The Role of Lipids in Allosteric Modulation of Dopamine D2 Receptor—In Silico Study
Abstract
1. Introduction
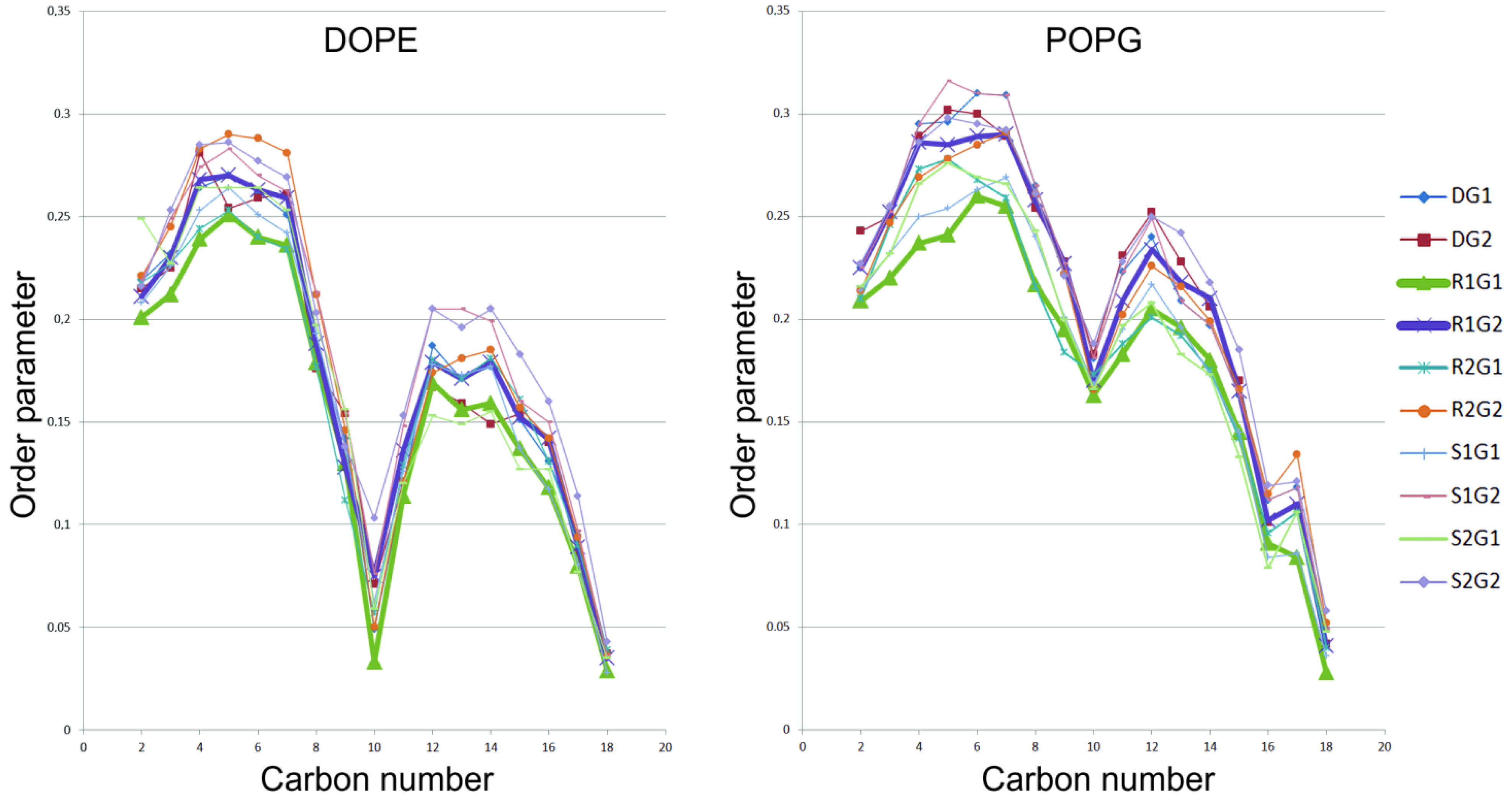
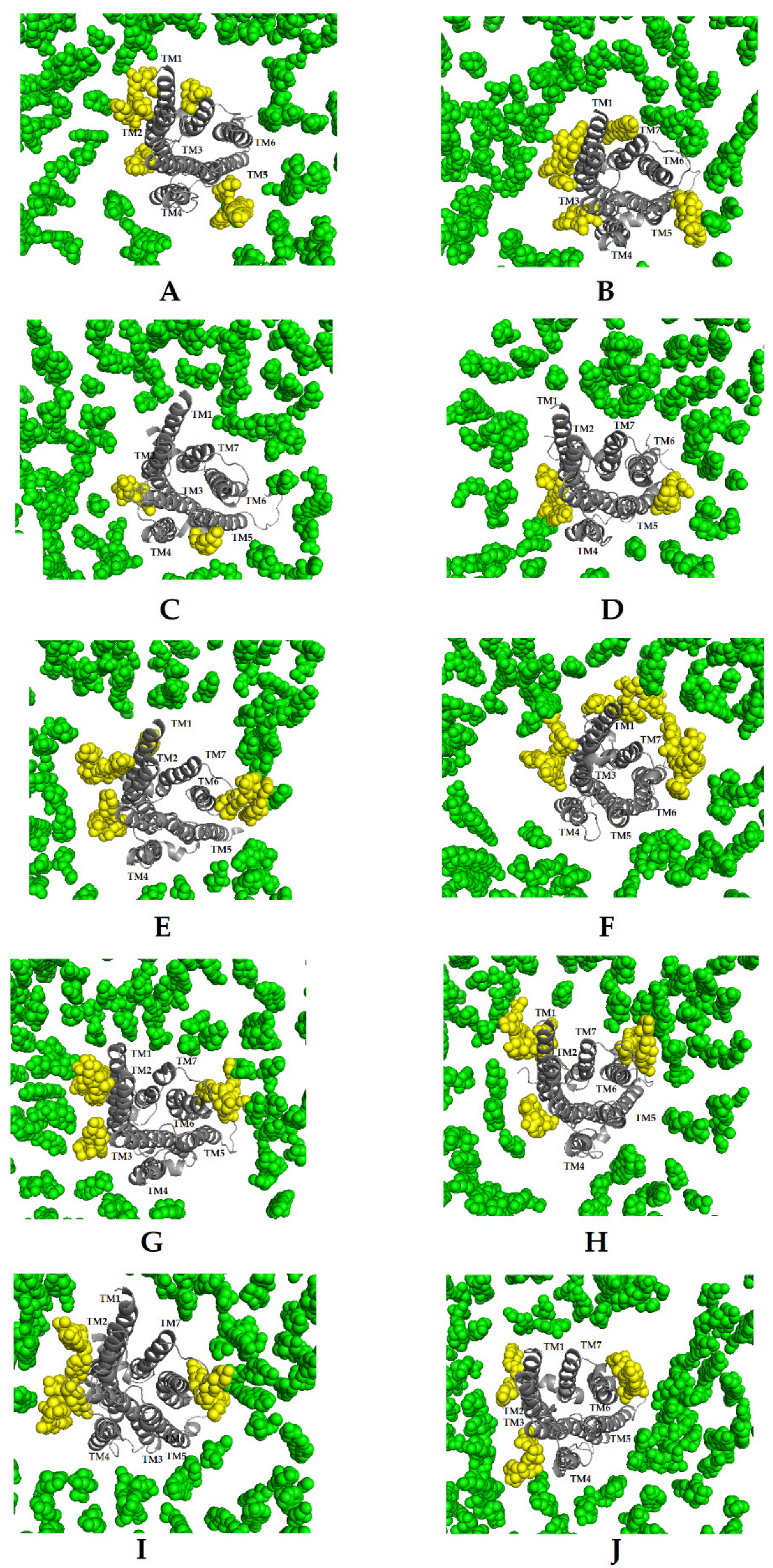
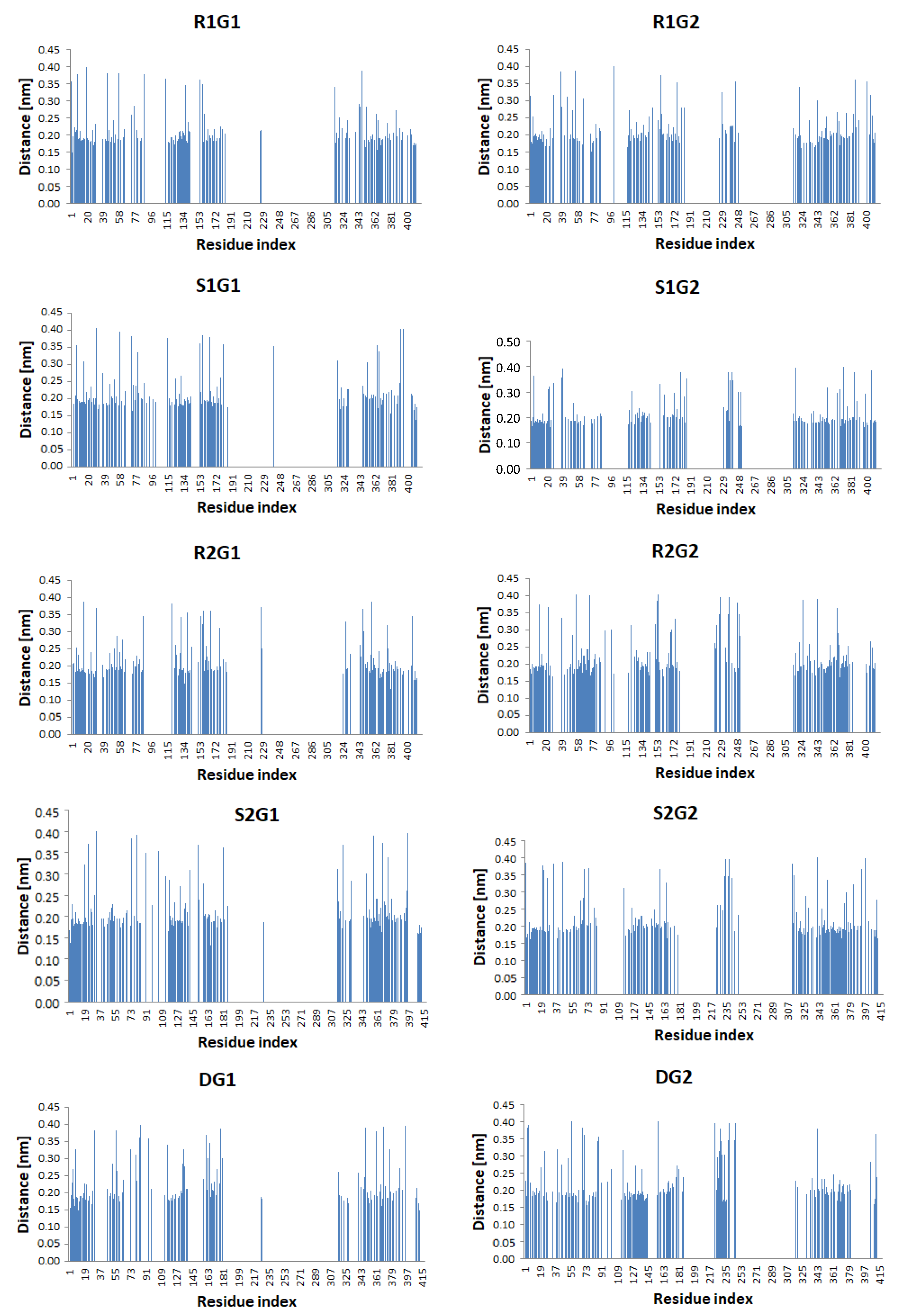
2. Results
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| DG1 | D2LONG receptor in complex with Gi1 protein |
| DG2 | D2LONG receptor in complex with Gi2 protein |
| DOPC | 1,2-dioleoyl-sn-glycero-3-phosphocholine |
| DOPE | 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOPS | 1,2-dioleoyl-sn-glycero-3-phospho-L-serine |
| ECL | Extracellular loop |
| GPCRs | G protein coupled receptors |
| ICL | Intracellular loop |
| MD | Molecular dynamics |
| NAM | Negative allosteric modulator |
| PAM | Positive allosteric modulator |
| POPC | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine |
| POPE | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanolamine |
| POPG | 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol |
| PLPC | 1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine |
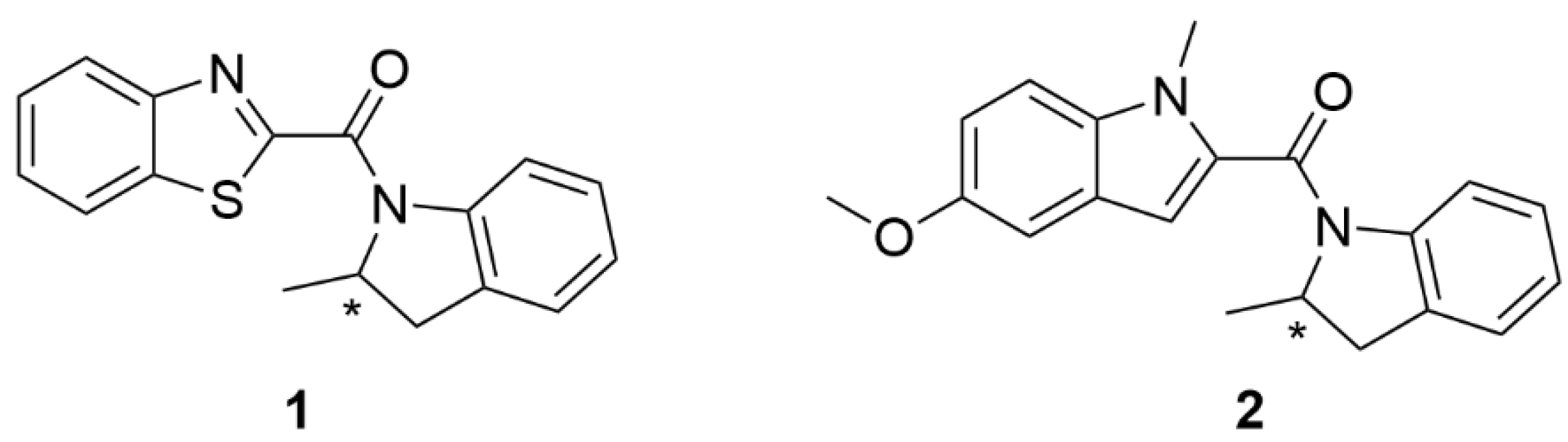
| R1 | (1,3-benzothiazol-2-yl(2-methyl-2,3-dihydro-indol-1-yl)methanone; enantiomer R |
| R1G1 | compound R1 bound to D2LONG dopamine receptor in complex with Gi1 protein |
| R1G2 | compound R1 bound to D2LONG dopamine receptor in complex with Gi2 protein |
| R2 | (4-methoxy-1-methyl-1H-indol-2-yl)(2-methyl-2,3-dihydro-1H-indol-1-yl)methanone; enantiomer R |
| R2G1 | compound R2 bound to D2LONG dopamine receptor in complex with Gi1 protein |
| R2G2 | compound R2 bound to D2LONG dopamine receptor in complex with Gi2 protein |
| S1 | (1,3-benzothiazol-2-yl(2-methyl-2,3-dihydro-indol-1-yl)methanone; enantiomer S |
| S1G1 | compound S1 bound to D2LONG dopamine receptor in complex with Gi1 protein |
| S1G2 | compound S1 bound to D2LONG dopamine receptor in complex with Gi2 protein |
| S2 | (4-methoxy-1-methyl-1H-indol-2-yl)(2-methyl-2,3-dihydro-1H-indol-1-yl)methanone; enantiomer S |
