Health-Promoting Properties of Medicinal Mushrooms and Their Bioactive Compounds for the COVID-19 Era—An Appraisal: Do the Pro-Health Claims Measure Up?
Abstract
:1. Introduction
2. Research Methods
3. SARS CoV-2 Virus and COVID-19 Infection
4. Immune and Inflammatory Responses to COVID-19
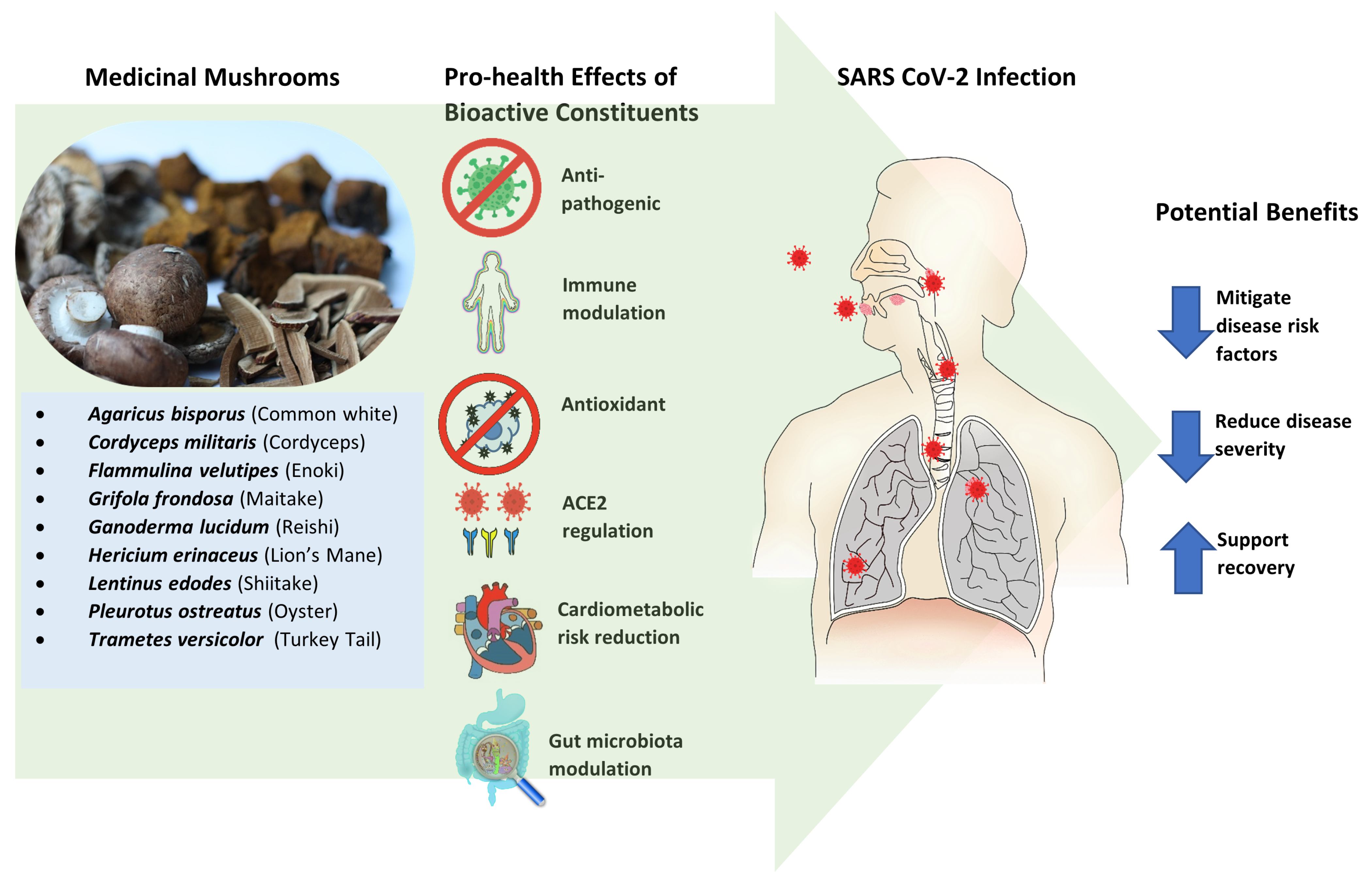
5. Mushrooms as Prevention or Treatment for COVID-19
5.1. General Features
5.2. Structural Elements and Bioactive Compounds
5.3. -Glucans
6. Systemic Pro-Health Responses and Activities Associated with Specific MMs
6.1. Anti-Pathogenic
6.2. Immune Modulation
6.3. Antioxidant
6.4. ACE2 Regulation
7. Comorbidities and Mortality Risk Reduction or Mitigation
7.1. Cardiometabolic Disorders Associated with COVID-19
7.2. Gut Microbiota Modulation as a COVID-19 Risk Reduction Consideration
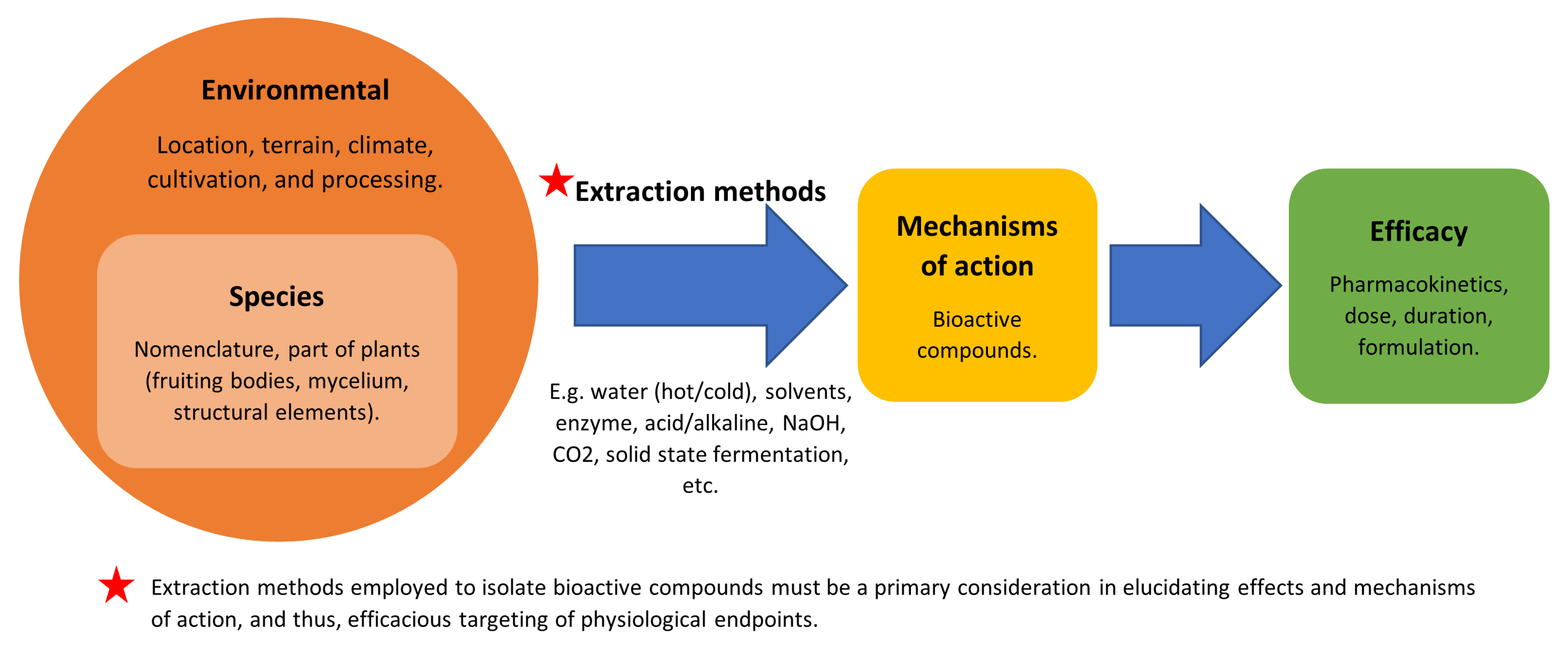
8. Considering Limitations or Barriers in the Application of Mushrooms as a Medicine
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AbM | Agaricus blazei Murril |
| ACE | angiotensin-converting enzyme |
| AGEs | advanced glycation end products |
| ARDS | acute respiratory distress syndrome |
| BP | blood pressure |
| BRMs | biological response modifiers |
| CD | cluster of differentiation |
| COVID-19 | corona virus disease 2019 |
| CVD | cardiovascular disease |
| DNA | deoxyribonucleic acid |
| EBV | Epstein–Barr virus |
| EMPs | edible mushroom polysaccharides |
| FS | fermented substrate |
| GLP | Ganoderma lucidum polysaccharide |
| GLUT4 | glucose transporter type 4 |
| GSK | glycogen synthase kinase |
| HbA1c | glycosylated haemoglobin A1c |
| HDL | high-density lipoprotein |
| HIV | human immunodeficiency virus |
| HMG-CoA | 3-hydroxy-3-methyl-glutaryl-coenzyme A |
| IL | interleukin |
| LDL | low-density lipoprotein |
| MMs | medicinal mushrooms |
| NK | natural killer |
| NO | nitric oxide |
| PE | pressure-assisted extractions |
| PPR | pattern recognition receptor |
| PSK | polysaccharide Krestin |
| PSP | polysaccharopeptides |
| RAAS | renin–angiotensin–aldosterone-system |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| RRTI | recurrent respiratory tract infection |
| SARS-CoV-2 | severe acute respiratory distress syndrome coronavirus-2 |
| SCFAs | short-chain fatty acids |
| STZ | streptozotocin |
| T2DM | type 2 diabetes mellitus |
| TC | total cholesterol |
| TG | triglycerides |
| Th | helper T cell |
| TNF | tumour necrosis factor |
| TvM | Trametes versicolor mycelium |
References
- Gargano, M.L.; van Griensven, L.J.L.D.; Isikhuemhen, O.S.; Lindequist, U.; Venturella, G.; Wasser, S.P.; Zervakis, G.I. Medicinal mushrooms: Valuable biological resources of high exploitation potential. Plant Biosyst. 2017, 151, 548–565. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal mushrooms: Bioactive compounds, use, and clinical trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef] [PubMed]
- Hetland, G.; Johnson, E.; Bernardshaw, S.V.; Grinde, B. Can medicinal mushrooms have prophylactic or therapeutic effect against COVID-19 and its pneumonic superinfection and complicating inflammation? Scand. J. Immunol. 2021, 93, e12937. [Google Scholar] [CrossRef] [PubMed]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.J.; Lim, K.T. Mushroom-derived bioactive molecules as immunotherapeutic agents: A review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Niedermeyer, T.H.J.; Jülich, W.D. The pharmacological potential of mushrooms. Evid.-Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansueto, G.; Niola, M.; Napoli, C. Can COVID 2019 induce a specific cardiovascular damage or it exacerbates pre-existing cardiovascular diseases? Pathol.-Res. Pract. 2020, 216, 153086. [Google Scholar] [CrossRef]
