Photoacoustic Effect of Near-Infrared Absorbing Organic Molecules via Click Chemistry
Abstract
:1. Introduction
2. Materials and Methods
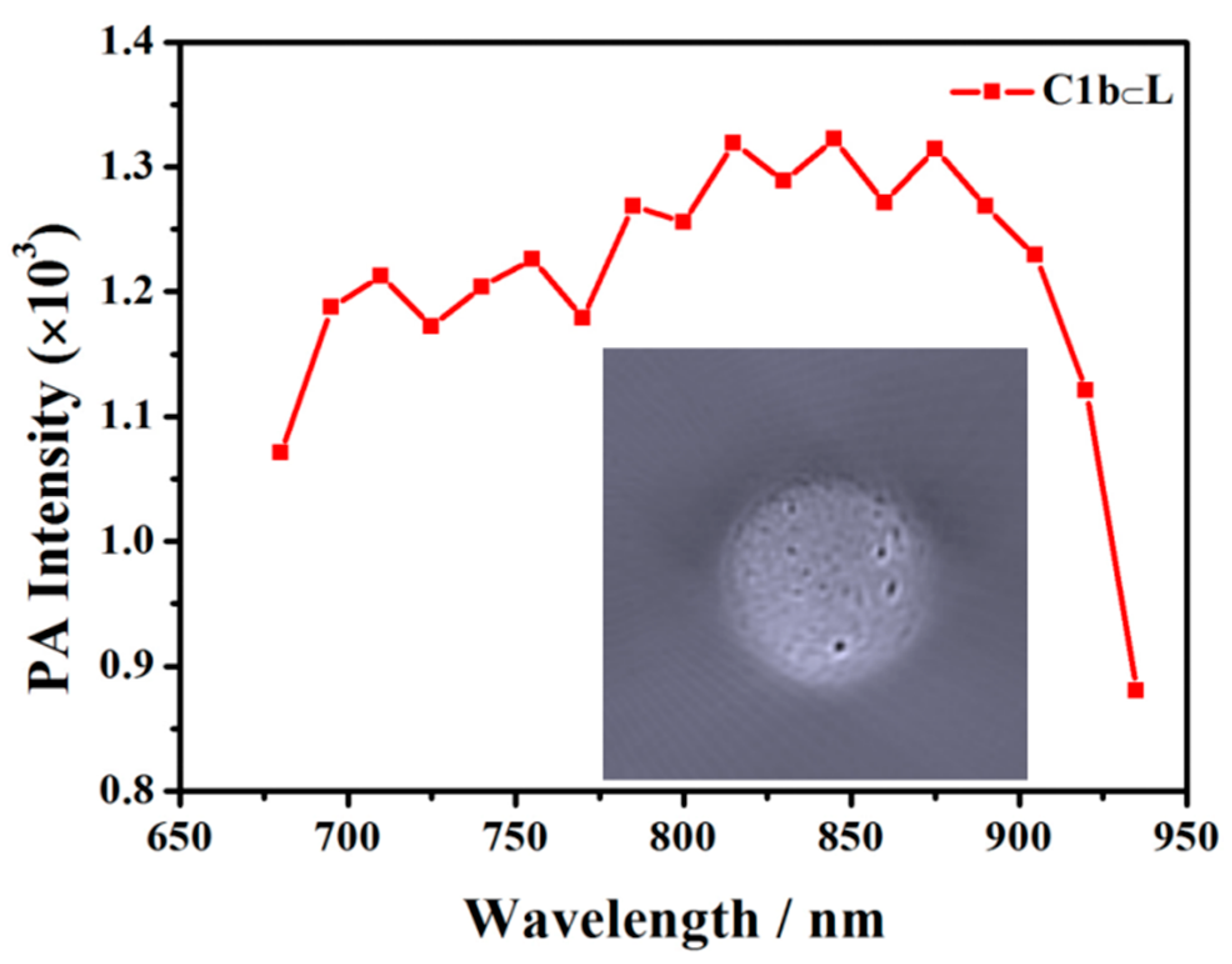
3. Discussion and Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dragulescu-Andrasi, A.; Kothapalli, S.-R.; Tikhomirov, G.A.; Rao, J.H.; Gambhir, S.S. Activatable Oligomerizable Imaging Agents for Photoacoustic Imaging of Furin-Like Activity in Living Subjects. J. Am. Chem. Soc. 2013, 135, 11015–11022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Attia, A.B.E.; Balasundaram, G.; Moothanchery, M.; Dinish, U.S.; Bi, R.Z.; Ntziachristos, V.; Olivo, M. A review of clinical photoacoustic imaging: Current and future trends. Photoacoustics 2019, 16, 100144. [Google Scholar] [CrossRef]
- Wang, L.V.; Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. Science 2012, 335, 1458–1462. [Google Scholar] [CrossRef] [Green Version]
- Ntziachristos, V.; Razansky, D. Molecular Imaging by Means of Multispectral Optoacoustic Tomography (MSOT). Chem. Rev. 2010, 110, 2783–2794. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.H.; Liu, M.Q.; You, B.H.; Luo, G.H.; Chen, Y.; Liu, B.L.; Jiang, Z.Y.; Chu, P.K.; Shao, J.D.; Yu, X.F. Biodegradable Bi2O2Se Quantum Dots for Photoacoustic Imaging-Guided Cancer Photothermal Therapy. Small 2020, 16, e1905208. [Google Scholar] [CrossRef]
- Tian, Y.; Younis, M.R.; Tang, Y.X.; Liao, X.; He, G.; Wang, S.J.; Teng, Z.G.; Huang, P.; Zhang, L.J.; Lu, G.M. Dye-loaded mesoporous polydopamine nanoparticles for multimodal tumor theranostics with enhanced immunogenic cell death. J. Nanobiotechnol. 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.S.; Zhou, J.J.; Song, Z.Y.; Mei, H.; Zhou, M.; Fu, Z.F.; Han, H.Y.; Zhao, L. Aptamer and RVG functionalized gold nanorods for targeted photothermal therapy of neurotropic virus infection in the mouse brain. Chem. Eng. J. 2021, 411, 128557. [Google Scholar] [CrossRef]