| S2G1 | compound S2 bound to D2LONG dopamine receptor in complex with Gi1 protein |
| S2G2 | compound S2 bound to D2LONG dopamine receptor in complex with Gi2 protein |
References
- Alberts, B.; Bray, D.; Hopkin, K.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Essential Cell Biology, 3rd ed.; Garland Science: New York, NY, USA, 2009. [Google Scholar]
- Tekpli, X.; Holme, J.A.; Sergent, O.; Lagadic-Gossmann, D. Role for Membrane Remodeling in Cell Death: Implication for Health and Disease. Toxicology 2012, 304, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.J.; Asico, L.D.; Jose, P.A.; Tiu, A.C. Lipid Rafts and Dopamine Receptor Signaling. Int. J. Mol. Sci. 2020, 21, 8909. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, L.H. Detergents as Tools for the Purification and Classification of Lipid Rafts. FEBS Lett. 2004, 559, 1–5. [Google Scholar] [CrossRef]
- Day, C.A.; Kenworthy, A.K. Tracking Microdomain Dynamics in Cell Membranes. Biochim. Biophys. Acta 2009, 1788, 245–253. [Google Scholar] [CrossRef]
- Delaunay, J.-L.; Breton, M.; Trugnan, G.; Maurice, M. Differential Solubilization of Inner Plasma Membrane Leaflet Components by Lubrol WX and Triton X-100. Biochim. Biophys. Acta 2008, 1778, 105–112. [Google Scholar] [CrossRef][Green Version]
- Hanzal-Bayer, M.F.; Hancock, J.F. Lipid Rafts and Membrane Traffic. FEBS Lett. 2007, 581, 2098–2104. [Google Scholar] [CrossRef]
- Lopez, C.; Madec, M.-N.; Jiménez-Flores, R. Lipid Rafts in the Bovine Milk Fat Globule Membrane Revealed by the Lateral Segregation of Phospholipids and Heterogeneous Distribution of Glycoproteins. Food Chem. 2010, 120, 22–33. [Google Scholar] [CrossRef]
- Mañes, S.; Martínez-A, C. Cholesterol Domains Regulate the Actin Cytoskeleton at the Leading Edge of Moving Cells. Trends Cell Biol. 2004, 14, 275–278. [Google Scholar] [CrossRef]
