Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K)
Abstract
1. Introduction
2. Experimental
2.1. Experimental: ICKC
2.2. Reagents (ICKC)
2.3. Experimental: NJIT
2.4. Reagents (NJIT)
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Baranov, A.N. Combustion-Fire-Explosion-Safety; Ministry of Russian Federation for Civil Defence, Emergencies and Elimination of Consequences of Natural Disasters (FGU VNIIPO EMERCOM of Russia): Moscow, Russia, 2004; p. 364. [Google Scholar]
- Hastie, J.W.; Bonnell, D.W. Molecular Chemistry of Inhibited Combustion Systems; National Bureau of Standards: Gaithersburg, MD, USA. [CrossRef]
- Tapscott, R.E.; Sheinson, R.S.; Babushok, V.; Nyden, M.R.; Gann, R.G. Alternative Fire Suppressant Chemicals: A Research Review with Recommendations. NIST Tech. Note. 2001, 1443, 13. [Google Scholar]
- Korobeinichev, O.P.; Shvartsberg, V.M.; Chernov, A.A. The Destruction Chemistry of Organophosphorus Compounds in Flames—II: Structure of a Hydrogen–Oxygen Flame Doped with Trimethylphosphate. Combust. Flame 1999, 118, 727–732. [Google Scholar] [CrossRef]
- Glaude, P.A.; Melius, C.; Pitz, W.J.; Westbrook, C.K. Detailed Chemical Kinetic Reaction Mechanisms for Incineration of Organophosphorus and Fluoro-Organophosphorus Compounds. Proc. Combust. Inst. 2002, 29, 2469–2476. [Google Scholar] [CrossRef]
- Jayaweera, T.M.; Melius, C.F.; Pitz, W.J.; Westbrook, C.K.; Korobeinichev, O.P.; Shvartsberg, V.M.; Shmakov, A.G.; Rybitskaya, I.V.; Curran, H.J. Flame Inhibition by Phosphorus-Containing Compounds over a Range of Equivalence Ratios. Combust. Flame 2005, 140, 103–115. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Shvartsberg, V.M.; Chernov, A.A.; Mokrushin, V.V. Hydrogen-Oxygen Flame Doped with Trimethyl Phosphate, its Structure and Trimethyl Phosphate Destruction Chemistry. Symp. Combust. 1996, 26, 1035–1042. [Google Scholar] [CrossRef]
