Screening and Structure–Activity Relationship of D2AAK1 Derivatives for Potential Application in the Treatment of Neurodegenerative Diseases
Abstract
:1. Introduction
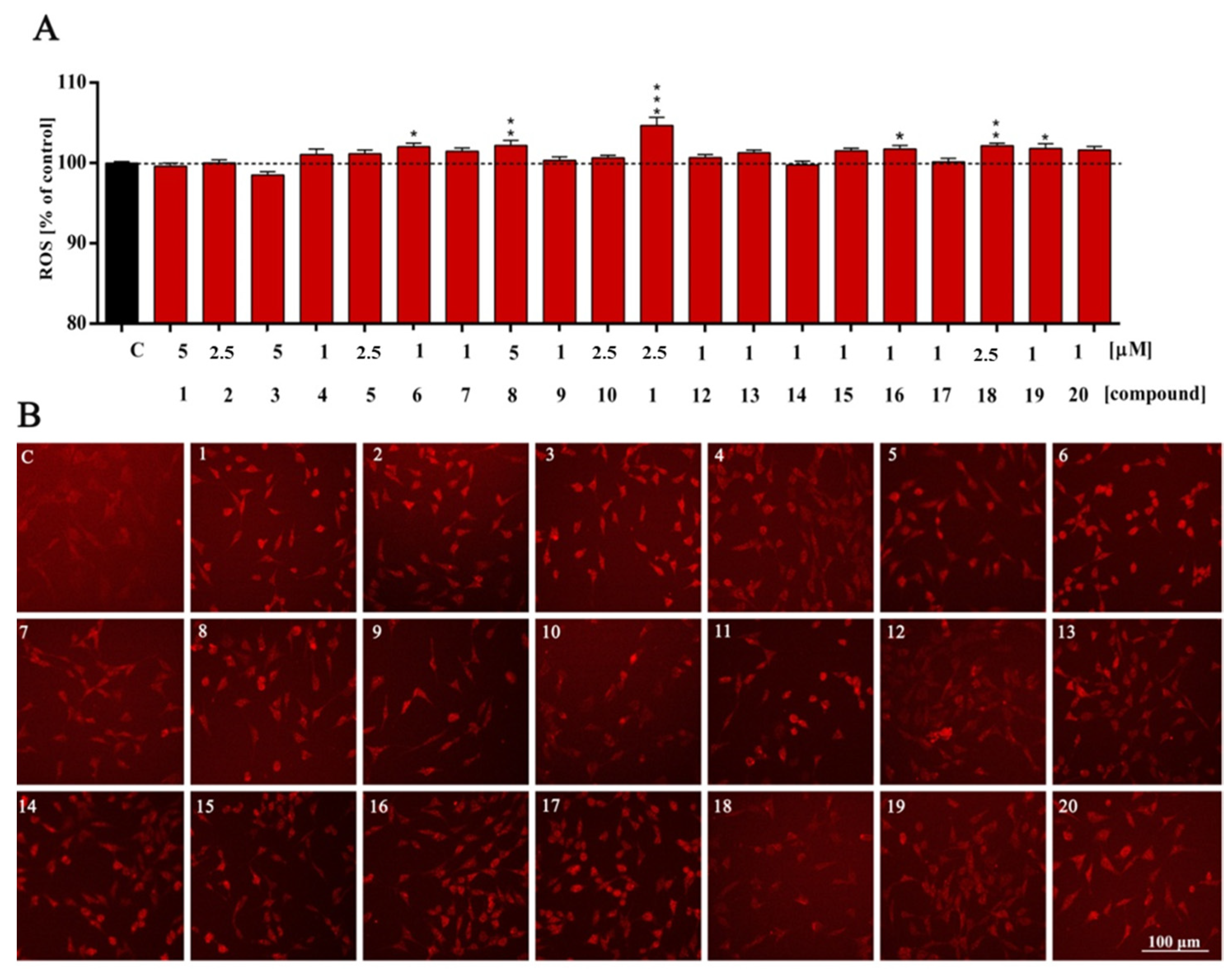
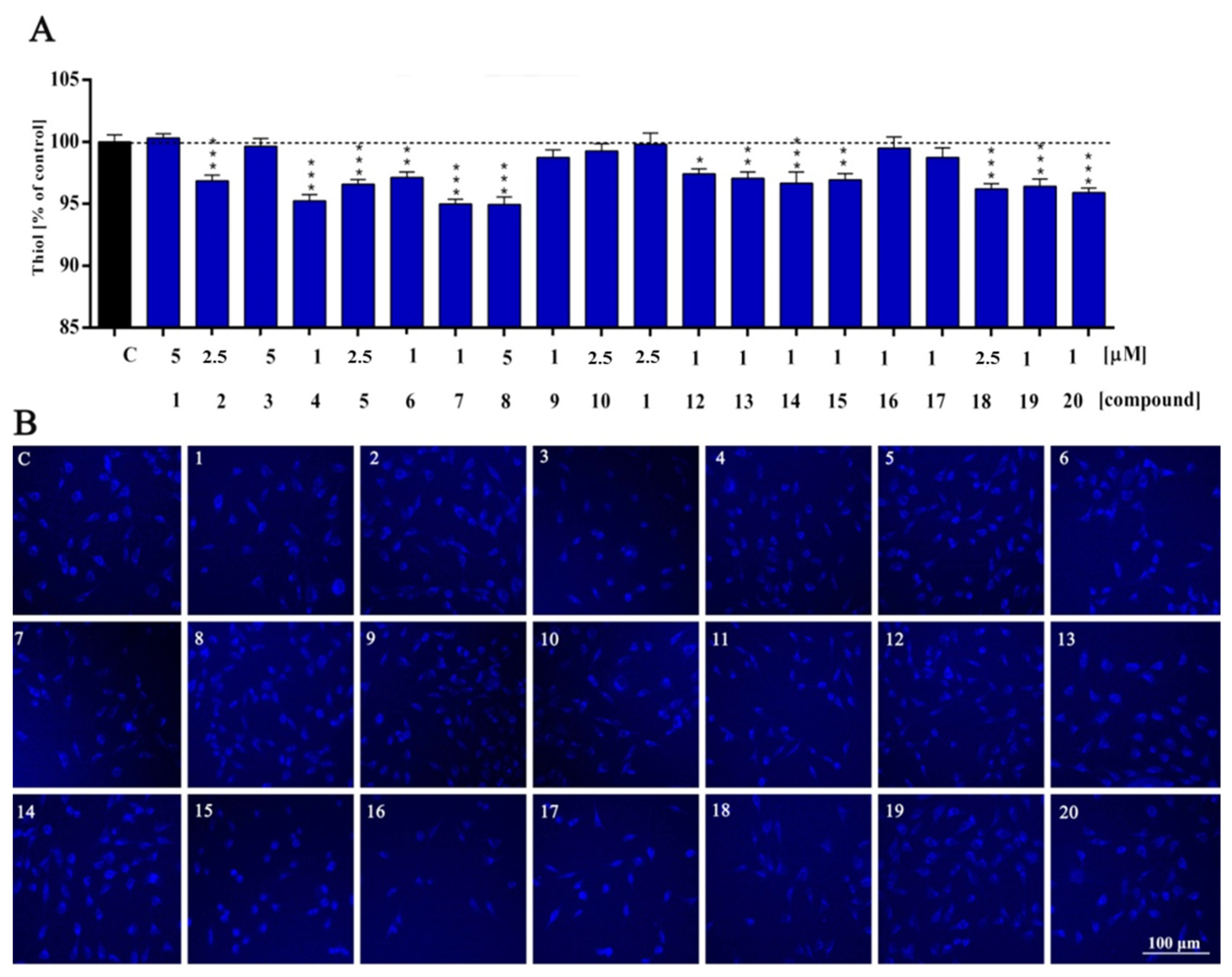
2. Results and Discussion
3. Materials and Methods
3.1. Hippocampal Neuronal Cell Line
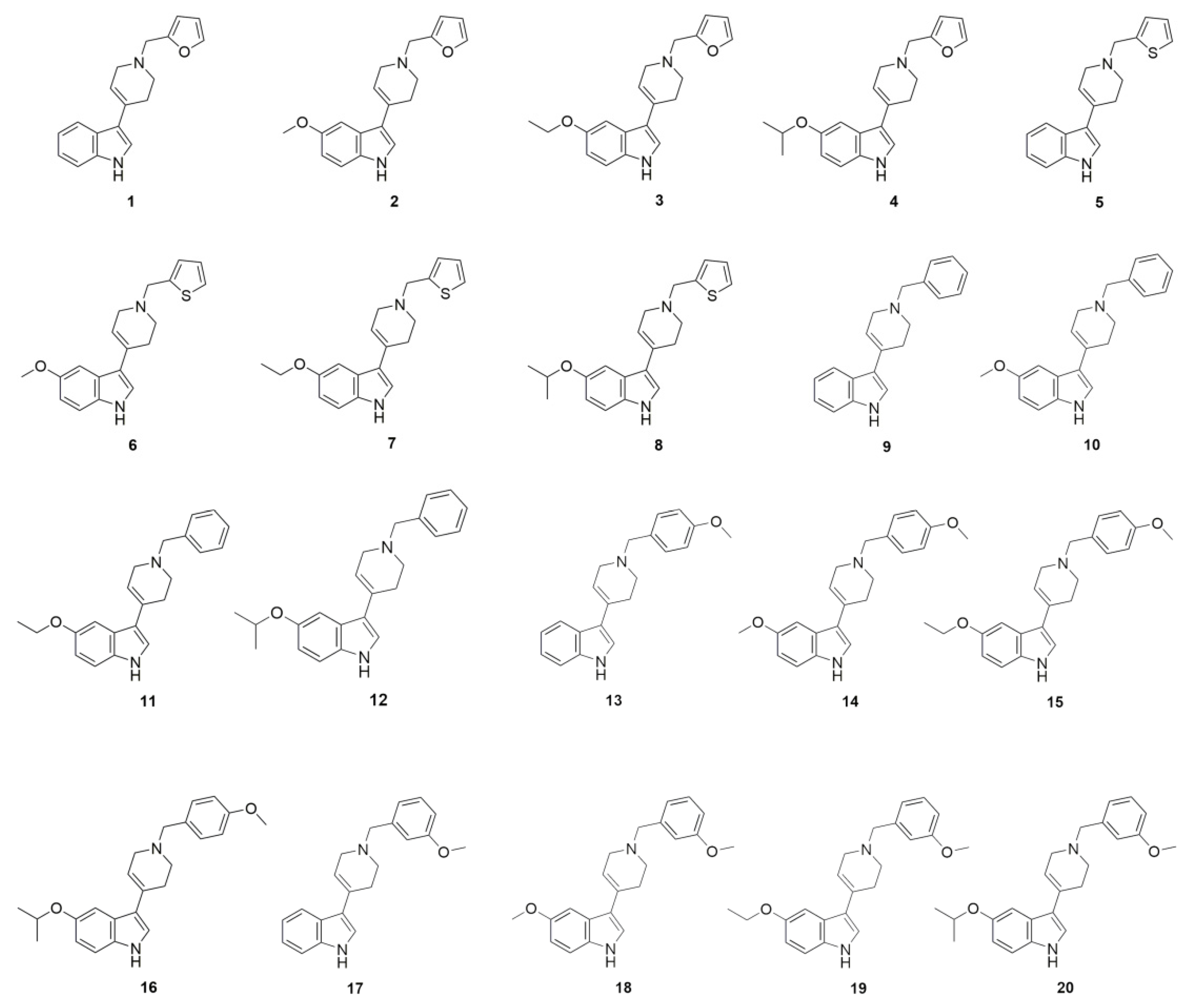
3.2. D2AAK1 Derivatives
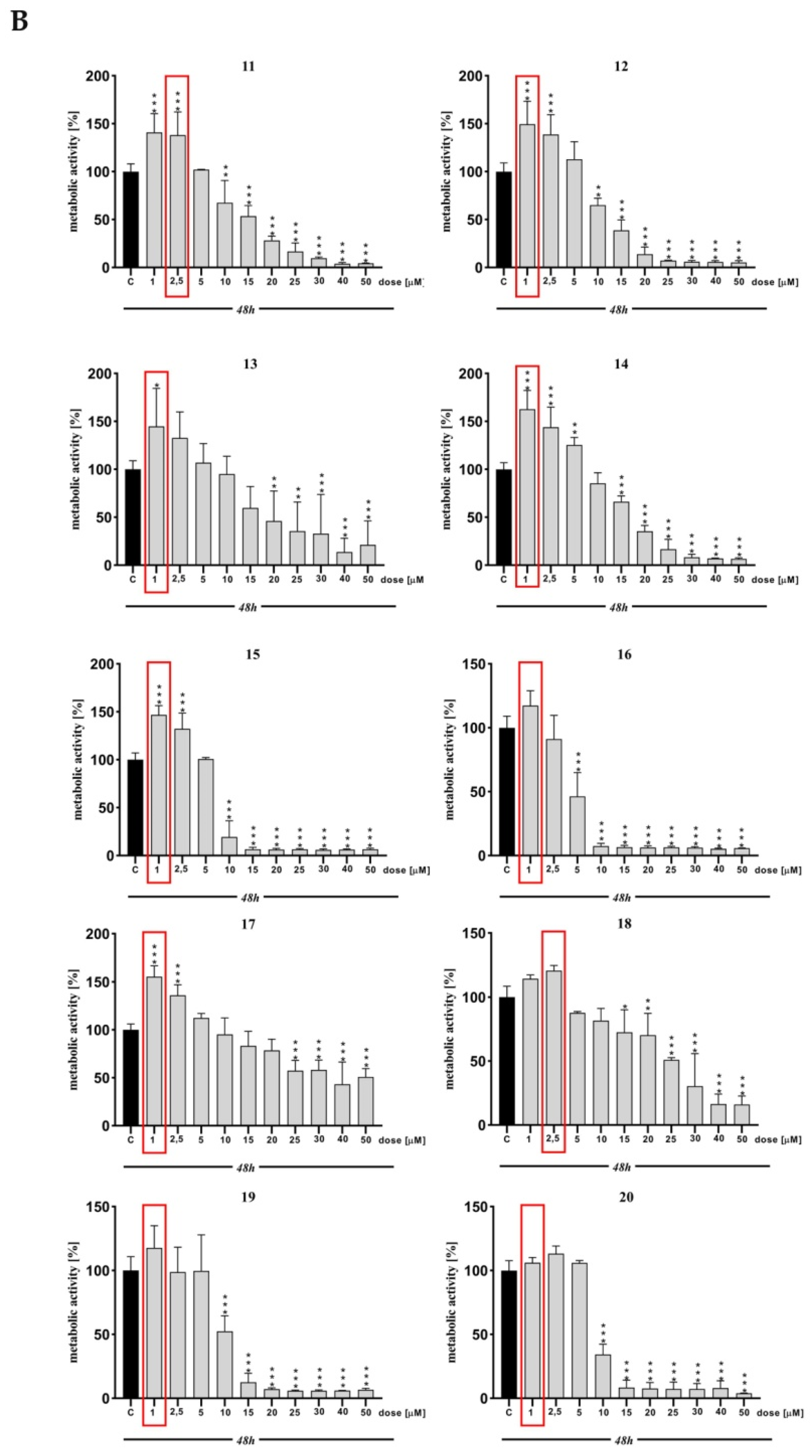
3.3. MTT Assay
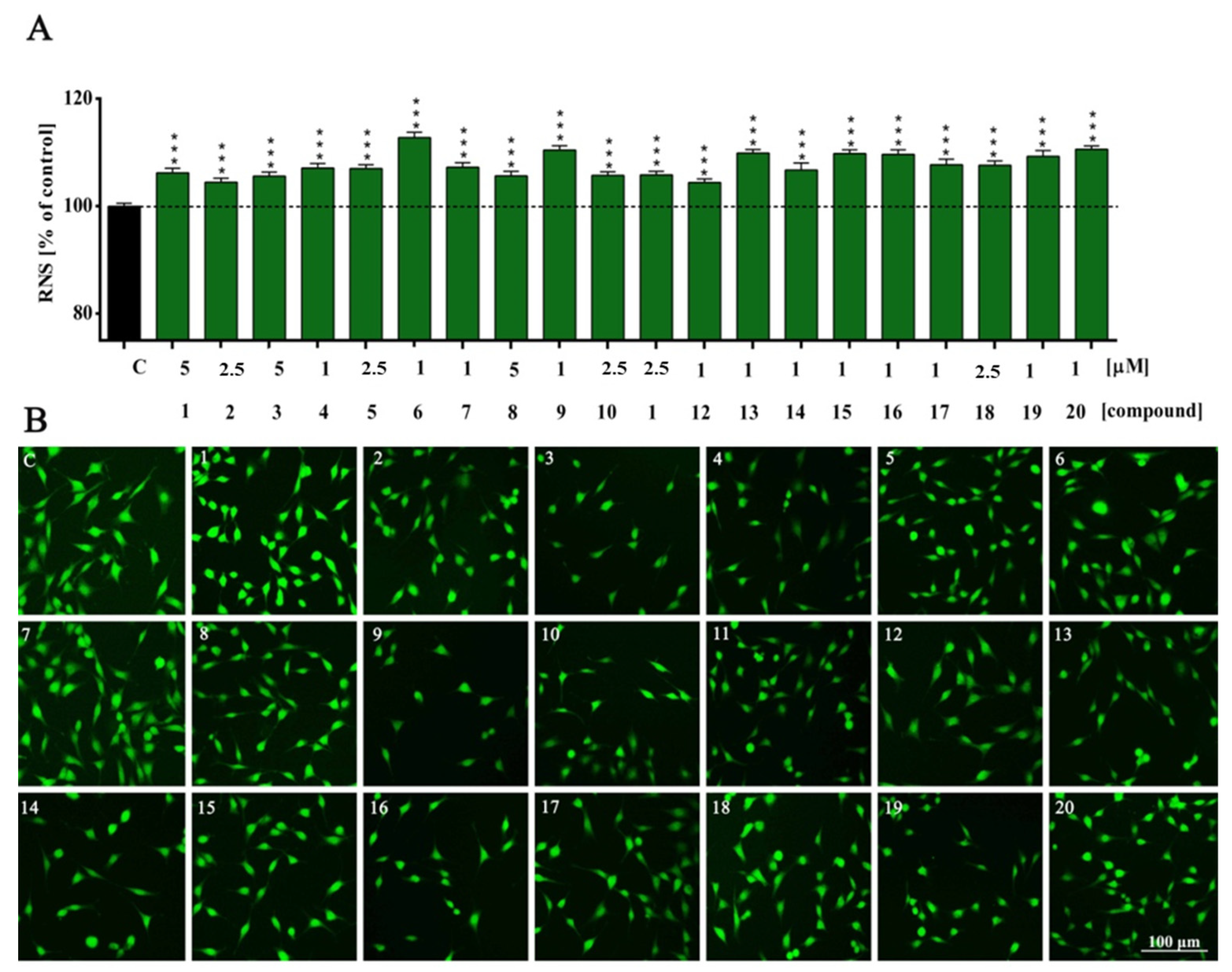
3.4. Cellular Redox Status and Antioxidant Capacity Assessment
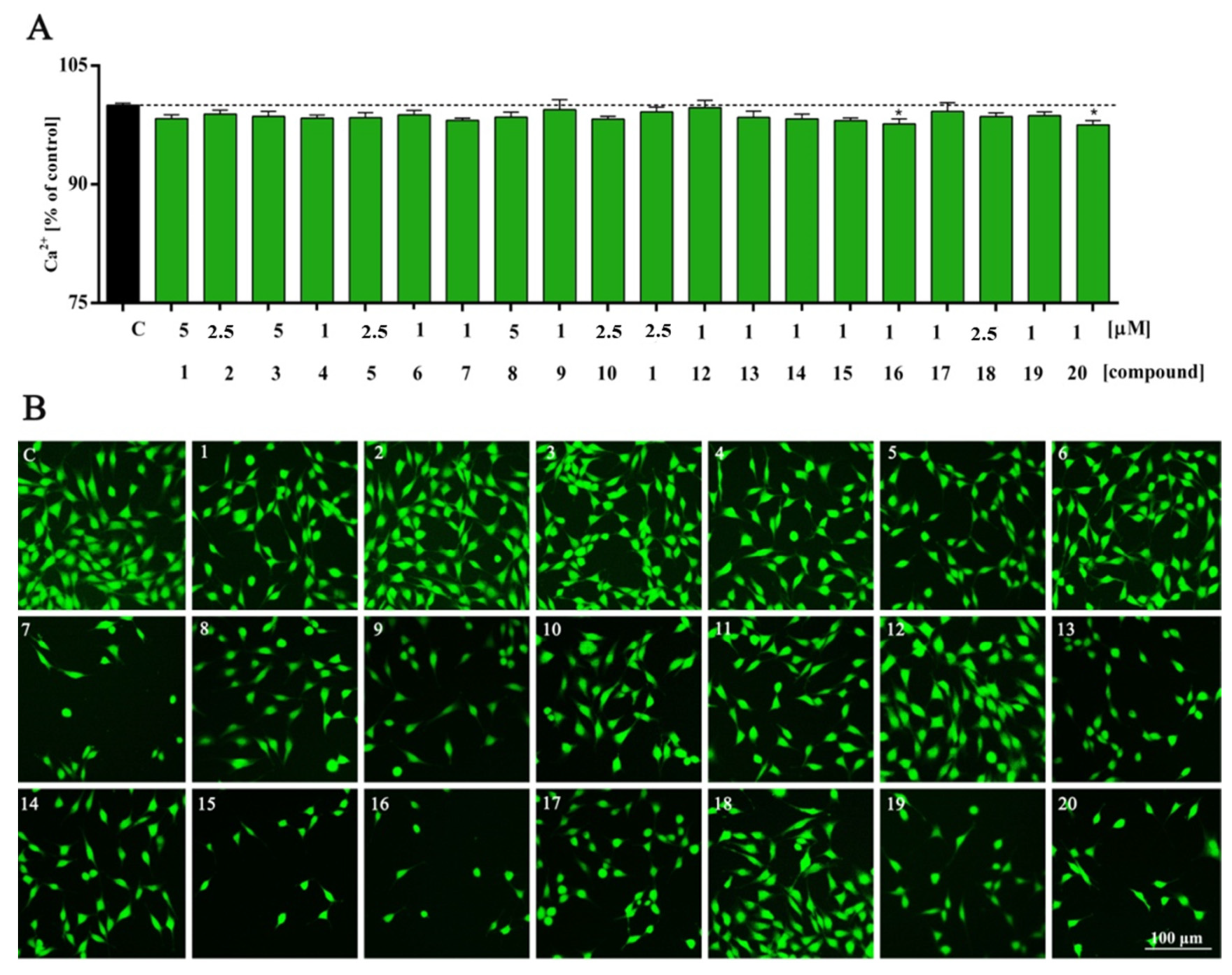
3.5. Intracellular Calcium Analysis
3.6. Statistical Analysis
4. Conclusions
D2AAK1 versus Its Analogs
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative Disease: Models, Mechanisms, and a New Hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linsley, J.W.; Reisine, T.; Finkbeiner, S. Cell Death Assays for Neurodegenerative Disease Drug Discovery. Expert Opin. Drug Discov. 2019, 14, 901–913. [Google Scholar] [CrossRef] [PubMed]