- Surma, M.A.; Klose, C.; Simons, K. Lipid-Dependent Protein Sorting at the Trans-Golgi Network. Biochim. Biophys. Acta (BBA)–Mol. Cell Biol. Lipids 2012, 1821, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Toomre, D. Lipid Rafts and Signal Transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Insel, P.A.; Head, B.P.; Patel, H.H.; Roth, D.M.; Bundey, R.A.; Swaney, J.S. Compartmentation of G-Protein-Coupled Receptors and Their Signalling Components in Lipid Rafts and Caveolae. Bioch. Soc. Trans. 2005, 33, 1131–1134. [Google Scholar] [CrossRef]
- Scheidt, H.A.; Huster, D. The Interaction of Small Molecules with Phospholipid Membranes Studied by 1H NOESY NMR under Magic-Angle Spinning. Acta Pharmacol. Sin. 2008, 29, 35–49. [Google Scholar] [CrossRef]
- Soubias, O.; Gawrisch, K. The Role of the Lipid Matrix for Structure and Function of the GPCR Rhodopsin. Biochim. Biophys. Acta 2012, 1818, 234–240. [Google Scholar] [CrossRef]
- Bruns, D.; Riedel, D.; Klingauf, J.; Jahn, R. Quantal Release of Serotonin. Neuron 2000, 28, 205–220. [Google Scholar] [CrossRef]
- Żuk, J.; Bartuzi, D.; Silva, A.G.; Pitucha, M.; Koszła, O.; Wróbel, T.M.; Matosiuk, D.; Castro, M.; Kaczor, A.A. Allosteric Modulation of Dopamine D2L Receptor in Complex with Gi1 and Gi2 Proteins: The Effect of Subtle Structural and Stereochemical Ligand Modifications. Pharmacol. Rep. 2022, 1–19. [Google Scholar] [CrossRef]
- Wood, M.; Ates, A.; Andre, V.M.; Michel, A.; Barnaby, R.; Gillard, M. In Vitro and In Vivo Identification of Novel Positive Allosteric Modulators of the Human Dopamine D2 and D3 Receptor. Mol. Pharmacol. 2016, 89, 303–312. [Google Scholar] [CrossRef]
- Ballesteros, J.A.; Weinstein, H. Integrated Methods for the Construction of Three-Dimensional Models and Computational Probing of Structure-Function Relations in G Protein-Coupled Receptors. In Methods in Neurosciences; Sealfon, S.C., Ed.; Receptor Molecular Biology; Academic Press: New York, NY, USA, 1995; Volume 25, pp. 366–428. [Google Scholar]