- Schieffer, E.; Schieffer, B.; Hilfiker-Kleiner, D. Cardiovascular diseases and COVID-19: Pathophysiology, complications and treatment. Herz 2021, 46, 107–114. [Google Scholar] [CrossRef]
- Downer, S.; Berkowitz, S.A.; Harlan, T.S.; Olstad, D.L.; Mozaffarian, D. Food is medicine: Actions to integrate food and nutrition into healthcare. BMJ 2020, 369, m2482. [Google Scholar] [CrossRef]
- Rózsa, S.; Gocan, T.M.; Lazăr, V.; Andreica, I.; Rózsa, M.; Măniuţiu, D.N.; Sima, R. The effect of processing on chemical constituents of Agaricus spp. mushrooms. Not. Bot. Horti Agrobot. Cluj-Napoca 2017, 45, 507–516. [Google Scholar] [CrossRef] [Green Version]
- Communicable Diseases Network Australia (CDNA). Coronavirus disease 2019 (COVID-19). CDNA Natl. Guidel. Public Health Units 2021, 4, 1–65. [Google Scholar]
- Raj, C.T.D.; Kandaswamy, D.K.; Danduga, R.C.S.R.; Rajasabapathy, R.; James, R.A. COVID-19: Molecular pathophysiology, genetic evolution and prospective therapeutics—A review. Arch. Microbiol. 2021, 203, 2043–2057. [Google Scholar] [CrossRef] [PubMed]
- Bohn, M.K.; Hall, A.; Sepiashvili, L.; Jung, B.; Steele, S.; Adeli, K. Pathophysiology of COVID-19: Mechanisms underlying disease severity and progression. Physiology 2020, 35, 288–301. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.A.; Rahman, M.S.; Bashir, N.M.B.; Mia, R.; Hossain, A.; Saha, S.K.; Kakon, A.J.; Sarker, N.C. Rationalization of mushroom-based preventive and therapeutic approaches to COVID-19: Review. Int. J. Med. Mushrooms 2021, 23, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, Q. Cellular immune response to COVID-19 and potential immune modulators. Front. Immunol. 2021, 12, 646333. [Google Scholar] [CrossRef]
- Shahzad, F.; Anderson, D.; Najafzadeh, M. The antiviral, anti-inflammatory effects of natural medicinal herbs and mushrooms and SARS-COV-2 infection. Nutrients 2020, 12, 2573. [Google Scholar] [CrossRef]
- Murphy, E.J.; Masterson, C.; Rezoagli, E.; O’toole, D.; Major, I.; Stack, G.D.; Lynch, M.; Laffey, J.G.; Rowan, N.J. β-Glucan extracts from the same edible shiitake mushroom Lentinus edodes produce differential in-vitro immunomodulatory and pulmonary cytoprotective effects-Implications for coronavirus disease (COVID-19) immunotherapies. Sci. Total Environ. 2020, 732, 139330. [Google Scholar] [CrossRef]
- Cerletti, C.; Esposito, S.; Iacoviello, L. Edible mushrooms and beta-glucans: Impact on human health. Nutrients 2021, 13, 2195. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Illescas-Montes, R.; Puerta-Puerta, J.M.; Ruiz, C.; Melguizo-Rodríguez, L. SARS-CoV-2 infection: The role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020, 54, 62–75. [Google Scholar] [CrossRef]
- Hassan, M.A.A.; Rouf, R.; Tiralongo, E.; May, T.W.; Tiralongo, J. Mushroom lectins: Specificity, structure and bioactivity relevant to human disease. Int. J. Mol. Sci. 2015, 16, 7802–7838. [Google Scholar] [CrossRef] [Green Version]
- Barroetaveña, C.; Toledo, C.V. The nutritional benefits of mushrooms. In Wild Plants, Mushrooms Nuts; Ferreira, I.C.F.R., Morales, P., Barros, L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 65–81. [Google Scholar] [CrossRef]