- Elufioye, T.O.; Berida, T.I.; Habtemariam, S. Plants-Derived Neuroprotective Agents: Cutting the Cycle of Cell Death through Multiple Mechanisms. Evid. Based Complement. Alternat. Med. 2017, 2017, 3574012. [Google Scholar] [CrossRef] [Green Version]
- Śliwka, L.; Wiktorska, K.; Suchocki, P.; Milczarek, M.; Mielczarek, S.; Lubelska, K.; Cierpiał, T.; Łyżwa, P.; Kiełbasiński, P.; Jaromin, A.; et al. The Comparison of MTT and CVS Assays for the Assessment of Anticancer Agent Interactions. PLoS ONE 2016, 11, e0155772. [Google Scholar] [CrossRef] [Green Version]
- Lapchak, P.A. Drug-like Property Profiling of Novel Neuroprotective Compounds to Treat Acute Ischemic Stroke: Guidelines to Develop Pleiotropic Molecules. Transl. Stroke Res. 2013, 4, 328–342. [Google Scholar] [CrossRef] [Green Version]
- Kaczor, A.A.; Targowska-Duda, K.M.; Budzyńska, B.; Biała, G.; Silva, A.G.; Castro, M. In Vitro, Molecular Modeling and Behavioral Studies of 3-{[4-(5-Methoxy-1H-Indol-3-Yl)-1,2,3,6-Tetrahydropyridin-1-Yl]Methyl}-1,2-Dihydroquinolin-2-One (D2AAK1) as a Potential Antipsychotic. Neurochem. Int. 2016, 96, 84–99. [Google Scholar] [CrossRef]