- Twarowski, A.J. The Influence of Phosphorus Oxides and Acids on Rate of H+OH Recombination. Combust. Flame 1993, 94, 91–107. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Bolshova, T.A.; Shvartsberg, V.M.; Chernov, A.A. Inhibition and Promotion of Combustion by Organophosphorus Compounds Added to Flames of CH4 or H2 in O2 and Ar. Combust. Flame 2001, 125, 744–751. [Google Scholar] [CrossRef]
- Glaude, P.A.; Curran, H.J.; Pitz, W.J.; Westbrook, C.K. Kinetic Study of the Combustion of Organophosphorus Compounds. Proc. Combust. Inst. 2000, 28, 1749–1756. [Google Scholar] [CrossRef]
- Azam, H.M.; Alam, T.; Hasan, M.; Yameogo, D.D.S.; Kannan, A.D.; Rahman, A.; Kwon, M.J. Phosphorous in the environment: Characteristics with distribution and effects, removal mechanisms, treatment technologies, and factors affecting recovery as minerals in natural and engineered systems. Environ. Sci. Pollut. Res. Int. 2019, 26, 20183–20207. [Google Scholar] [CrossRef]
- Tuazon, E.C.; Atkinson, R.; Aschumann, S.M.; Arey, J.; Winer, A.M.; Pitts, J.N., Jr. Atmospheric Loss Processes of 1,2-Dibromo-3-Chloropropane and Trimethyl Phosphate”. Environ. Sci. Technol. 1986, 20, 1043–1046. [Google Scholar] [CrossRef]
- Aschmann, S.M.; Long, W.D.; Atkinson, R. Rate Constants for the gas-phase reactions of OH radicals with dimethyl phosphonate over the temperature range of 278–351 K and for a series of other organophosphorus compounds at similar to 280 K. J. Phys. Chem. A 2008, 112, 4793–4799. [Google Scholar] [CrossRef] [PubMed]
- Koshlyakov, P.V.; Barkova, D.A.; Gerasimov, I.E.; Chesnokov, E.N.; Zhang, X.; Krasnoperov, L.N. Kinetics of the gas-phase reaction of hydroxyl radicals with trimethyl phosphate over the 273–837 K temperature range. RSC Adv. 2021, 11, 14121–14131. [Google Scholar] [CrossRef]