- Fulgoni, V.L.; Agarwal, S. Nutritional impact of adding a serving of mushrooms on usual intakes and nutrient adequacy using National Health and Nutrition Examination Survey 2011–2016 data. Food Sci. Nutr. 2021, 9, 1504–1511. [Google Scholar] [CrossRef]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, 1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalač, P. A review of chemical composition and nutritional value of wild-growing and cultivated mushrooms. J. Sci. Food Agric. 2013, 93, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Du, H.; Hu, Q.; Yang, W.; Pei, F.; Xiao, H. Health benefits of edible mushroom polysaccharides and associated gut microbiota regulation. Crit. Rev. Food Sci. Nutr. 2021, 2021, 1–18. [Google Scholar] [CrossRef]
- Benson, K.F.; Stamets, P.; Davis, R.; Nally, R.; Taylor, A.; Slater, S.; Jensen, G.S. The mycelium of the Trametes versicolor (Turkey tail) mushroom and its fermented substrate each show potent and complementary immune activating properties in vitro. BMC Complement. Altern. Med. 2019, 19, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badalyan, S.M.; Barkhudaryan, A.; Rapior, S. Recent progress in research on the pharmacological potential of mushrooms and prospects for their clinical application. In Medicinal Mushrooms; Agrawal, D., Dhanasekaran, M., Eds.; Springer: Singapore, 2019; pp. 1–70. [Google Scholar] [CrossRef]
- Cheung, P.C.K. The nutritional and health benefits of mushrooms. Nutr. Bull. 2010, 35, 292–299. [Google Scholar] [CrossRef]
- Friedman, M. Chemistry, nutrition, and health-promoting properties of Hericium erinaceus (Lion’s Mane) mushroom fruiting bodies and mycelia and their bioactive compounds. J. Agric. Food Chem. 2015, 64, 7108–7123. [Google Scholar] [CrossRef]
- Abdullah, N.; Ismail, S.M.; Aminudin, N.; Shuib, A.S.; Lau, B.F. Evaluation of selected culinary-medicinal mushrooms for antioxidant and ACE inhibitory activities. Evid.-Based Complement. Altern. Med. 2012, 2012, 464238. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Baruah, K.; Cox, E.; Vanrompay, D.; Bossier, P. Structure-functional activity relationship of β-glucans from the perspective of immunomodulation: A mini-review. Front. Immunol. 2020, 11, 658. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Ikewaki, N.; Iwasaki, M.; Kurosawa, G.; Rao, K.S.; Lakey-Beitia, J.; Preethy, S.; Abraham, S.J. β-glucans: Wide-spectrum immune-balancing food-supplement-based enteric (β-WIFE) vaccine adjuvant approach to COVID-19. Hum. Vaccines Immunother. 2021, 17, 2808–2813. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Hrubisko, M.; Majtan, J.; Rennerova, Z.; Banovcin, P. Anti-allergic effect of pleuran (β-glucan from pleurotus ostreatus) in children with recurrent respiratory tract infections. Phytother. Res. 2014, 28, 471–474. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Qin, T.; Qiu, F.; Song, Y.; Lin, D.; Ma, Y.; Li, J.; Huang, Y. Immunomodulatory effects of hydroxyethylated Hericium erinaceus polysaccharide on macrophages RAW264.7. Int. J. Biol. Macromol. 2017, 105, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Geller, A.; Yan, J. Could the induction of trained immunity by β-glucan serve as a defense against COVID-19? Front. Immunol. 2020, 11, 1782. [Google Scholar] [CrossRef]
- Liu, H.; Meng, Z.; Wang, H.; Zhang, S.; Huang, Z.; Geng, X.; Guo, R.; Wu, Z.; Hong, Z. Robust immune responses elicited by a hybrid adjuvant based on β-glucan particles from yeast for the hepatitis B vaccine. ACS Appl. Bio Mater. 2021, 4, 3614–3622. [Google Scholar] [CrossRef]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Chitosan, alginate, hyaluronic acid, gums, and β-glucan as potent adjuvants and vaccine delivery systems for viral threats including SARS-CoV-2: A review. Int. J. Biol. Macromol. 2021, 182, 1931–1940. [Google Scholar] [CrossRef]