- Huang, P.; Lin, J.; Li, W.; Rong, P.; Wang, Z.; Wang, S.; Wang, X.; Sun, X.; Aronova, M.; Niu, G.; et al. Biodegradable Gold Nanovesicles with an Ultrastrong Plasmonic Coupling Effect for Photoacoustic Imaging and Photothermal Therapy. Angew. Chem. Int. Ed. 2013, 52, 13958–13964. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Shieh, M.-J. Platinum(II) Drug-Loaded Gold Nanoshells for Chemo-Photothermal Therapy in Colorectal Cancer. ACS Appl. Mater. Interfaces 2020, 12, 4254–4264. [Google Scholar] [CrossRef]
- Homan, K.A.; Souza, M.; Truby, R.; Luke, G.P.; Green, C.; Vreeland, E.; Emelianov, S. Silver Nanoplate Contrast Agents for in Vivo Molecular Photoacoustic Imaging. ACS Nano 2012, 6, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Zhang, Z.; Guo, Q.; Zhang, L.; Fan, F.; Qin, Y.; Wang, H.; Zhou, S.; Ou-Yang, W.; Sun, H.; et al. A Dual-Model Imaging Theragnostic System Based on Mesoporous Silica Nanoparticles for Enhanced Cancer Phototherapy. Adv. Health Mater. 2019, 8, e1900840. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.L.; Cheng, K.; Yang, Z.; Zhang, R.P.; Yang, M.; Hu, X.; Ma, X.W.; Bu, L.H.; Lu, X.M.; Xiong, X.X.; et al. Perylene-Diimide-Based Nanoparticles as Highly Efficient Photoacoustic Agents for Deep Brain Tumor Imaging in Living Mice. Adv. Mater. 2015, 27, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Chi, J.N.; Xia, J.F.; Zhang, Y.R.; Han, S.C.; Sun, Y. Iodinated Cyanine Dyes for Fast Near-Infrared-Guided Deep Tissue Synergistic Phototherapy. ACS Appl. Mater. Interfaces 2019, 11, 25720–25729. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M.; Hamblin, M.R. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. [Google Scholar] [CrossRef]