- Krasnoperov, L.N.; Peng, J.; Marshall, P. Modified Transition State Theory and Negative Apparent Activation Energies of Simple Metathesis Reactions. In Proceedings of the 16th International Symposium on Gas Kinetics, Cambridge, UK, 23–27 July 2000; p. PA9. [Google Scholar]
- Krasnoperov, L.N.; Peng, J.; Marshall, P. Modified Transition State Theory and Negative Apparent Activation Energies of Simple Metathesis Reactions: Application to the Reaction CH3 + HBr → CH4 + Br. J. Phys. Chem. A 2005, 110, 3110–3120. [Google Scholar] [CrossRef] [PubMed]
- Aguila, A.; O’Shea, K.E.; Tobien, T.; Asmus, K.-D. Reactions of Hydroxyl Radical with Dimethyl Methylphosphonate and Diethyl Methylphosphonate. A Fundamental Mechanistic Study. J. Phys. Chem. A 2001, 105, 7834–7839. [Google Scholar] [CrossRef]
- Dunlea, E.J.; Ravishankara, A.R. Kinetic Studies of the Reactions of O(1D) with Several Atmospheric Molecules. Phys. Chem. Chem. Phys. 2004, 6, 2152–2161. [Google Scholar] [CrossRef]
- Dunlea, E.J.; Ravishankara, A.R. Measurement of the Rate Coefficient for the Reaction of O(1D) with H2O and Re-Evaluation of the Atmospheric OH Production Rate. Phys. Chem. Chem. Phys. 2004, 6, 3333–3340. [Google Scholar] [CrossRef]
- Zhang, X.; Sangwan, M.; Yan, C.; Koshlyakov, P.V.; Chesnokov, E.N.; Bedjanian, Y.; Krasnoperov, L.N. Disproportionation Channel of the Self-reaction of Hydroxyl Radical, OH + OH → H2O + O, Revisited. J. Phys. Chem. A 2020, 124, 3993–4005. [Google Scholar] [CrossRef]
- Sangwan, M.; Chesnokov, E.N.; Krasnoperov, L.N. Reaction OH + OH Studied over the 298–834 K Temperature and 1–100 bar Pressure Ranges. J. Phys. Chem. A 2012, 116, 6282–6294. [Google Scholar] [CrossRef]