- Soares, E.; Groothuismink, Z.M.A.; Boonstra, A.; Borges, O. Glucan particles are a powerful adjuvant for the HBsAg, favoring antiviral immunity. Mol. Pharm. 2019, 16, 1971–1981. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Antiviral bioactive compounds of mushrooms and their antiviral mechanisms: A review. Viruses 2021, 13, 350. [Google Scholar] [CrossRef]
- Ryu, E.; Son, M.; Lee, M.; Lee, K.; Cho, J.Y.; Cho, S.; Lee, S.K.; Lee, Y.M.; Cho, H.; Sung, G.H.; et al. Cordycepin is a novel chemical suppressor of Epstein-Barr virus replication. Oncoscience 2014, 1, 866–881. [Google Scholar] [CrossRef] [Green Version]
- El-Mekkawy, S.; Meselhy, M.R.; Nakamura, N.; Tezuka, Y.; Hattori, M.; Kakiuchi, N.; Shimotohno, K.; Kawahata, T.; Otake, T. Anti-HIV-1 and anti-HIV-1-protease substances from Ganoderma lucidum. Phytochemistry 1998, 49, 1651–1657. [Google Scholar] [CrossRef]
- Hearst, R.; Nelson, D.; Mccollum, G.; Millar, B.C.; Maeda, Y.; Goldsmith, C.E.; Rooney, P.J.; Loughrey, A.; Rao, J.R.; Moore, J.E. An examination of antibacterial and antifungal properties of constituents of Shiitake (Lentinula edodes) and Oyster (Pleurotus ostreatus) mushrooms. Complement. Ther. Clin. Pract. 2008, 15, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Xu, L.; Lu, T.; Yin, J. Structural characterization and antiviral activity of lentinan from Lentinus edodes mycelia against infectious hematopoietic necrosis virus. Int. J. Biol. Macromol. 2018, 115, 1202–1210. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Sun, Y.; Chen, S.; Zhang, J.; Kang, J.; Wang, Y.; Wang, H.; Xia, G.; Liu, Q.; Kang, Y. Mushroom lectin enhanced immunogenicity of HBV DNA vaccine in C57BL/6 and HBsAg-transgenic mice. Vaccine 2013, 31, 2273–2280. [Google Scholar] [CrossRef] [PubMed]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef]
- Jesenak, M.; Urbancikova, I.; Banovcin, P. Respiratory tract infections and the role of biologically active polysaccharides in their management and prevention. Nutrients 2017, 9, 779. [Google Scholar] [CrossRef] [PubMed]
- Pasnik, J.; Slemp, A.; Cyswinska-Bernas, A.; Zeman, K.; Jezenak, M. Preventative effect of pleuran (β-glucan isolated from Pleurotus ostreatus) in children with recurrent respiratory tract infections-open-label prospective study. Curr. Paediatr. Res. 2017, 21, 99–104. [Google Scholar]
- Johnson, E.; Førland, D.T.; Saetre, L.; Bernardshaw, S.V.; Lyberg, T.; Hetland, G. Effect of an extract based on the medicinal mushroom Agaricus blazei murill on release of cytokines, chemokines and leukocyte growth factors in human blood ex vivo and in vivo. Scand. J. Immunol. 2009, 69, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Hetland, G.; Johnson, E.; Lybergà, T.; Bernardshaw, S.; Tryggestad, A.M.A.; Grinde, B. Effects of the medicinal mushroom Agaricus blazei Murill on immunity, infection and cancer. Scand. J. Immunol. 2008, 68, 363–370. [Google Scholar] [CrossRef]
- Bernardshaw, S.; Johnson, E.; Hetland, G. An extract of the mushroom Agaricus blazei Murill administered orally protects against systemic Streptococcus pneumoniae infection in mice. Scand. J. Immunol. 2005, 62, 393–398. [Google Scholar] [CrossRef]
- Hetland, G.; Tangen, J.M.; Mahmood, F.; Mirlashari, M.R.; Nissen-Meyer, L.S.H.; Nentwich, I.; Therkelsen, S.P.; Tjønnfjord, G.E.; Johnson, E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients 2020, 12, 1339. [Google Scholar] [CrossRef]
- Wu, S.J.; Chen, Y.W.; Wang, C.Y.; Shyu, Y.T. Anti-inflammatory properties of high pressure-assisted extracts of Grifola frondosa in lipopolysaccharide-activated RAW 264.7 macrophages. Int. J. Food Sci. Technol. 2017, 52, 671–678. [Google Scholar] [CrossRef]