- Zhen, X.; Pu, K.; Jiang, X. Photoacoustic Imaging and Photothermal Therapy of Semiconducting Polymer Nanoparticles: Signal Amplification and Second Near-Infrared Construction. Small 2021, 17, e2004723. [Google Scholar] [CrossRef]
- Ntziachristos, V. Going deeper than microscopy: The optical imaging frontier in biology. Nat. Methods 2010, 7, 603–614. [Google Scholar] [CrossRef]
- Liang, P.X.; Li, Z.Q.; Mi, Y.S.; Yang, Z.; Wang, D.; Cao, H.; He, W.; Yang, H. Pyrene-Based Small Molecular Nonlinear Optical Materials Modified by “Click-Reaction”. J. Electron. Mater. 2015, 44, 2883–2889. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.S.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.L.; Wang, H.H.; Gu, J.M.; Hu, H.Y. The application of double click to synthesize a third-order nonlinear polymer containing donor-acceptor chromophores. Polym. Chem. 2016, 7, 3714–3721. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.L.; Wang, H.H.; Gu, J.M.; Hu, H.Y. Nonlinear optical properties of symmetrical and asymmetrical porphyrin derivatives with click chemistry modification. Dye. Pigment. 2016, 134, 155–163. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, R.R.; Gao, H.; Wang, X.K.; Wang, H.H.; Yang, Z.; He, W.L.; Cao, H.M.; Gu, J.M.; Hu, H.Y.; et al. Energy-level tuning of poly(p-phenylenebutadiynylene) derivatives by click chemistry-type postfunctionalization of side-chain alkynes. React. Funct. Polym. 2016, 105, 114–121. [Google Scholar] [CrossRef]
- Wang, D.; Han, H.H.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.L.; Wang, H.H.; Gu, J.M.; Hu, H.Y. Synthesis and evaluation of simple molecules for dye sensitized solar cells. Synth. Met. 2016, 220, 41–47. [Google Scholar] [CrossRef]