- Sangwan, M.; Krasnoperov, L.N. Disproportionation Channel of Self-Reaction of Hydroxyl Radical, OH + OH → H2O + O, Studied by Time-Resolved Oxygen Atom Trapping. J. Phys. Chem. A 2012, 116, 11817–11822. [Google Scholar] [CrossRef]
- Yan, C.; Kocevska, S.; Krasnoperov, L.N. Kinetics of the Reaction of CH3O2 Radicals with OH Studied over the 292–526 K Temperature Range. J. Phys. Chem. A 2016, 120, 6111–6121. [Google Scholar] [CrossRef]
- Sangwan, M.; Krasnoperov, L.N. Kinetics of the Gas Phase Reaction CH3 + HO2. J. Phys. Chem. A 2013, 117, 2916–2923. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, M.; Yan, C.; Chesnokov, E.N.; Krasnoperov, L.N. Reaction CH3 + CH3 → C2H6 Studied over the 292–714 K and 1–100 bar Pressure Ranges. J. Phys. Chem. A 2015, 119, 7847–7857. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Alecu, I.M.; Hsieh, P.-C.; Morgan, B.P.; Marshall, P.; Krasnoperov, L.N. Thermochemistry Is Not a Lower Bound to the Activation Energy of Endothermic Reactions: A Kinetic Study of the Gas-Phase Reaction of Atomic Chlorine with Ammonia. J. Phys. Chem. A 2006, 110, 6844–6850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Manion, J.A.; Huie, R.E.; Levin, R.D., Jr.; Burgess, D.R.; Orkin, V.L.; Tsang, W.; McGivern, W.S.; Hudgens, J.W.; Knyazev, V.D.; Atkinson, D.B.; et al. NIST Chemical Kinetics Database, NIST Standard Reference Database 17, Version 7.0 (Web Version), Release 1.4.3, Data version 2008.12; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2008; pp. 20899–28320. [Google Scholar]
- Burns, D.S.; Cory, M.G.; Taylor, D.E.; Bunte, S.W.; Runge, K.; Vasey, J.L. A Comparison of Primary and Secondary Hydrogen Abstraction from Organophosphates by Hydroxyl Radical. Int. J. Chem. Kinet. 2013, 45, 187–201. [Google Scholar] [CrossRef]