- Roncero-Ramos, I.; Delgado-Andrade, C. The beneficial role of edible mushrooms in human health. Curr. Opin. Food Sci. 2017, 14, 122–128. [Google Scholar] [CrossRef]
- Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Chang, C.J.; Lin, C.S.; Lai, H.C.; Young, J.D. Immunomodulatory properties of plants and mushrooms. Trends Pharmacol. Sci. 2017, 38, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Nakamura, Y.; Tagata, M.; Tokuda, H.; Yasukawa, K.; Uchiyama, E.; Suzuki, T.; Kimura, Y. Anti-inflammatory and anti-tumor-promoting effects of triterpene acids and sterols from the fungus Ganoderma lucidum. Chem. Biodivers. 2007, 4, 224–231. [Google Scholar] [CrossRef]
- Saleh, M.H.; Rashedi, I.; Keating, A. Immunomodulatory properties of Coriolus versicolor: The role of polysaccharopeptide. Front. Immunol. 2017, 8, 1087. [Google Scholar] [CrossRef] [Green Version]
- Teng, J.F.; Lee, C.H.; Hsu, T.H.; Lo, H.C. Potential activities and mechanisms of extracellular polysaccharopeptides from fermented Trametes versicolor on regulating glucose homeostasis in insulin-resistant HepG2 cells. PLoS ONE 2018, 13, e0201131. [Google Scholar] [CrossRef]
- Chang, Y.; Zhang, M.; Jiang, Y.; Liu, Y.; Luo, H.; Hao, C.; Zeng, P.; Zhang, L. Preclinical and clinical studies of Coriolus versicolor polysaccharopeptide as an immunotherapeutic in China. Discov. Med. 2017, 23, 207–219. [Google Scholar]
- Fritz, H.; Kennedy, D.A.; Ishii, M.; Fergusson, D.; Fernandes, R.; Cooley, K.; Seely, D. Polysaccharide K and Coriolus versicolor extracts for lung cancer: A systematic review. Integr. Cancer Ther. 2015, 14, 201–211. [Google Scholar] [CrossRef]
- Jedrzejewski, T.; Piotrowski, J.; Kowalczewska, M.; Wrotek, S.; Kozak, W. Polysaccharide peptide from Coriolus versicolor induces interleukin 6-related extension of endotoxin fever in rats. Int. J. Hyperth. 2015, 31, 626–634. [Google Scholar] [CrossRef]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.; van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [Green Version]
- Cheah, I.K.; Halliwell, B. Could ergothioneine aid in the treatment of coronavirus patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Nikšić, M.; Vrvić, M.M.; Todorović, N.; Jakovljević, D.; Van Griensven, L.J. Antioxidative activities and chemical characterization of polysaccharide extracts from the widely used mushrooms Ganoderma applanatum, Ganoderma lucidum, Lentinus edodes and Trametes versicolor. J. Food Compos. Anal. 2012, 26, 144–153. [Google Scholar] [CrossRef]
- Podkowa, A.; Kryczyk-Poprawa, A.; Opoka, W.; Muszyńska, B. Culinary–medicinal mushrooms: A review of organic compounds and bioelements with antioxidant activity. Eur. Food Res. Technol. 2021, 247, 513–533. [Google Scholar] [CrossRef]
- Muszyńska, B.; Kała, K.; Rojowski, J.; Grzywacz, A.; Opoka, W. Composition and biological properties of Agaricus bisporus fruiting bodies—A Review. Pol. J. Food Nutr. Sci. 2017, 67, 173–181. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.Y.; Ho, K.J.; Hsieh, Y.J.; Wang, L.T.; Mau, J.L. Contents of lovastatin, γ-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Weigand-Heller, A.J.; Kris-Etherton, P.M.; Beelman, R.B. The bioavailability of ergothioneine from mushrooms (Agaricus bisporus) and the acute effects on antioxidant capacity and biomarkers of inflammation. Prev. Med. 2012, 54, S75–S78. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abdullah, N.; Aminudin, N. Inhibitory effect on in vitro LDL oxidation and HMG Co-A reductase activity of the liquid-liquid partitioned fractions of Hericium erinaceus (Bull.) persoon (Lion’s Mane mushroom). BioMed Res. Int. 2014, 2014, 828149. [Google Scholar] [CrossRef] [Green Version]