- Vermeer, L.S.; de Groot, B.L.; Réat, V.; Milon, A.; Czaplicki, J. Acyl Chain Order Parameter Profiles in Phospholipid Bilayers: Computation from Molecular Dynamics Simulations and Comparison with 2H NMR Experiments. Eur. Biophys. J. 2007, 36, 919–931. [Google Scholar] [CrossRef]
- Davis, J.H. The Description of Membrane Lipid Conformation, Order and Dynamics by 2H-NMR. Biochim. Biophys. Acta 1983, 737, 117–171. [Google Scholar] [CrossRef]
- Hodzic, A.; Zoumpoulakis, P.; Pabst, G.; Mavromoustakos, T.; Rappolt, M. Losartan’s Affinity to Fluid Bilayers Modulates Lipid–Cholesterol Interactions. Phys. Chem. Chem. Phys. 2012, 14, 4780–4788. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.A.; Cherezov, V.; Roth, C.B.; Griffith, M.T.; Jaakola, V.-P.; Chien, E.Y.T.; Velasquez, J.; Kuhn, P.; Stevens, R.C. A Specific Cholesterol Binding Site Is Established by the 2.8 Å Structure of the Human Β2-Adrenergic Receptor in an Alternate Crystal Form. Structure 2008, 16, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Gimpl, G. Interaction of G Protein Coupled Receptors and Cholesterol. Chem. Phys. Lipids 2016, 199, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Taghon, G.J.; Rowe, J.B.; Kapolka, N.J.; Isom, D.G. Predictable Cholesterol Binding Sites in GPCRs Lack Consensus Motifs. Structure 2021, 29, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, P.; Chattopadhyay, A. Cholesterol Interaction Motifs in G Protein-Coupled Receptors: Slippery Hot Spots? Wiley Interdiscip. Rev. Syst. Biol. Med. 2020, 12, e1481. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Schultz, G.; Gudermann, T. Structural Basis of G Protein-Coupled Receptor Function. Mol. Cell. Endocrinol. 1999, 151, 181–193. [Google Scholar] [CrossRef]
- Barnett-Norris, J.; Lynch, D.; Reggio, P.H. Lipids, Lipid Rafts and Caveolae: Their Importance for GPCR Signaling and Their Centrality to the Endocannabinoid System. Life Sci. 2005, 77, 1625–1639. [Google Scholar] [CrossRef]