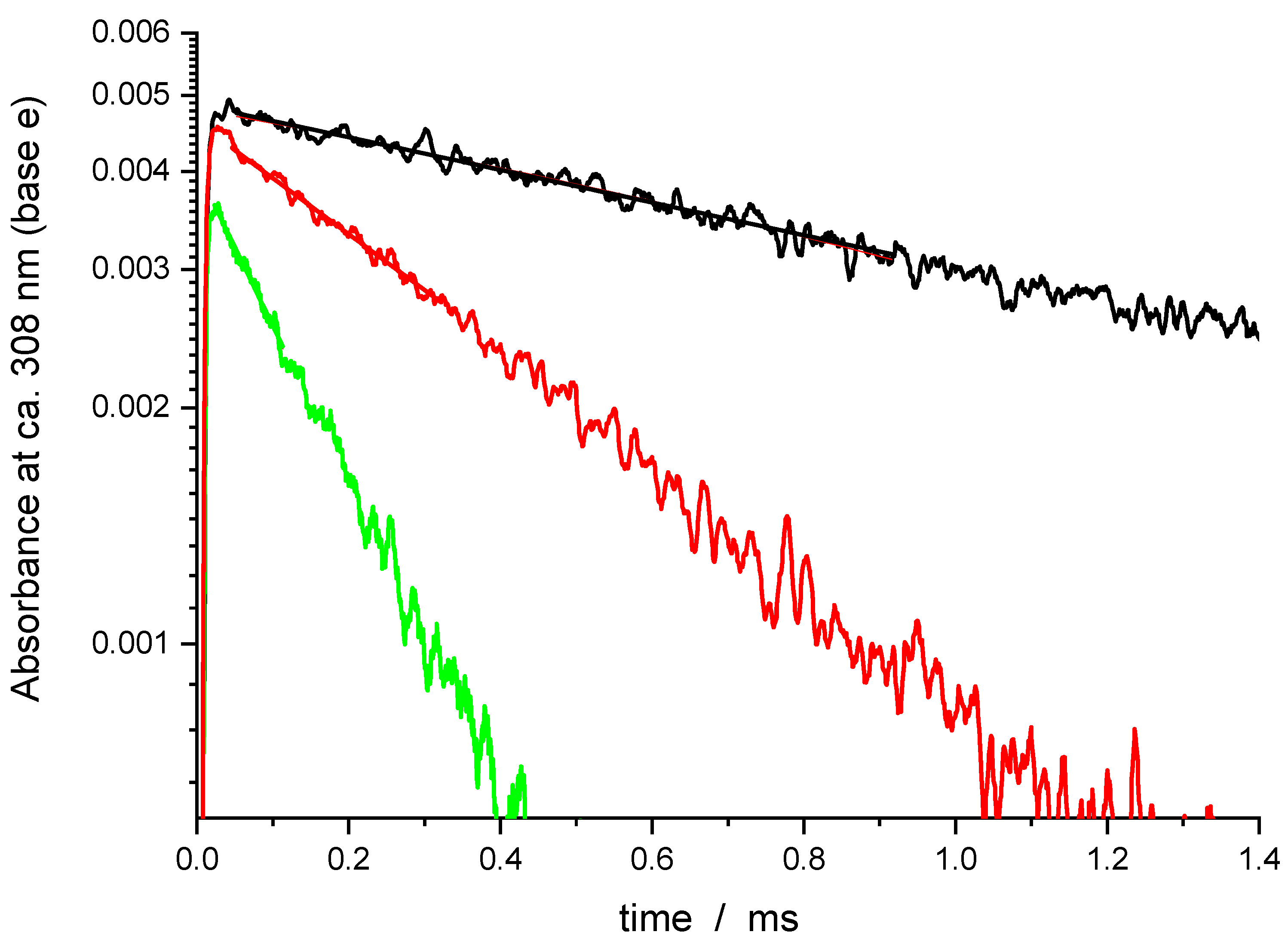
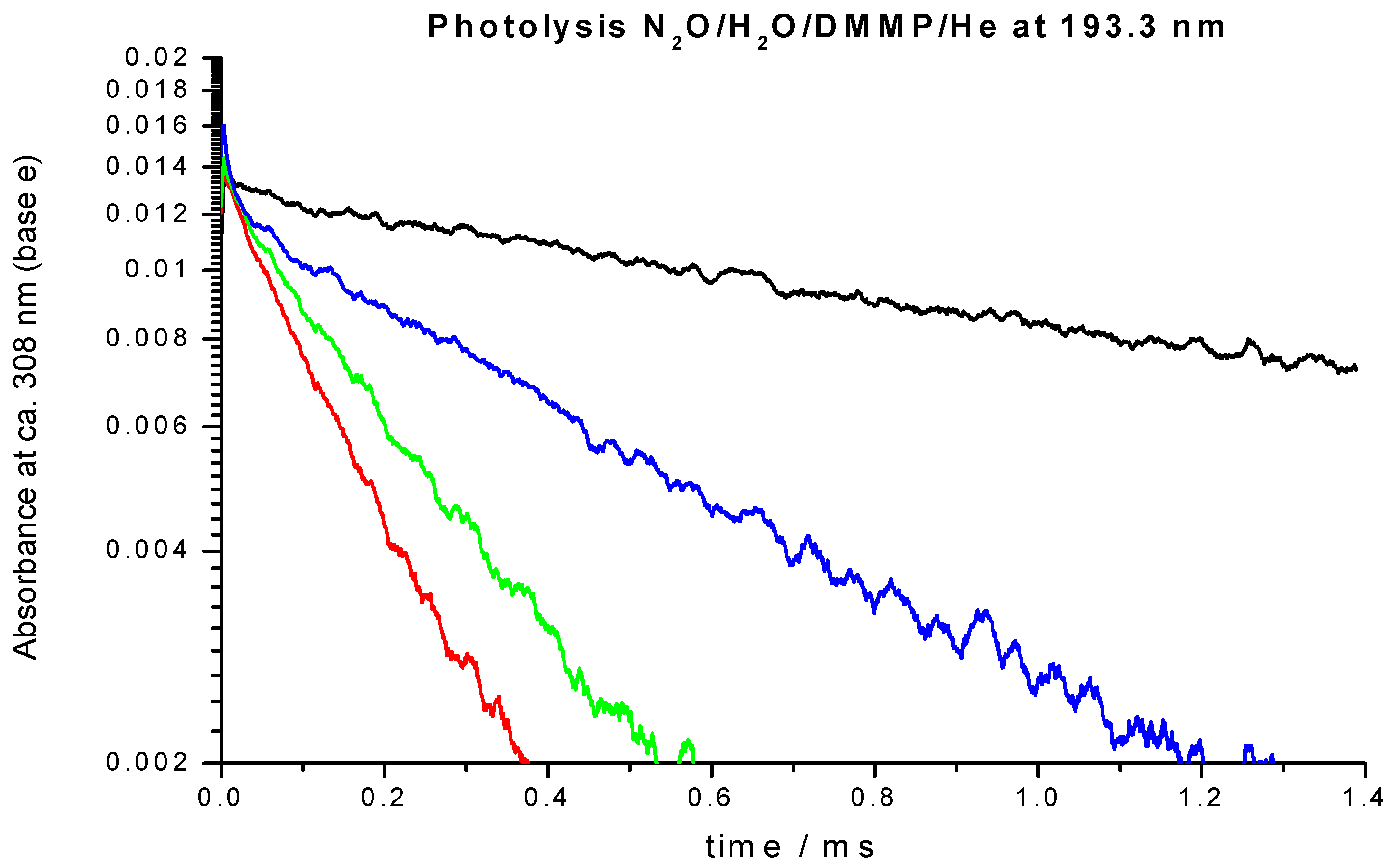
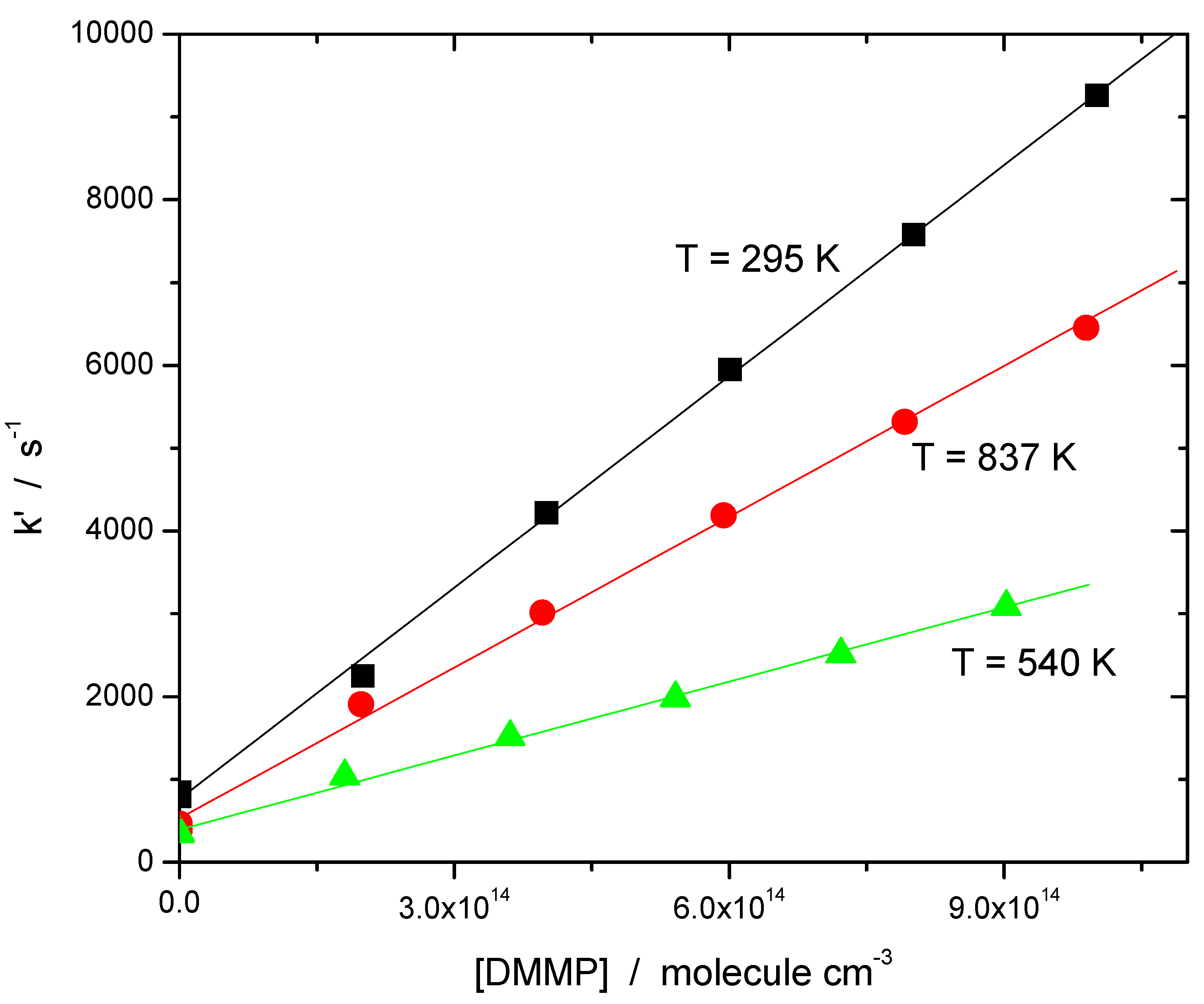


| Temperature/K | [O3]/1014 Molecule cm−3 | [O2]/1015 Molecule cm−3 | [H2O]/1016 Molecule cm−3 | [He]/1019 Molecule cm−3 | [DMMP]/1014 Molecule cm−3 | k’/103s−1 | k/10−12 cm3 Molecule−1 s−1 |
|---|---|---|---|---|---|---|---|
| 299 | 4.57 | 9.80 | 3.38 | 2.44 | 0 | 0.467 | 10.5 |
| 299 | 4.57 | 9.80 | 3.38 | 2.44 | 4.70 | 5.03 | |
| 299 | 4.57 | 9.80 | 3.38 | 2.44 | 9.41 | 9.98 | |
| 299 | 4.57 | 9.77 | 3.37 | 2.44 | 14.1 | 15.2 | |
| 299 | 4.57 | 9.75 | 3.37 | 2.44 | 18.73 | 19.9 | |
| 299 | 4.57 | 9.68 | 3.34 | 2.44 | 23.2 | 25.4 | |
| 299 | 4.54 | 9.69 | 3.42 | 2.44 | 0 | 0.511 | 9.85 |
| 299 | 4.54 | 9.62 | 3.4 | 2.44 | 4.73 | 5.55 | |
| 299 | 4.54 | 9.46 | 3.38 | 2.44 | 9.42 | 9.83 | |
| 299 | 4.54 | 9.32 | 3.33 | 2.44 | 13.9 | 14.3 | |
| 299 | 4.54 | 10.31 | 3.51 | 2.44 | 19.6 | 19.1 | |
| 299 | 4.54 | 10.35 | 3.49 | 2.44 | 24.3 | 23.9 | |
| 328 | 2.78 | 9.05 | 3.30 | 2.26 | 0 | 0.450 | 6.70 |
| 328 | 2.78 | 8.79 | 3.14 | 2.26 | 4.37 | 3.22 | |
| 328 | 2.78 | 8.66 | 3.10 | 2.26 | 8.61 | 6.25 | |
| 328 | 2.78 | 8.51 | 3.08 | 2.26 | 12.8 | 8.33 | |
| 328 | 2.78 | 8.47 | 3.07 | 2.26 | 17.0 | 13.2 | |
| 328 | 2.78 | 8.29 | 3.04 | 2.26 | 21.1 | 14.2 | |
| 365 | 1.78 | 8.19 | 2.77 | 2.02 | 0 | 0.452 | 4.75 |
| 365 | 1.78 | 8.18 | 2.74 | 2.02 | 3.97 | 1.95 | |
| 365 | 1.78 | 8.22 | 2.67 | 2.02 | 7.98 | 4.16 | |
| 365 | 1.78 | 8.08 | 2.65 | 2.02 | 11.9 | 5.88 | |
| 365 | 1.78 | 8.03 | 2.63 | 2.02 | 15.7 | 7.69 | |
| 365 | 1.78 | 7.83 | 2.60 | 2.02 | 19.4 | 8.33 | |
| 365 | 3.97 | 8.15 | 2.57 | 2.00 | 0 | 0.452 | 5.06 |
| 365 | 3.97 | 7.99 | 2.63 | 2.00 | 4.02 | 2.32 | |