- Koszła, O.; Sołek, P.; Woźniak, S.; Kędzierska, E.; Wróbel, T.M.; Kondej, M.; Archała, A.; Stępnicki, P.; Biała, G.; Matosiuk, D.; et al. The Antipsychotic D2AAK1 as a Memory Enhancer for Treatment of Mental and Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 8849. [Google Scholar] [CrossRef]
- Koszła, O.; Sołek, P.; Kędzierska, E.; Listos, P.; Castro, M.; Agnieszka, A.K. In Vitro and In Vivo Evaluation of Antioxidant and Neuroprotective Properties of Antipsychotic D2AAK1. Neurochem. Res. 2022; in press. [Google Scholar] [CrossRef]
- Kondej, M.; Wróbel, T.M.; Silva, A.G.; Stępnicki, P.; Koszła, O.; Kędzierska, E.; Bartyzel, A.; Biała, G.; Matosiuk, D.; Loza, M.I.; et al. Synthesis, Pharmacological and Structural Studies of 5-Substituted-3-(1-Arylmethyl-1,2,3,6-Tetrahydropyridin-4-Yl)-1H-Indoles as Multi-Target Ligands of Aminergic GPCRs. Eur. J. Med. Chem. 2019, 180, 673–689. [Google Scholar] [CrossRef]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of Oxidative Stress in Alzheimer’s Disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.