- Han, P.B.; Yang, Z.; Cao, H.; He, W.L.; Wang, D.; Zhang, J.J.; Xing, Y.; Gao, H. Nonlinear optical properties of the novel kind of organic donor-acceptor thiophene derivatives with click chemistry modification. Tetrahedron 2017, 73, 6210–6216. [Google Scholar] [CrossRef]
- Han, P.B.; Wang, D.; Gao, H.; Zhang, J.J.; Xing, Y.; Yang, Z.; Cao, H.; He, W.L. Third-order nonlinear optical properties of cyanine dyes with click chemistry modification. Dye. Pigment. 2018, 149, 8–15. [Google Scholar] [CrossRef]
- Li, L.L.; Wang, D.; Wang, L.; Ramella, D.; Wang, H.; Gao, H.; Zhang, J.J.; Xing, Y.; Li, B.N.; Yang, Z.; et al. The photoacoustic effect of near-infrared absorbing porphyrin derivatives prepared via click chemistry. Dye. Pigment. 2018, 148, 501–507. [Google Scholar] [CrossRef]
- Çağatay, D. Polycyclic aromatic hydrocarbon-substituted push–pull chromophores: An investigation of optoelectronic and nonlinear optical properties using experimental and theoretical approaches. Turk. J. Chem. 2021, 45, 1375–1390. [Google Scholar] [CrossRef]
- Zhang, W.S.; Wang, D.; Cao, H.; Yang, H.I. Energy level tunable pre-click functionalization of [60]fullerene for nonlinear optics. Tetrahedron 2014, 70, 573–577. [Google Scholar] [CrossRef]
- Rao, P.S.; Brixi, S.; Shaikh, D.B.; Al Kobaisi, M.; Lessard, B.H.; Bhosale, S.V.; Bhosale, S.V. The Effect of TCNE and TCNQ Acceptor Units on Triphenylamine-Naphthalenediimide Push-Pull Chromophore Properties. Eur. J. Org. Chem. 2021, 2021, 2615–2624. [Google Scholar] [CrossRef]
