Nyctanthes arbortristis flower extract. Bioprocess Biosyst. Eng. 2011, 34, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Adu, M.O.; Cobbinah, T.; Asare, P.A.; Yawson, D.O.; Taah, K.J. Demucilaging Freshly Stored Seeds of Cocoa (Theobroma cacao L.) Improves Seedling Emergence and Growth. J. Bot. 2017, 2017, 1938359. [Google Scholar] [CrossRef]
- García-Osuna, H.T.; Bocardo, L.E.; Robledo-Torres, V.; Mendoza, A.B.; Godina, F.R. Germinación y micropropagación de tomate de cáscara (Physalis ixocarpa) tetraploide. Rev. Mex. Cienc. Agrícolas 2015, 6, 2301–2311. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Xiong, Y.; Wei-Ning, W.; Quinl, D.; Pratim, B. Standardization of size, shape and concentration of nanoparticle for plant application. Appl. Biol. Res. 2012, 14, 35–44. [Google Scholar]
- Lira Saldivar, R.H.; Méndez Argüello, B.; de los Santos Villarreal, G.; Vera Reyes, I. Potencial de la nanotecnología en la agricultura. Acta Univ. 2018, 28, 9–24. [Google Scholar] [CrossRef]
- Wierzbicka, M.S.; Obidzińska, J. The effect of lead on seed imbibition and germination in different plant species. Plant Sci. 1998, 137, 155–171. [Google Scholar] [CrossRef]
- Munzuroglu, O.; Geckil, H. Effects of Metals on Seed Germination, Root Elongation, and Coleoptile and Hypocotyl Growth in Triticum aestivum and Cucumis sativus. Arch. Environ. Contam. Toxicol. 2001, 43, 203–213. [Google Scholar] [CrossRef]
- Wang, X.; Sun, C.; Gao, S.; Wang, L.; Shuokui, H. Validation of germination rate and root elongation as indicator to assess phytotoxicity with Cucumis sativus. Chemosphere 2001, 44, 1711–1721. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Mashwani, Z.U.R.; Iqbal, M.; Ejaz, M.; Yasmeen, F.; Sohail, S. In vitro germination and biochemical profiling of citrus reticulata in response to green synthesised zinc and copper nanoparticles. IET Nanobiotechnol. 2017, 11, 790–796. [Google Scholar] [CrossRef]
- Regni, L.; del Buono, D.; Micheli, M.; Facchin, S.L.; Tolisano, C.; Proietti, P. Effects of Biogenic ZnO Nanoparticles on Growth, Physiological, Biochemical Traits and Antioxidants on Olive Tree In Vitro. Horticulturae 2022, 8, 161. [Google Scholar] [CrossRef]
- Hernández-López, A.; Beltrán-Peña, G.; Elenir, A.; Oliveira, A.; Nuñez Gaona, O.; Avila-Alejandre, A.X. Preacondicionamiento del agua en la germinación y emergencia de Capsicum chinense Jacq. Rev. Mex. Cienc. Agrícolas 2018, 9, 1703–1714. [Google Scholar] [CrossRef]
- Auld, D.S. Zinc coordination sphere in biochemical zinc sites. Biometals 2001, 14, 271–313. [Google Scholar] [CrossRef] [PubMed]
- Kornarzyński, K.; Sujak, A.; Czernel, G.; Wiącek, D. Effect of Fe3O4 nanoparticles on germination of seeds and concentration of elements in Helianthus annuus L. under constant magnetic field. Sci. Rep. 2020, 10, 8068. [Google Scholar] [CrossRef] [PubMed]
- Intriago Rojas, L.E. Efecto del Tratamiento de Semillas con zn Sobre la Germinaciòn y Vigor de Plàntulas de Maìz Dulce (Zea Mays L.) var. Bandit; Universidad de las Fuerzas Armadas ESPE. IASA I. Carrera de Ingeniería de Ciencias Agropecuarias: El Prado, Ecuador, 2021. [Google Scholar]
- De Souza, J.; Júnior, A.; Baudet, L.M.; de Araújo Rufino, C.; Fernandes Veira, J.; Martín-Gil, J.; Martín-Ramos, P. Efectos del recubrimiento con sulfato de zinc sobre tasas de germinación y niveles de isoenzimas en semillas de algodón. In Proceedings of the X Congreso Ibérico de Agroingeniería, Huesca, España, 3–6 September 2019; pp. 1–11. [Google Scholar]
- Neto, M.E.; Britt, D.W.; Lara, L.M.; Cartwright, A.; dos Santos, R.F.; Inoue, T.T.; Batista, M.A. Initial Development of Corn Seedlings after Seed Priming with Nanoscale Synthetic Zinc Oxide. Agronmy 2020, 10, 307. [Google Scholar] [CrossRef]
- Espitia, M.; Cardona, C.; Araméndiz, H. Pruebas de germinación de semillas de forestales nativos de Córdoba, Colombia, en laboratorio y casa-malla. Rev. UDCA Actual. Divulg. Científica 2016, 19, 307–315. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Norouzi, M.; Saeri, M. Impact of zinc and zinc oxide nanoparticles on the physiological and biochemical processes in tomato and wheat. Botany 2016, 95, 441–455. [Google Scholar] [CrossRef]