-D.; Dunnavant, F.D.; Jarman, T.; Deutch, A.Y. Effects of Antipsychotic Drugs on Neurogenesis in the Forebrain of the Adult Rat. Neuropsychopharmacology 2004, 29, 1230–1238. [Google Scholar] [CrossRef] [PubMed]
- Keilhoff, G.; Fusar-Poli, P.; Becker, A. Effects of Antipsychotics on Dentate Gyrus Stem Cell Proliferation and Survival in Animal Models: A Critical Update. Neural Plast. 2012, 2012, 832757. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; He, X.-Q.; Peng, L.; Li, A.-P.; Wang, X.-R.; Zhou, J.-W.; Liu, Q.-Z. The Role of Oxidative Stress in Hormesis Induced by Sodium Arsenite in Human Embryo Lung Fibroblast (HELF) Cellular Proliferation Model. J. Toxicol. Environ. Health Part A 2007, 70, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Frosali, S.; Leonini, A.; Ettorre, A.; Di Maio, G.; Nuti, S.; Tavarini, S.; Di Simplicio, P.; Di Stefano, A. Role of Intracellular Calcium and S-Glutathionylation in Cell Death Induced by a Mixture of Isothiazolinones in HL60 Cells. Biochim. Biophys. Acta–Mol. Cell Res. 2009, 1793, 572–583. [Google Scholar] [CrossRef] [Green Version]