- Oates, J.; Watts, A. Uncovering the Intimate Relationship between Lipids, Cholesterol and GPCR Activation. Curr. Opin. Struct. Biol. 2011, 21, 802–807. [Google Scholar] [CrossRef]
- Gutierrez, M.G.; Deyell, J.; White, K.L.; Ore, L.C.D.; Cherezov, V.; Stevens, R.C.; Malmstadt, N. The Lipid Phase Preference of the Adenosine A2A Receptor Depends on Its Ligand Binding State. Chem. Commun. 2019, 55, 5724–5727. [Google Scholar] [CrossRef]
- Yin, J.; Chen, K.-Y.M.; Clark, M.J.; Hijazi, M.; Kumari, P.; Bai, X.; Sunahara, R.K.; Barth, P.; Rosenbaum, D.M. Structure of a D2 Dopamine Receptor–G-Protein Complex in a Lipid Membrane. Nature 2020, 584, 125–129. [Google Scholar] [CrossRef]
- Żuk, J.; Bartuzi, D.; Matosiuk, D.; Kaczor, A.A. Preferential Coupling of Dopamine D2S and D2L Receptor Isoforms with Gi1 and Gi2 Proteins-In Silico Study. Int. J. Mol. Sci. 2020, 21, 436. [Google Scholar] [CrossRef] [PubMed]
- Manna, M.; Nieminen, T.; Vattulainen, I. Understanding the Role of Lipids in Signaling Through Atomistic and Multiscale Simulations of Cell Membranes. Annu. Rev. Biophys. 2019, 48, 421–439. [Google Scholar] [CrossRef]
- Bruzzese, A.; Gil, C.; Dalton, J.A.R.; Giraldo, J. Structural Insights into Positive and Negative Allosteric Regulation of a G Protein-Coupled Receptor through Protein-Lipid Interactions. Sci. Rep. 2018, 8, 4456. [Google Scholar] [CrossRef] [PubMed]
- Kučerka, N.; Nieh, M.-P.; Katsaras, J. Fluid Phase Lipid Areas and Bilayer Thicknesses of Commonly Used Phosphatidylcholines as a Function of Temperature. Biochim. Biophys. Acta (BBA)-Biomembr. 2011, 1808, 2761–2771. [Google Scholar] [CrossRef]
- Rawicz, W.; Olbrich, K.C.; McIntosh, T.; Needham, D.; Evans, E. Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophys. J. 2000, 79, 328–339. [Google Scholar] [CrossRef]
- Jones, A.J.Y.; Gabriel, F.; Tandale, A.; Nietlispach, D. Structure and Dynamics of GPCRs in Lipid Membranes: Physical Principles and Experimental Approaches. Molecules 2020, 25, 4729. [Google Scholar] [CrossRef]