- Sturikova, H.; Krystofova, O.; Huska, D.; Adam, V. Zinc, zinc nanoparticles and plants. J. Hazard. Mater. 2018, 349, 101–110. [Google Scholar] [CrossRef]
- Garciá-López, J.I.; Zavala-Garcia, F.; Olivares-Saénz, E.; Lira-Saldivar, R.H.; Barriga-Castro, E.D.; Ruiz-Torres, N.A.; Ramos-Cortez, E.; Vázquez-Alvarado, R.; Ninõ-Medina, G. Zinc Oxide Nanoparticles Boosts Phenolic Compounds and Antioxidant Activity of Capsicum annuum L. during Germination. Agronmy 2018, 8, 215. [Google Scholar] [CrossRef]
- Tondey, M.; Kalia, A.; Singh, A.; Dheri, G.S.; Taggar, M.S.; Nepovimova, E.; Krejcar, O.; Kuca, K. Seed Priming and Coating by Nano-Scale Zinc Oxide Particles Improved Vegetative Growth, Yield and Quality of Fodder Maize (Zea mays). Agronmy 2021, 11, 729. [Google Scholar] [CrossRef]
- Acharya, P.; Jayaprakasha, G.K.; Crosby, K.M.; Jifon, J.L.; Patil, B.S. Nanoparticle-Mediated Seed Priming Improves Germination, Growth, Yield, and Quality of Watermelons (Citrullus lanatus) at multi-locations in Texas. Sci. Rep. 2020, 10, 5037. [Google Scholar] [CrossRef] [PubMed]
- Changmei, L.; Chaoying, Z.; Junqiang, W.; Guorong, W.; Mingxuan, T. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 2001, 3, 168–171. [Google Scholar]
- Spanò, C.; Bottega, S.; Bellani, L.; Muccifora, S.; Sorce, C.; Ruffini Castiglione, M. Effect of Zinc Priming on Salt Response of Wheat Seedlings: Relieving or Worsening? Plants 2020, 9, 1514. [Google Scholar] [CrossRef] [PubMed]
- El-Zohri, M.; Al-Wadaani, N.A.; Bafeel, S.O. Foliar Sprayed Green Zinc Oxide Nanoparticles Mitigate Drought-Induced Oxidative Stress in Tomato. Plants 2021, 10, 2400. [Google Scholar] [CrossRef]
- Awad, A.A.M.; Sweed, A.A.A.; Rady, M.M.; Majrashi, A.; Ali, E.F. Rebalance the Nutritional Status and the Productivity of High CaCO3-Stressed Sweet Potato Plants by Foliar Nourishment with Zinc Oxide Nanoparticles and Ascorbic Acid. Agronmy 2021, 11, 1443. [Google Scholar] [CrossRef]
- Elhawat, N.; Alshaal, T.; Hamad, E.; El-Nahrawy, E.; Omara, A.E.D.; El-Nahrawy, S.; Elsakhawy, T.; Ghazi, A.; Abdalla, N.; Domokos-Szabolcsy, É. Nanoparticle-Associated Phytotoxicity and Abiotic Stress under Agroecosystems. Phytotoxicity Nanoparticles 2018, 241–268. [Google Scholar] [CrossRef]
- Brunner, T.J.; Wick, P.; Manser, P.; Spohn, P.; Grass, R.N.; Limbach, L.K.; Bruinink, A.; Stark, W.J. In vitro cytotoxicity of oxide nanoparticles: Comparison to asbestos, silica, and the effect of particle solubility. Environ. Sci. Technol. 2006, 40, 4374–4381. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asmat-Campos, D.; López-Medina, E.; Montes de Oca-Vásquez, G.; Gil-Rivero, E.; Delfín-Narciso, D.; Juárez-Cortijo, L.; Villena-Zapata, L.; Gurreonero-Fernández, J.; Rafael-Amaya, R. ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum. Molecules 2022, 27, 2343. https://doi.org/10.3390/molecules27072343
Asmat-Campos D, López-Medina E, Montes de Oca-Vásquez G, Gil-Rivero E, Delfín-Narciso D, Juárez-Cortijo L, Villena-Zapata L, Gurreonero-Fernández J, Rafael-Amaya R. ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum. Molecules. 2022; 27(7):2343. https://doi.org/10.3390/molecules27072343
Chicago/Turabian StyleAsmat-Campos, David, Eloy López-Medina, Gabriela Montes de Oca-Vásquez, Efraín Gil-Rivero, Daniel Delfín-Narciso, Luisa Juárez-Cortijo, Luigi Villena-Zapata, Julio Gurreonero-Fernández, and Roly Rafael-Amaya. 2022. "ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum" Molecules 27, no. 7: 2343. https://doi.org/10.3390/molecules27072343
APA StyleAsmat-Campos, D., López-Medina, E., Montes de Oca-Vásquez, G., Gil-Rivero, E., Delfín-Narciso, D., Juárez-Cortijo, L., Villena-Zapata, L., Gurreonero-Fernández, J., & Rafael-Amaya, R. (2022). ZnO Nanoparticles Obtained by Green Synthesis as an Alternative to Improve the Germination Characteristics of L. esculentum. Molecules, 27(7), 2343. https://doi.org/10.3390/molecules27072343