| 365 | 3.97 | 7.95 | 2.62 | 2.00 | 8.00 | 4.16 | |
| 365 | 3.97 | 7.84 | 2.61 | 2.00 | 11.9 | 6.66 | |
| 365 | 3.97 | 7.70 | 2.60 | 2.00 | 15.8 | 8.33 | |
| 365 | 3.97 | 7.53 | 2.62 | 2.00 | 19.6 | 9.09 | |
| 365 | 3.97 | 8.72 | 2.67 | 2.00 | 0 | 0.413 | 5.04 |
| 365 | 3.97 | 8.63 | 2.64 | 2.00 | 12.4 | 6.66 | |
| 365 | 3.97 | 8.46 | 2.62 | 2.00 | 16.4 | 9.09 | |
| 365 | 3.97 | 8.30 | 2.60 | 2.00 | 20.4 | 11.1 | |
| 365 | 3.97 | 8.16 | 2.58 | 2.00 | 24.3 | 12.5 | |
| 365 | 3.97 | 7.93 | 2.54 | 2.00 | 11.9 | 6.66 | |
| 365 | 3.97 | 7.79 | 2.53 | 2.00 | 23.8 | 12.7 | |
| 411 | 3.44 | 7.50 | 2.31 | 1.80 | 0 | 0.450 | 3.99 |
| 411 | 3.44 | 7.64 | 2.30 | 1.80 | 3.67 | 1.75 | |
| 411 | 3.44 | 7.34 | 2.29 | 1.80 | 7.30 | 3.03 | |
| 411 | 3.44 | 7.20 | 2.27 | 1.80 | 10.8 | 4.54 | |
| 411 | 3.44 | 7.06 | 2.25 | 1.80 | 14.3 | 6.25 | |
| 411 | 3.44 | 7.02 | 2.24 | 1.80 | 17.8 | 7.14 | |
| 470 | 1.88 | 6.44 | 1.99 | 1.59 | 0 | 0.478 | 3.16 |
| 470 | 1.88 | 6.39 | 1.99 | 1.59 | 3.22 | 1.38 | |
| 470 | 1.88 | 6.28 | 1.99 | 1.59 | 6.40 | 2.22 | |
| 470 | 1.88 | 6.56 | 1.98 | 1.59 | 9.55 | 3.33 | |
| 470 | 1.88 | 5.39 | 1.96 | 1.58 | 12.6 | 4.16 | |
| 470 | 1.88 | 6.23 | 1.95 | 1.59 | 15.6 | 5.55 |
| Temperature/K | [H2O]/1017 Molecule cm−3 | [N2O]/1016 Molecule cm−3 | [DMMP]/1014 Molecule cm−3 | k’/103 s−1 | k/10−12 cm3 Molecule −1 s−1 |
|---|---|---|---|---|---|
| 295 | 3.03 | 3.23 | 0 | 0.847 | 8.51 |
| 295 | 3.03 | 3.23 | 10.0 | 9.25 | |
| 295 | 3.03 | 3.23 | 8.0 | 7.57 | |
| 295 | 3.03 | 3.23 | 6.0 | 5.95 | |
| 295 | 3.03 | 3.23 | 4.0 | 4.21 | |
| 295 | 3.03 | 3.23 | 2.0 | 2.24 | |
| 295 | 3.03 | 3.23 | 0 | 0.794 | |
| 323 | 2.98 | 7.70 | 0 | 0.448 | 6.82 |
| 323 | 2.98 | 7.70 | 7.56 | 5.61 | |
| 323 | 2.98 | 7.70 | 6.05 | 4.60 | |
| 323 | 2.98 | 7.70 | 4.54 | 3.43 | |