- Rao, P.S.; More, V.G.; Jangale, A.D.; Bhosale, S.V.; Bhosale, R.S.; Puyad, A.L.; Chen, J.Y.; Li, J.L.; Bhosale, S.V.; Gupta, A.; et al. A series of V-shaped small molecule non-fullerene electron acceptors for efficient bulk-heterojunction devices. Dye. Pigment. 2019, 171, 107677. [Google Scholar] [CrossRef]
- Tian, S.; Cao, H.; Yang, Z.; Zhao, Y.; He, W.; Gao, H. Synthesis of pyrene-based materials with third-order nonlinearity optical property by click chemistry modification. Pigment Resin Technol. 2021; ahead-of-print. [Google Scholar] [CrossRef]
- Banziger, S.D.; Clendening, R.A.; Oxley, B.M.; Ren, T. Spectroelectrochemical and Computational Analysis of a Series of Cycloaddition–Retroelectrocyclization-Derived Donor–Acceptor Chromophores. J. Phys. Chem. B 2020, 124, 11901–11909. [Google Scholar] [CrossRef]
- Raheem, A.A.; Kumar, C.; Shanmugam, R.; Murugan, P.; Praveen, C. Molecular engineering of twisted dipolar chromophores for efficiency boosted BHJ solar cells. J. Mater. Chem. C 2021, 9, 4562–4575. [Google Scholar] [CrossRef]
- Michinobu, T. Development of N-Type Semiconducting Polymers for Transistor Applications. J. Photopolym. Sci. Technol. 2019, 32, 563–570. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Wang, D.; Gao, H.; Yang, Z.; Xing, Y.; Cao, H.; He, W.; Wang, H.; Gu, J.; Hu, H. Click chemistry functionalization improving the wideband optical-limiting performance of fullerene derivatives. Phys. Chem. Chem. Phys. 2016, 18, 7341–7348. [Google Scholar] [CrossRef]
- Rout, Y.; Mobin, S.M.; Misra, R.; Rout, Y. Tetracyanobutadiene (TCBD) functionalized benzothiadiazole derivatives: Effect of donor strength on the [2+2] cycloaddition–retroelectrocyclization reaction. New J. Chem. 2019, 43, 12299–12307. [Google Scholar] [CrossRef]
- Cheng, S.; Li, K.; Hu, J.; He, J.; Zeller, M.; Xu, Z. Building Conjugated Donor-Acceptor Cross-Links into Metal-Organic Frameworks for Photo-and Electroactivity. ACS Appl. Mater. Interfaces 2020, 12, 19201–19209. [Google Scholar] [CrossRef]