- Bhunia, A.; Bera, S.; Korshavn, K.; Kar, R.; Lim, M.; Ramamoorthy, A. Biophysical Insights into the Membrane Interaction of the Core Amyloid-Forming Aβ40 Fragment K16-K28 and Its Role in the Pathogenesis of Alzheimer’s Disease. Phys. Chem. Chem. Phys. 2016, 18, 16890–16901. [Google Scholar] [CrossRef]
- Šali, A.; Blundell, T.L. Comparative Protein Modelling by Satisfaction of Spatial Restraints. J. Mol. Biol. 1993, 234, 779–815. [Google Scholar] [CrossRef]
- Ozvoldik, K.; Stockner, T.; Rammner, B.; Krieger, E. Assembly of Biomolecular Gigastructures and Visualization with the Vulkan Graphics API. J. Chem. Inf. Model. 2021, 61, 5293–5303. [Google Scholar] [CrossRef]
- Jo, S.; Kim, T.; Im, W. Automated Builder and Database of Protein/Membrane Complexes for Molecular Dynamics Simulations. PLoS ONE 2007, 2, e880. [Google Scholar] [CrossRef]
- Pike, L.J.; Han, X.; Chung, K.-N.; Gross, R.W. Lipid Rafts Are Enriched in Arachidonic Acid and Plasmenylethanolamine and Their Composition Is Independent of Caveolin-1 Expression: A Quantitative Electrospray Ionization/Mass Spectrometric Analysis. Biochemistry 2002, 41, 2075–2088. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High Performance Molecular Simulations through Multi-Level Parallelism from Laptops to Supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Spartan; Version 10 VI.01; WavefunctionInc: California, CA, USA, 2016.
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef]
- Jämbeck, J.P.M.; Lyubartsev, A.P. Another Piece of the Membrane Puzzle: Extending Slipids Further. J. Chem. Theory Comput. 2013, 9, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and Testing of a General Amber Force Field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- The PyMOL Molecular Graphics System; Version 4.6; Schrödinger, LLC.: New York, NY, USA, 2002.
- Schrödinger Release 2020-2: Maestro; Schrödinger, LLC.: New York, NY, USA, 2020.