- Rizzuto, R. Intracellular Ca2+ Pools in Neuronal Signalling. Curr. Opin. Neurobiol. 2001, 11, 306–311. [Google Scholar] [CrossRef]
- Brini, M.; Calì, T.; Ottolini, D.; Carafoli, E. Neuronal Calcium Signaling: Function and Dysfunction. Cell. Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef]
- Trebak, M.; Ginnan, R.; Singer, H.A.; Jourd’heuil, D. Interplay between Calcium and Reactive Oxygen/Nitrogen Species: An Essential Paradigm for Vascular Smooth Muscle Signaling. Antioxid. Redox Signal. 2010, 12, 657–674. [Google Scholar] [CrossRef] [Green Version]
- Mattson, M.P.; Gleichmann, M.; Cheng, A. Mitochondria in Neuroplasticity and Neurological Disorders. Neuron 2008, 60, 748–766. [Google Scholar] [CrossRef] [Green Version]
- Feno, S.; Butera, G.; Vecellio Reane, D.; Rizzuto, R.; Raffaello, A. Crosstalk between Calcium and ROS in Pathophysiological Conditions. Oxid. Med. Cell. Longev. 2019, 2019, 9324018. [Google Scholar] [CrossRef] [Green Version]
- Hidalgo, C.; Carrasco, M.A.; Muñoz, P.; Núñez, M.T. A Role for Reactive Oxygen/Nitrogen Species and Iron on Neuronal Synaptic Plasticity. Antioxid. Redox Signal. 2007, 9, 245–255. [Google Scholar] [CrossRef]
- Yermolaieva, O.; Brot, N.; Weissbach, H.; Heinemann, S.H.; Hoshi, T. Reactive Oxygen Species and Nitric Oxide Mediate Plasticity of Neuronal Calcium Signaling. Proc. Natl. Acad. Sci. USA 2000, 97, 448–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milkovic, L.; Cipak Gasparovic, A.; Cindric, M.; Mouthuy, P.-A.; Zarkovic, N. Short Overview of ROS as Cell Function Regulators and Their Implications in Therapy Concepts. Cells 2019, 8, 793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Glutathione Homeostasis and Functions: Potential Targets ForMedical Interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef] [Green Version]
- Kerksick, C.; Willoughby, D. The Antioxidant Role of Glutathione and N-Acetyl-Cysteine Supplements and Exercise-Induced Oxidative Stress. J. Int. Soc. Sports Nutr. 