- Sharma, R.; Thomas, M.B.; Misra, R.; D’Souza, F. Strong Ground- and Excited-State Charge Transfer in C 3 -Symmetric Truxene-Derived Phenothiazine-Tetracyanobutadine and Expanded Conjugates. Angew. Chem. Int. Ed. 2019, 58, 4350–4355. [Google Scholar] [CrossRef] [PubMed]
- Mi, Y.S.; Liang, P.X.; Yang, Z.; Wang, D.; He, W.L.; Cao, H.; Yang, H. Synthesis and co-assembly of gold nanoparticles functionalized by a pyrene-thiol derivative. RSC Adv. 2015, 5, 140–145. [Google Scholar] [CrossRef]
- Wang, D.; Guo, Q.S.; Gao, H.; Yang, Z.; Cao, H.; He, W.L.; Wang, H.H. Facile synthesis of functional poly(vinylene sulfide)s containing donor-acceptor chromophores by a double click reaction. RSC Adv. 2016, 6, 59327–59332. [Google Scholar] [CrossRef]
- Liu, W.Y.; Li, B.N.; Gao, H.; Wang, D.; Wang, L.; Yang, Z.; Cao, H.; He, W.L.; Wang, H.; Zhang, J.J.; et al. NIR absorbing thiophene derivatives with photoacoustic and photothermal effect used in drug release. Dye. Pigment. 2018, 162, 331–338. [Google Scholar] [CrossRef]
- Ramnivasmirtha, P.; Balaji, J.; Sneha, X.C.M.; Karthik, P.S.; Gajalaskhmi, D.; Vinitha, G. Studies on growth, optical, dielectric, and third-order nonlinearity of 4-methyl N-(4-chlorobenzylidene)aniline (4CBT) crystal. J. Mater. Sci. Mater. Electron. 2020, 31, 18234–18247. [Google Scholar] [CrossRef]
- Tsuda, Y.; Kojima, M.; Matsuda, T.; Oh, J.M. Soluble Polyimides Based on Long-chain Alkyl Groups via Amide Linkages. Polym. J. 2008, 40, 354–366. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.