- Guixà-González, R.; Rodriguez-Espigares, I.; Ramírez-Anguita, J.M.; Carrió-Gaspar, P.; Martinez-Seara, H.; Giorgino, T.; Selent, J. MEMBPLUGIN: Studying Membrane Complexity in VMD. Bioinformatics 2014, 30, 1478–1480. [Google Scholar] [CrossRef]
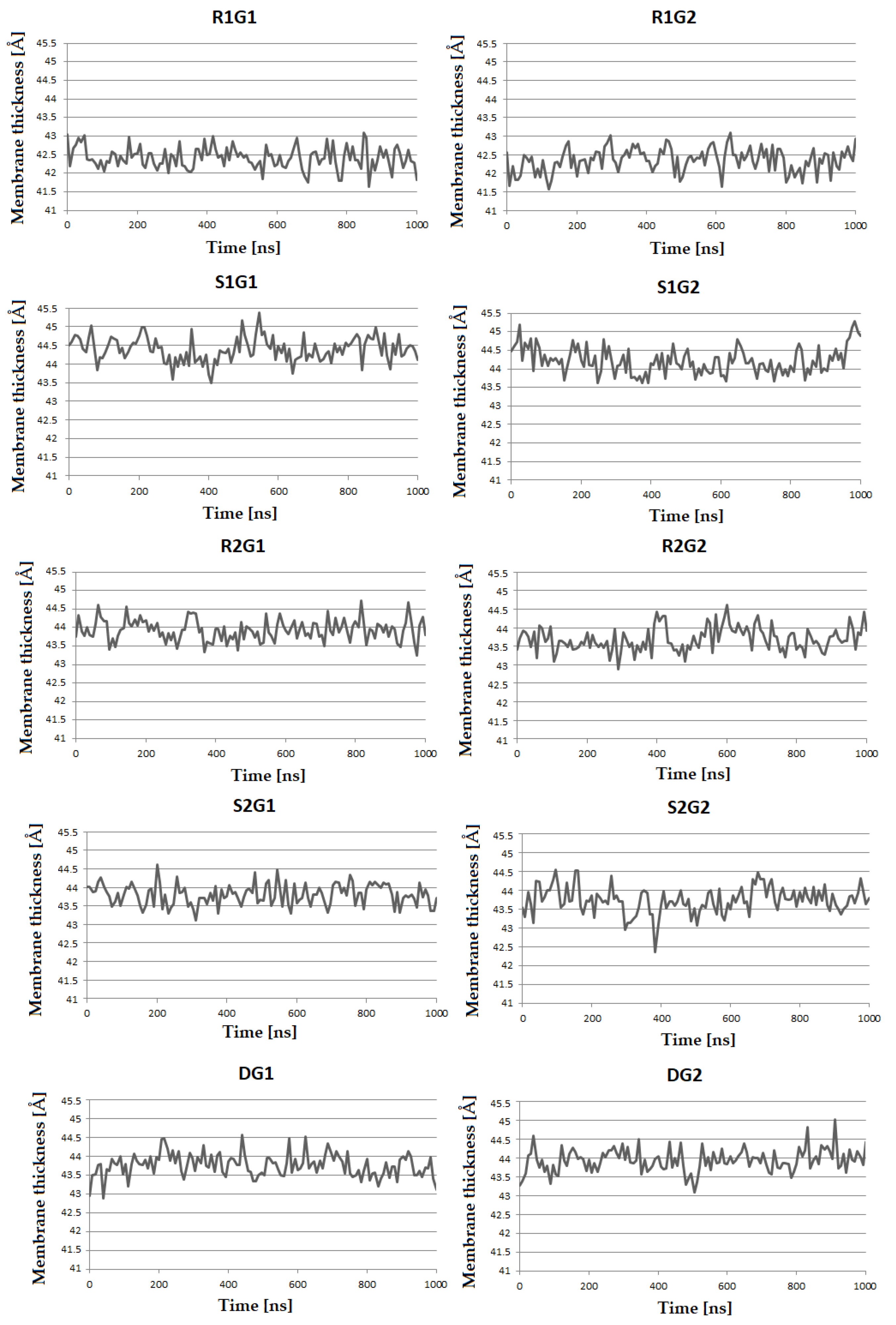
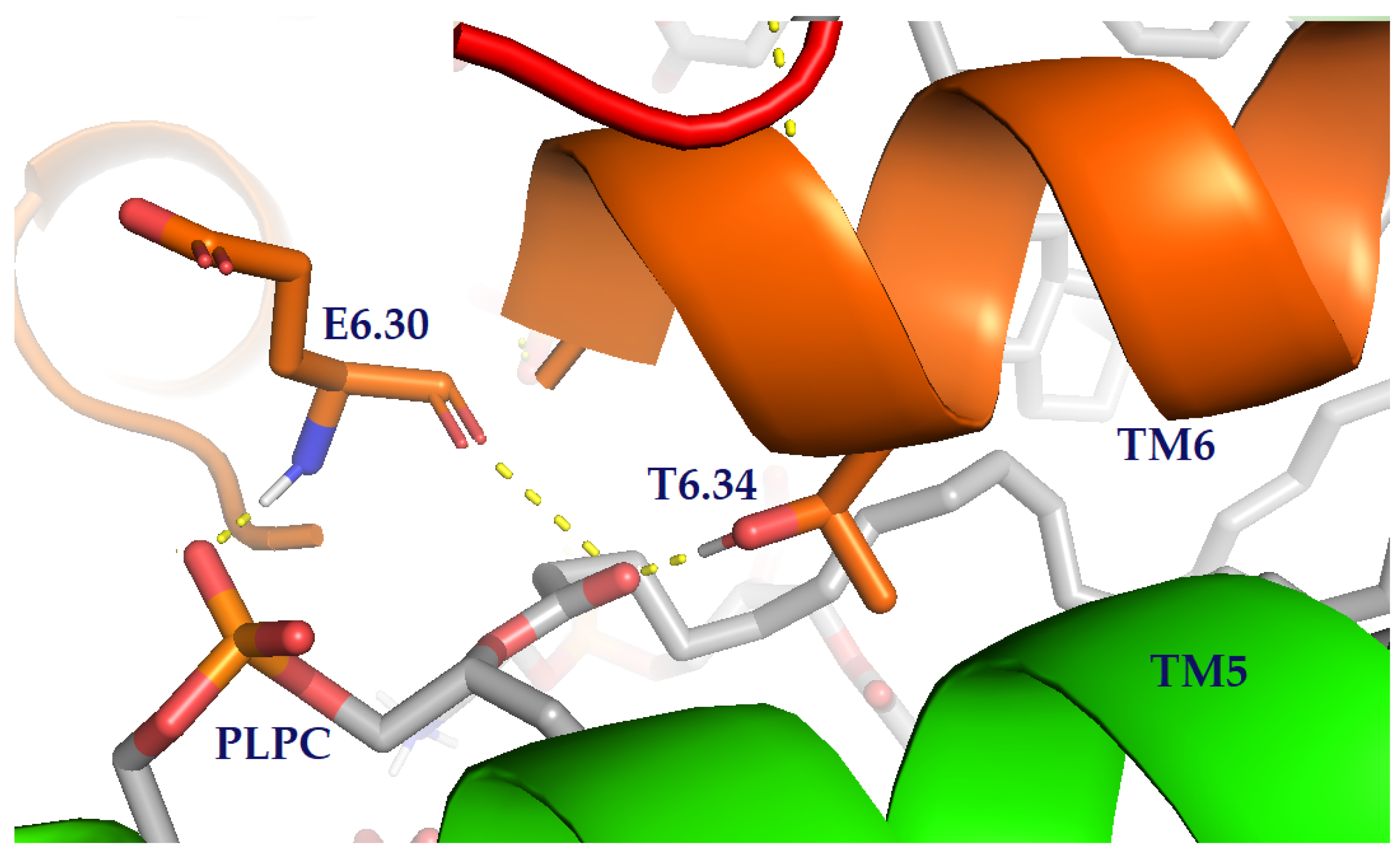
| Area per Lipid (Å2) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CHL | SM | DOPE | DOPC | DOPS | PLPC | POPC | POPE | POPG | Total | |
| R1G1 | 31.02 ± 0.94 | 42.06 ± 1.03 | 62.10 ± 1.34 | 62.32 ± 0.98 | 58.40 ± 1.05 | 67.17 ± 1.10 | 61.18 ± 1.14 | 54.23 ± 1.32 | 63.15 ± 1.19 | 58.46 ± 0.20 |
| R1G2 | 31.50 ± 0.82 | 42.59 ± 1.09 | 62.82 ± 1.09 | 62.42 ± 0.98 | 54.01 ± 0.98 | 66.19 ± 1.11 | 59.33 ± 1.05 | 55.46 ± 1.32 | 56.16 ± 1.23 | 56.89 ± 0.27 |