2005, 2, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Lu, M.; Mei, M.; Wang, H.; Han, Z.; Chen, M.; Yao, H.; Song, N.; Ding, X.; Ding, J.; et al. Pyridoxine Induces Glutathione Synthesis via PKM2-Mediated Nrf2 Transactivation and Confers Neuroprotection. Nat. Commun. 2020, 11, 941. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| HT-22 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D2AAK1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | |
| MTT | *** | ** | *** | *** | *** | *** | *** | * | ns | *** | *** | *** | *** | * | *** | *** | ns | *** | ns | ns | ns |
| Ca2+ | *** | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | ns | * |
| NO | ns | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| ROS | ns | ns | ns | ns | ns | ns | * | ns | ** | ns | ns | *** | ns | ns | ns | ns | * | ns | ** | * | ns |
| Thiol | ns | ns | *** | ns | *** | *** | ** | *** | *** | ns | ns | ns | * | ** | *** | ** | ns | ns | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koszła, O.; Sołek, P.; Stępnicki, P.; Kaczor, A.A. Screening and Structure–Activity Relationship of D2AAK1 Derivatives for Potential Application in the Treatment of Neurodegenerative Diseases. Molecules 2022, 27, 2239. https://doi.org/10.3390/molecules27072239
Koszła O, Sołek P, Stępnicki P, Kaczor AA. Screening and Structure–Activity Relationship of D2AAK1 Derivatives for Potential Application in the Treatment of Neurodegenerative Diseases. Molecules. 2022; 27(7):2239. https://doi.org/10.3390/molecules27072239
Chicago/Turabian StyleKoszła, Oliwia, Przemysław Sołek, Piotr Stępnicki, and Agnieszka A. Kaczor. 2022. "Screening and Structure–Activity Relationship of D2AAK1 Derivatives for Potential Application in the Treatment of Neurodegenerative Diseases" Molecules 27, no. 7: 2239. https://doi.org/10.3390/molecules27072239
APA StyleKoszła, O., Sołek, P., Stępnicki, P., & Kaczor, A. A. (2022). Screening and Structure–Activity Relationship of D2AAK1 Derivatives for Potential Application in the Treatment of Neurodegenerative Diseases. Molecules, 27(7), 2239. https://doi.org/10.3390/molecules27072239