| 323 | 2.98 | 7.70 | 3.02 | 2.34 | |
| 323 | 2.98 | 7.70 | 1.51 | 1.51 | |
| 323 | 2.98 | 7.70 | 0 | 0.442 | |
| 365 | 2.91 | 4.49 | 0 | 0.463 | 5.36 |
| 365 | 2.91 | 4.49 | 9.59 | 5.68 | |
| 365 | 2.91 | 4.49 | 7.68 | 4.58 | |
| 365 | 2.91 | 4.49 | 5.76 | 3.71 | |
| 365 | 2.91 | 4.49 | 3.84 | 2.77 | |
| 365 | 2.91 | 4.49 | 1.92 | 1.60 | |
| 411 | 3.03 | 5.99 | 0 | 0.360 | 4.17 |
| 411 | 3.03 | 5.99 | 10.0 | 4.566 | |
| 411 | 3.03 | 5.99 | 8.0 | 3.77 | |
| 411 | 3.03 | 5.99 | 6.0 | 3.04 | |
| 411 | 3.03 | 5.99 | 4.0 | 2.24 | |
| 411 | 3.03 | 5.99 | 2.0 | 1.324 | |
| 411 | 3.03 | 5.99 | 0 | 0.465 | |
| 455 | 3.24 | 5.44 | 0 | 0.341 | 3.59 |
| 455 | 3.24 | 5.44 | 9.62 | 3.83 | |
| 455 | 3.24 | 5.44 | 7.70 | 3.09 | |
| 455 | 3.24 | 5.44 | 5.77 | 2.52 | |
| 455 | 3.24 | 5.44 | 3.85 | 1.89 | |
| 455 | 3.24 | 5.44 | 1.92 | 1.05 | |
| 455 | 3.24 | 5.44 | 0 | 0.394 | |
| 500 | 2.81 | 4.23 | 0 | 0.371 | 3.16 |
| 500 | 2.81 | 4.23 | 9.27 | 3.26 | |
| 500 | 2.81 | 4.23 | 7.42 | 2.74 | |
| 500 | 2.81 | 4.23 | 5.56 | 2.16 | |
| 500 | 2.81 | 4.23 | 3.71 | 1.48 | |
| 500 | 2.81 | 4.23 | 1.85 | 1.00 | |
| 500 | 2.81 | 4.23 | 0 | 0.350 | |
| 540 | 2.46 | 3.64 | 0 | 0.34 | 2.98 |
| 540 | 2.46 | 3.64 | 9.02 | 3.08 | |
| 540 | 2.46 | 3.64 | 7.22 | 2.51 | |
| 540 | 2.46 | 3.64 | 5.41 | 1.98 | |
| 540 | 2.46 | 3.64 | 3.61 | 1.51 | |
| 540 | 2.46 | 3.64 | 1.80 | 1.03 | |
| 540 | 2.46 | 3.64 | 0 | 0.353 | |
| 591 | 2.73 | 4.17 | 0 | 0.388 | 3.17 |
| 591 | 2.73 | 4.17 | 9.82 | 3.50 | |
| 591 | 2.73 | 4.17 | 7.86 | 2.91 | |
| 591 | 2.73 | 4.17 | 5.90 | 2.27 | |
| 591 | 2.73 | 4.17 | 3.93 | 1.70 | |
| 591 | 2.73 | 4.17 | 1.96 | 1.03 | |
| 591 | 2.73 | 4.17 | 0 | 0.427 | |
| 673 | 2.42 | 4.85 | 0 | 0.355 | 3.85 |
| 673 | 2.42 | 4.85 | 8.70 | 3.65 | |