-Y.; Yao, S.; Wang, X.; Belfield, K.D. Near-Infrared-Emitting Squaraine Dyes with High 2PA Cross-Sections for Multiphoton Fluorescence Imaging. ACS Appl. Mater. Interfaces 2012, 4, 2847–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, E.; Jin, C.S.; Wilson, B.C.; Zheng, G. Aggregate Enhanced Trimodal Porphyrin Shell Microbubbles for Ultrasound, Photoacoustic, and Fluorescence Imaging. Bioconjug. Chem. 2014, 25, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, L.V. Photoacoustic imaging in biomedicine. Rev. Sci. Instrum. 2006, 77, 41101. [Google Scholar] [CrossRef] [Green Version]
- Braun, S.; Salaneck, W.R. Fermi level pinning at interfaces with tetrafluorotetracyanoquinodimethane (F4-TCNQ): The role of integer charge transfer states. Chem. Phys. Lett. 2007, 438, 259–262. [Google Scholar] [CrossRef]
- Thomas, A.P.; Babu, P.S.S.; Nair, S.A.; Ramakrishnan, S.; Ramaiah, D.; Chandrashekar, T.K.; Srinivasan, A.; Pillai, M.R. meso-Tetrakis(p-sulfonatophenyl)N-Confused Porphyrin Tetrasodium Salt: A Potential Sensitizer for Photodynamic Therapy. J. Med. Chem. 2012, 55, 5110–5120. [Google Scholar] [CrossRef]
- An, H.-W.; Qiao, S.-L.; Hou, C.-Y.; Lin, Y.-X.; Li, L.-L.; Xie, H.-Y.; Wang, Y.; Wang, L.; Wang, H. Self-assembled NIR nanovesicles for long-term photoacoustic imaging in vivo. Chem. Commun. 2015, 51, 13488–13491. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wang, L.; Zhang, J.-P.; Wang, H. Bis-pyrene-based supramolecular aggregates with reversibly mechanochromic and vapochromic responsiveness. J. Mater. Chem. C 2014, 2, 1887–1892. [Google Scholar] [CrossRef]
- Eisele, D.M.; Knoester, J.; Kirstein, S.; Rabe, J.P.; Bout, D.A.V. Uniform exciton fluorescence from individual molecular nanotubes immobilized on solid substrates. Nat. Nanotechnol. 2009, 4, 658–663. [Google Scholar] [CrossRef] [Green Version]