- Savoie, J.M.; Minivielle, N.; Largeteau, M. Radical-scavenging properties of extracts from the white button mushroom, Agaricus bisporus. J. Sci. Food Agric. 2007, 88, 970–975. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Usman, D.; Batiha, G.E.S.; Cruz-Martins, N.; Malami, I.; Ibrahim, K.G.; Abubakar, B.; Bello, M.B.; Muhammad, A.; Gan, S.H.; et al. Natural products modulating angiotensin converting enzyme 2 (ACE2) as potential COVID-19 therapies. Front. Pharmacol. 2021, 12, 629935. [Google Scholar] [CrossRef]
- Lichtenberger, L.M.; Vijayan, K.V. Is COVID-19–induced platelet activation a cause of concern for patients with cancer? Cancer Res. 2021, 81, 1209–1211. [Google Scholar] [CrossRef]
- Ansor, N.M.; Abdullah, N.; Aminudin, N. Anti-angiotensin converting enzyme (ACE) proteins from mycelia of Ganoderma lucidum (Curtis) P. Karst. Complement. Altern. Med. 2013, 13, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed Yahaya, N.F.; Rahman, M.A.; Abdullah, N. Therapeutic potential of mushrooms in preventing and ameliorating hypertension. Trends Food Sci. Technol. 2014, 39, 104–115. [Google Scholar] [CrossRef]
- Dicks, L.; Ellinger, S. Effect of the intake of oyster mushrooms (Pleurotus ostreatus) on cardiometabolic parameters—A systematic review of clinical trials. Nutrients 2020, 12, 1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kała, K.; Kryczyk-Poprawa, A.; Rzewińska, A.; Muszyńska, B. Fruiting bodies of selected edible mushrooms as a potential source of lovastatin. Eur. Food Res. Technol. 2020, 246, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Atlý, B.; Yamaç, M.; Yýldýz, Z.; Sölener, M. Solid state fermentation optimization of Pleurotus ostreatus for lovastatin production. Pharm. Chem. J. 2019, 53, 858–864. [Google Scholar] [CrossRef]
- Aramabašić Jovanović, J.; Mihailović, M.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidaković, M. The effects of major mushroom bioactive compounds on mechanisms that control blood glucose level. J. Fungi 2021, 7, 58. [Google Scholar] [CrossRef]
- Jeong, S.C.; Jeong, Y.T.; Yang, B.K.; Islam, R.; Koyyalamudi, S.R.; Pang, G.; Cho, K.Y.; Song, C.H. White button mushroom (Agaricus bisporus) lowers blood glucose and cholesterol levels in diabetic and hypercholesterolemic rats. Nutr. Res. 2010, 30, 49–56. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Hu, C.; Wang, J.; Zhang, J.; Ren, Z.; Song, X.; Jia, L. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci. Rep. 2017, 7, 10847. [Google Scholar] [CrossRef]
- Wang, L.; Xu, N.; Zhang, J.; Zhao, H.; Lin, L.; Jia, S.; Jia, L. Antihyperlipidemic and hepatoprotective activities of residue polysaccharide from Cordyceps militaris SU-12. Carbohydr. Polym. 2015, 131, 355–362. [Google Scholar] [CrossRef]
- Preuss, H.G.; Echard, B.; Fu, J.; Perricone, N.V.; Bagchi, D.; Kaylor, M.; Zhuang, C. Fraction SX of maitake mushroom favorably influences blood glucose levels and blood pressure in streptozotocin-induced diabetic rats. J. Med. Food 2012, 15, 901–908. [Google Scholar] [CrossRef]