| 673 | 2.42 | 4.85 | 6.96 | 3.05 | |
| 673 | 2.42 | 4.85 | 5.22 | 2.51 | |
| 673 | 2.42 | 4.85 | 3.48 | 1.74 | |
| 673 | 2.42 | 4.85 | 1.74 | 1.06 | |
| 673 | 2.42 | 4.85 | 0 | 0.346 | |
| 773 | 2.10 | 3.55 | 0 | 0.424 | 5.16 |
| 773 | 2.10 | 3.55 | 7.60 | 4.34 | |
| 773 | 2.10 | 3.55 | 6.06 | 3.57 | |
| 773 | 2.10 | 3.55 | 4.54 | 2.84 | |
| 773 | 2.10 | 3.55 | 3.03 | 2.14 | |
| 773 | 2.10 | 3.55 | 1.51 | 1.33 | |
| 773 | 2.10 | 3.55 | 0 | 0.412 | |
| 837 | 1.94 | 3.27 | 0 | 0.465 | 6.07 |
| 837 | 1.94 | 3.27 | 9.89 | 6.45 | |
| 837 | 1.94 | 3.27 | 7.91 | 5.31 | |
| 837 | 1.94 | 3.27 | 5.94 | 4.18 | |
| 837 | 1.94 | 3.27 | 3.96 | 3.01 | |
| 837 | 1.94 | 3.27 | 1.98 | 1.90 | |
| 837 | 1.94 | 3.27 | 0 | 0.385 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Barkova, D.A.; Koshlyakov, P.V.; Gerasimov, I.E.; Chesnokov, E.N.; Krasnoperov, L.N. Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K). Molecules 2022, 27, 2301. https://doi.org/10.3390/molecules27072301
Zhang X, Barkova DA, Koshlyakov PV, Gerasimov IE, Chesnokov EN, Krasnoperov LN. Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K). Molecules. 2022; 27(7):2301. https://doi.org/10.3390/molecules27072301
Chicago/Turabian StyleZhang, Xiaokai, Daria A. Barkova, Pavel V. Koshlyakov, Ilya E. Gerasimov, Evgeni N. Chesnokov, and Lev N. Krasnoperov. 2022. "Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K)" Molecules 27, no. 7: 2301. https://doi.org/10.3390/molecules27072301
APA StyleZhang, X., Barkova, D. A., Koshlyakov, P. V., Gerasimov, I. E., Chesnokov, E. N., & Krasnoperov, L. N. (2022). Kinetics of the Gas-Phase Reaction of Hydroxyl Radicals with Dimethyl Methylphosphonate (DMMP) over an Extended Temperature Range (273–837 K). Molecules, 27(7), 2301. https://doi.org/10.3390/molecules27072301