- Yao, H.; Kagoshima, Y.; Kitamura, S.-I.; Isohashi, T.; Ozawa, A.Y.; Kimura, K. Superstructures of Mesoscopic Monomolecular Sheets of Thiacyanine J Aggregates in Solution. Langmuir 2003, 19, 8882–8887. [Google Scholar] [CrossRef]
- Barni, E.; Savarino, P.; Pelizzetti, E.; Rothenberger, G. Synthesis, Surface Activity and Micelle Formation of Novel Cyanine Dyes. Helv. Chim. Acta 1981, 64, 1943–1948. [Google Scholar] [CrossRef]

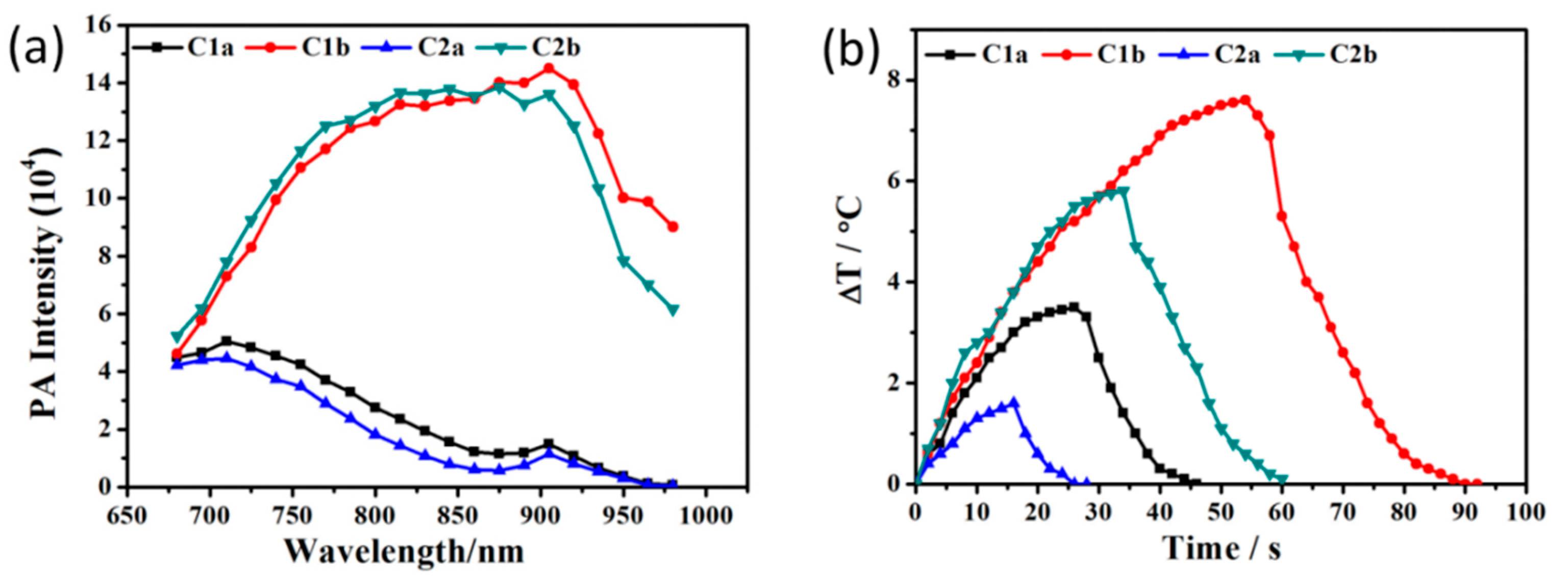
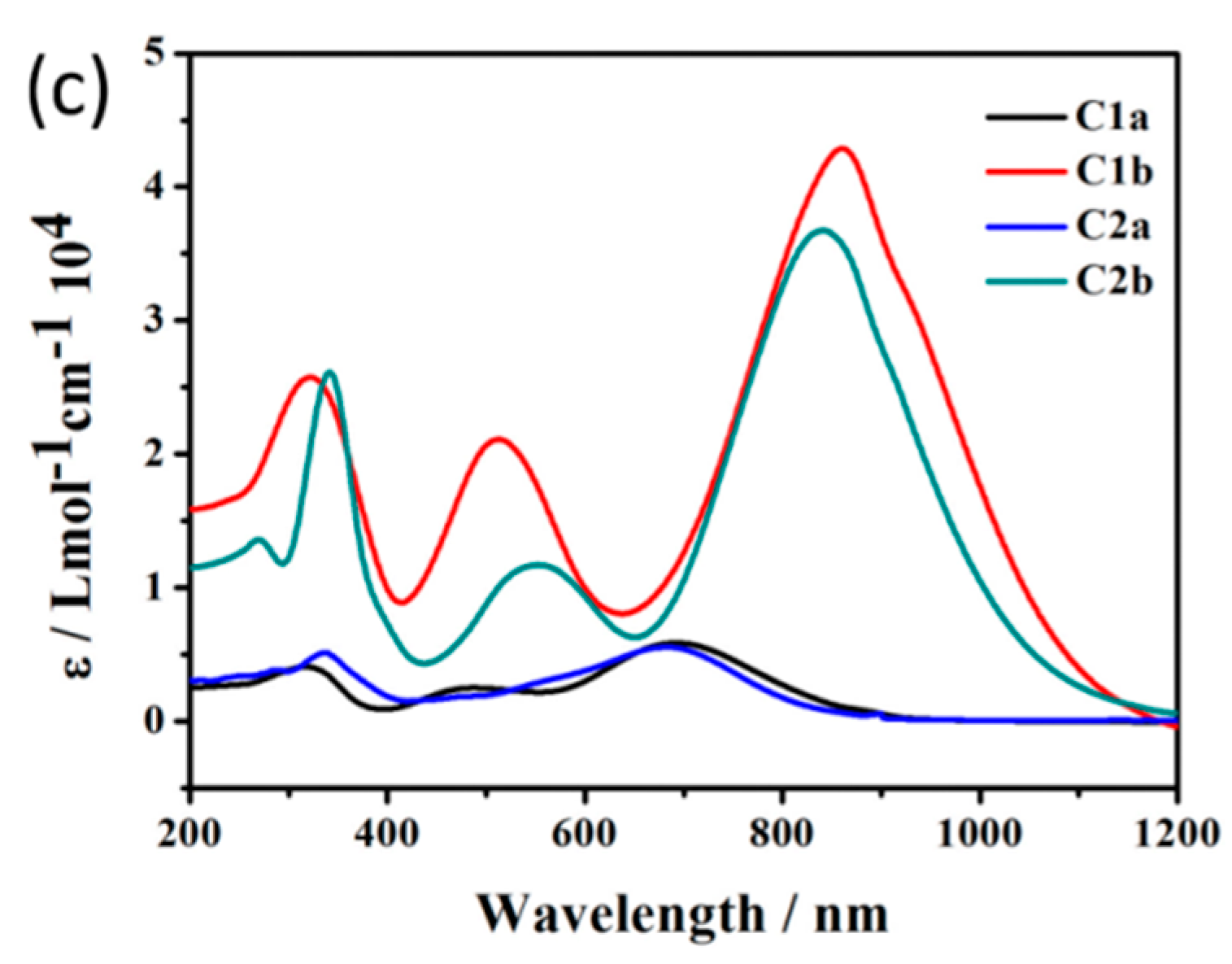
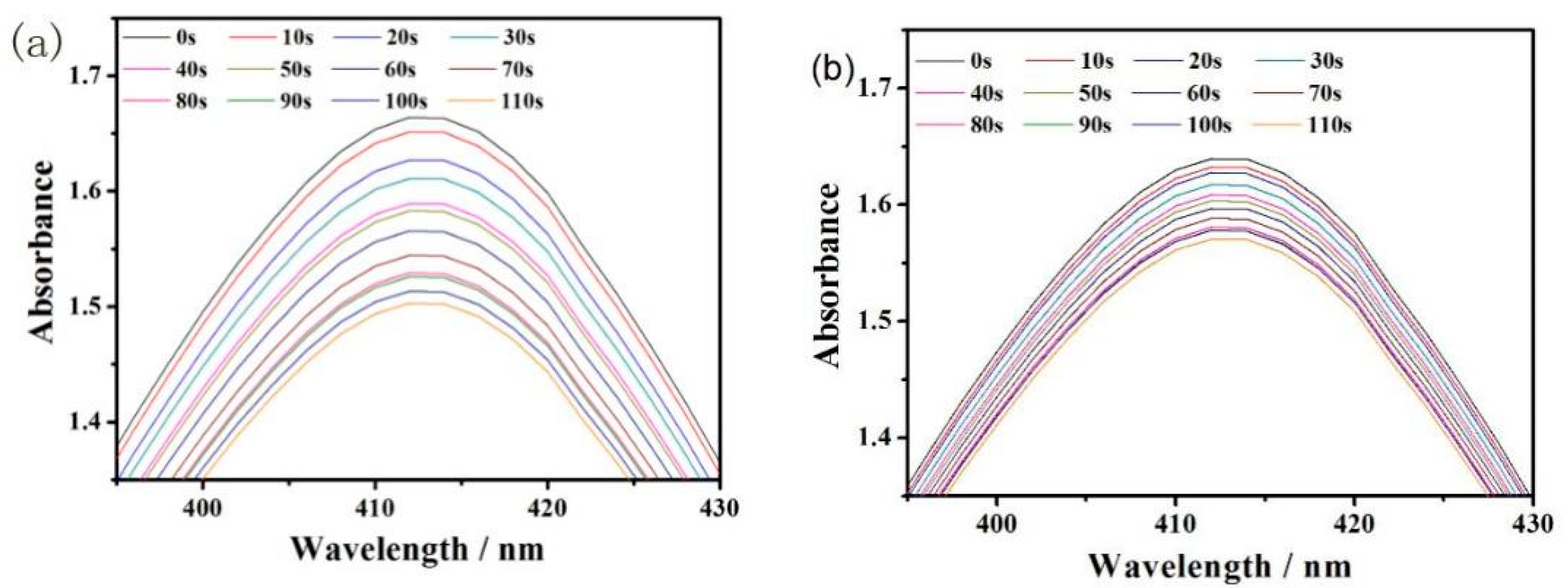
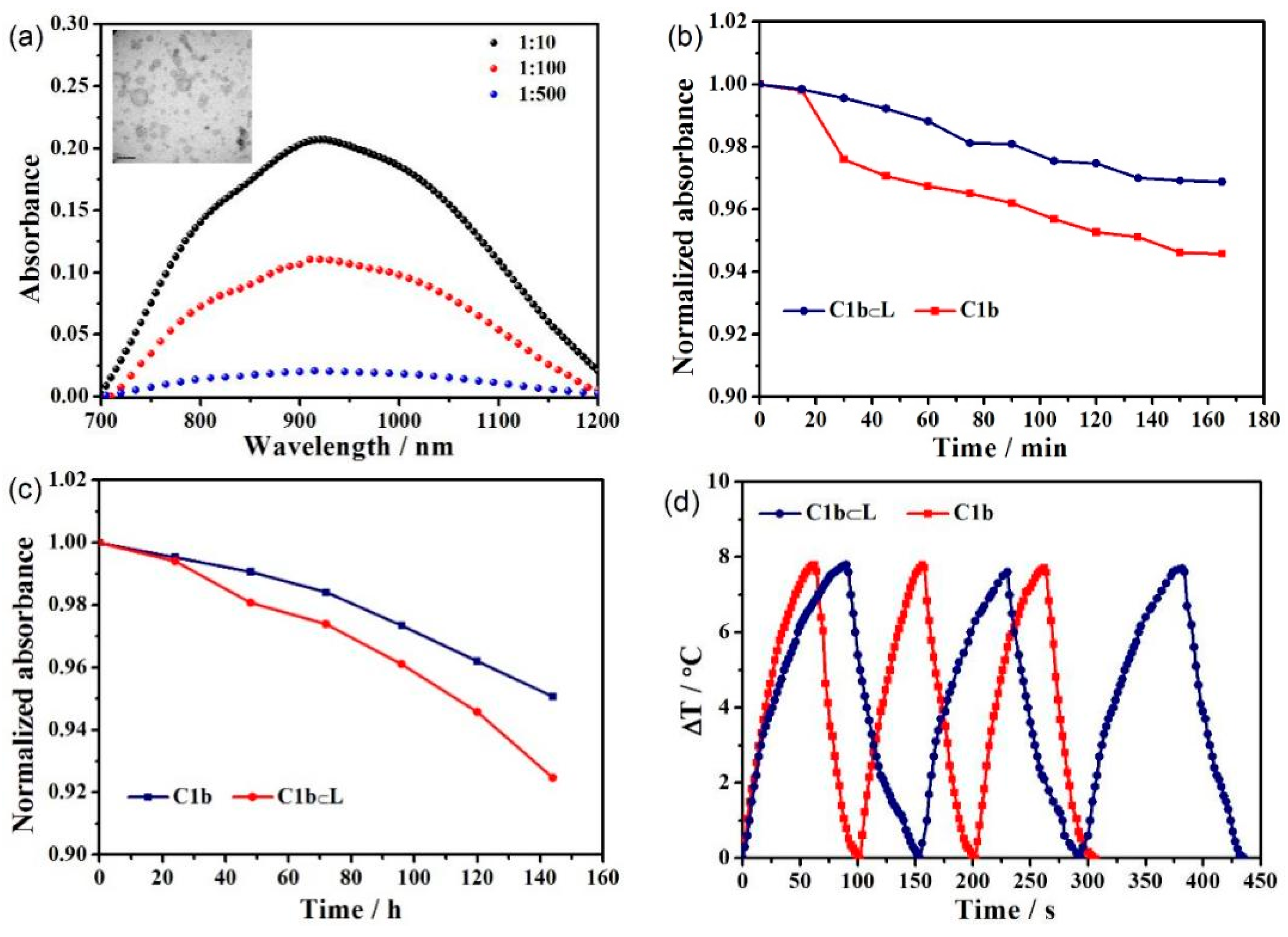
| PA (104) | ε (104/mol/cm) | ΔT (°C) | τs | η (%) | |
|---|---|---|---|---|---|
| C1a | 5.1 | 0.6 | 3.5 | 5.5 | 55.9 |
| C1b | 14.5 | 4.3 | 7.6 | 13.0 | 38.4 |
| C2a | 4.5 | 0.6 | 1.6 | 3.8 | 39.2 |
| C2b | 13.9 | 3.7 | 5.8 | 11.5 | 33.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Miao, Z.; Chu, Y.; Li, L.; Wang, L.; Wang, D. Photoacoustic Effect of Near-Infrared Absorbing Organic Molecules via Click Chemistry. Molecules 2022, 27, 2329. https://doi.org/10.3390/molecules27072329
Zhu W, Miao Z, Chu Y, Li L, Wang L, Wang D. Photoacoustic Effect of Near-Infrared Absorbing Organic Molecules via Click Chemistry. Molecules. 2022; 27(7):2329. https://doi.org/10.3390/molecules27072329
Chicago/Turabian StyleZhu, Wenqing, Zongcheng Miao, Yaqin Chu, Liaoliao Li, Lei Wang, and Dong Wang. 2022. "Photoacoustic Effect of Near-Infrared Absorbing Organic Molecules via Click Chemistry" Molecules 27, no. 7: 2329. https://doi.org/10.3390/molecules27072329
APA StyleZhu, W., Miao, Z., Chu, Y., Li, L., Wang, L., & Wang, D. (2022). Photoacoustic Effect of Near-Infrared Absorbing Organic Molecules via Click Chemistry. Molecules, 27(7), 2329. https://doi.org/10.3390/molecules27072329