- Yeh, M.Y.; Ko, W.C.; Lin, L.Y. Hypolipidemic and antioxidant activity of enoki mushrooms (Flammulina velutipes). BioMed Res. Int. 2014, 2014, 352385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Tang, J.; Gao, H.; Xu, Y.; Han, Y.; Shang, H.; Lu, Y.; Qin, C. Ganoderma lucidum triterpenoids and polysaccharides attenuate atherosclerotic plaque in high-fat diet rabbits. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A double-blind, randomised, placebo-controlled trial of Ganoderma lucidum for the treatment of cardiovascular risk factors of metabolic syndrome. Sci. Rep. 2016, 6, 29540. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.S.; Mehrotra, A.; Beelman, R.B.; Nadkarni, G.; Wang, L.; Cai, W.; Boon, C.; Goh, C.; Kalaras, M.D.; Uribarri, J. A retrospective study in adults with metabolic syndrome: Diabetic risk factor response to daily consumption of Agaricus bisporus (white button mushrooms). Plant Foods Hum. Nutr. 2016, 71, 245–251. [Google Scholar] [CrossRef]
- Li, K.; Zhuo, C.; Teng, C.; Yu, S.; Wang, X.; Hu, Y.; Ren, G.; Yu, M.; Qu, J. Effects of Ganoderma lucidum polysaccharides on chronic pancreatitis and intestinal microbiota in mice. Int. J. Biol. Macromol. 2016, 93, 904–912. [Google Scholar] [CrossRef]
- Hess, J.; Wang, Q.; Gould, T.; Slavin, J. Impact of Agaricus bisporus mushroom consumption on gut health markers in healthy adults. Nutrients 2018, 10, 1402. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.M.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef] [Green Version]
- Ganesan, K.; Xu, B. Anti-obesity effects of medicinal and edible mushrooms. Molecules 2018, 23, 2880. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Xiao, D.; Liu, W.; Song, Y.; Zou, B.; Li, L.; Li, P.; Cai, Y.; Liu, D.; Liao, Q.; et al. Intake of Ganoderma lucidum polysaccharides reverses the disturbed gut microbiota and metabolism in type 2 diabetic rats. Int. J. Biol. Macromol. 2020, 155, 890–902. [Google Scholar] [CrossRef]
- Lee, D.H.; Yang, M.; Giovannucci, E.L.; Sun, Q.; Chavarro, J.E. Mushroom consumption, biomarkers, and risk of cardiovascular disease and type 2 diabetes: A prospective cohort study of US women and men. Am. J. Clin. Nutr. 2019, 110, 666. [Google Scholar] [CrossRef]
- Atila, F.; Tüzel, Y.; Pekşen, A.; Cano, A.F.; Fernández, J.A. The effect of different fruiting temperatures on the yield and nutritional parameters of some wild and hybrid Hericium isolates. Sci. Hortic. 2021, 280, 109915. [Google Scholar] [CrossRef]
- Feeney, M.J.; Miller, A.M.; Roupas, P. Mushrooms—Biologically distinct and nutritionally unique. Nutr. Today 2014, 49, 301–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cohen, N.; Cohen, J.; Asatiani, M.D.; Varshney, V.K.; Yu, H.T.; Yang, Y.C.; Li, Y.H.; Mau, J.L.; Wasser, S.P. Chemical composition and nutritional and medicinal value of fruit bodies and submerged cultured mycelia of culinary-medicinal higher Basidiomycetes mushrooms. Int. J. Med. Mushrooms 2014, 16, 273–291. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Puia, I.C.; Puia, A.; Chedea, V.S.; Leopold, N.; Bocsan, I.C.; Buzoianu, A.D. Characterization of Trametes versicolor: Medicinal mushroom with important health benefits. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 343–349. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Seoane, P.; Díaz-Reinoso, B.; González-Muñoz, M.J.; Fernández de Ana Portela, C.; Domínguez, H. Innovative technologies for the extraction of saccharidic and phenolic fractions from Pleurotus eryngii. LWT 2019, 101, 774–782. [Google Scholar] [CrossRef]
| Mushroom | Bioactive Compound | Pro-Health Effects | References |
|---|---|---|---|
| A. bisporus (Common white) | Phenolics e.g., Ergothioneine | Antioxidant; increased ORAC activity; increased adiponectin; reduced AGEs; increased glutathione reductase and catalase activities | [63,70,86] |
| Polysaccharides | Gut microbiota regulation; intestinal barrier integrity | [25] | |
| Secondary metabolites | Anti-hyperglycaemic; inhibitory effects on LDL oxidation; reduced HMG-CoA reductase activity; LPS reduction; ACE2 inhibition; cardiometabolic parameters improvement | [30,66,79,80] | |
| Andosan™ | Ergosterol | Cytotoxic | [51] |
| Commercial extract AbM + H. erinaceus and G. frondosa | Immunomodulatory; anti-inflammatory; anti-tumour | [49] | |
| Tomeka™ | Commercial extract A. bisporus + soy | Anti-pathogenic | [3,29] |
| C. militaris (Cordyceps) | Cordycepin | Antiviral; RNA synthesis inhibition; suppressed EB viral replication; cytotoxic | [14,41] |
| Antioxidant; anti-hyperlipidaemic; hepatoprotective | [81] | ||
| SCFAs | Immune regulation and health promoting | [25] | |
| F. velutipes (Enoki) | Polysaccharides/dietary fibre | Reduced cardiometabolic parameters | [83] |
| Antioxidant | Mycosterol | [83] | |
| G. frondosa (Maitake) | Polysaccharides | Anti-inflammatory; antioxidant; immunomodulatory | [31,53] |
| Increased insulin sensitivity; decreased systolic BP; decreased RAAS; increased NO | [82] | ||
| G. lucidum (Reishi) | Polysaccharides | SCFAs production; gut microbiota regulation; anti-obesity; anti-inflammation; reduced metabolic endotoxaemia; decreased FBG and insulin levels | [87,89,90,91] |
| Ganodermic compounds—triterpenoids, other phenolics | Antioxidant; atherosclerotic plaque attenuation; anti-tumour; anti-inflammation; antiviral HIV-1 and HIV-1 protease inhibition | [30,42,56,64,84] | |
| Mycelia fractions | ACE inhibition | [73] | |
| H. erinaceus (Lion’s Mane) | Mycelia polysaccharide fractions | Anti-hyperglycaemic; improved antioxidant enzymatic activities | [69,80] |
| Fruiting body solvent fractions | LDL oxidation inhibition; HMG-CoA reductase inhibition | ||
| L. edodes (Shiitake) | Polysaccharides; -glucans | Antiviral; antioxidant; immunomodulatory; cytotoxic; anti-inflammatory; microbiome regulation | [1,2,6,17,25,44] |
| Aqueous extract | Anti-bacterial; anti-fungal | [43] | |
| Lovastatin | Hypolipidaemic | [76] | |
| P. ostreatus (Oyster) | -glucans-pleuran | Immunomodulatory | [36,46,47,48] |
| Lectins | Vaccine adjuvant | [20,45] | |
| Phenolics, peptides | ACE2 inhibition | [30,75] | |
| Lovastatin | HMG-CoA reductase inhibition | [75,76,77] | |
| Polysaccharides | Cardiometabolic parameter improvements | [75,78] | |
| T. versicolor (Turkey Tail) | PSP, PSK | Cancer therapy/adjuvants; Immunomodulatory | [57,59,60,61] |
| Gut microbiota modulation | [25] | ||
| Glucans, phenolics | Antioxidant | [62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phillips, J.M.; Ooi, S.L.; Pak, S.C. Health-Promoting Properties of Medicinal Mushrooms and Their Bioactive Compounds for the COVID-19 Era—An Appraisal: Do the Pro-Health Claims Measure Up? Molecules 2022, 27, 2302. https://doi.org/10.3390/molecules27072302
Phillips JM, Ooi SL, Pak SC. Health-Promoting Properties of Medicinal Mushrooms and Their Bioactive Compounds for the COVID-19 Era—An Appraisal: Do the Pro-Health Claims Measure Up? Molecules. 2022; 27(7):2302. https://doi.org/10.3390/molecules27072302
Chicago/Turabian StylePhillips, Jennifer Mary, Soo Liang Ooi, and Sok Cheon Pak. 2022. "Health-Promoting Properties of Medicinal Mushrooms and Their Bioactive Compounds for the COVID-19 Era—An Appraisal: Do the Pro-Health Claims Measure Up?" Molecules 27, no. 7: 2302. https://doi.org/10.3390/molecules27072302
APA StylePhillips, J. M., Ooi, S. L., & Pak, S. C. (2022). Health-Promoting Properties of Medicinal Mushrooms and Their Bioactive Compounds for the COVID-19 Era—An Appraisal: Do the Pro-Health Claims Measure Up? Molecules, 27(7), 2302. https://doi.org/10.3390/molecules27072302