| S1G1 | 30.91 ± 0.87 | 42.65 ± 1.05 | 53.57 ± 1.11 | 57.60 ± 0.99 | 43.47 ± 1.07 | 63.90 ± 0.94 | 52.14 ± 1.02 | 50.41 ± 1.15 | 53.60 ± 1.25 | 48.55 ± 0.33 |
| S1G2 | 30.60 ± 1.00 | 43.44 ± 1.12 | 52.81 ± 1.09 | 59.14 ± 1.02 | 51.57 ± 0.92 | 63.86 ± 1.06 | 55.23 ± 1.05 | 50.95 ± 1.19 | 54.62 ± 1.21 | 48.40 ± 0.20 |
| R2G1 | 30.97 ± 1.90 | 42.42 ± 1.15 | 57.69 ± 1.43 | 56.57 ± 1.13 | 54.12 ± 0.14 | 63.97 ± 1.26 | 60.95 ± 1.22 | 53.24 ± 1.13 | 54.35 ± 1.40 | 50.89 ± 0.21 |
| R2G2 | 31.11 ± 0.82 | 43.68 ± 1.12 | 52.65 ± 1.09 | 57.78 ± 0.93 | 53.57 ± 1.32 | 62.97 ± 1.01 | 55.23 ± 0.98 | 55.51 ± 0.94 | 54.35 ± 1.24 | 50.13 ± 0.32 |
| S2G1 | 30.89 ± 1.13 | 42.98 ± 1.09 | 57.60 ± 1.20 | 56.97 ± 0.87 | 52.41 ± 1.07 | 63.81 ± 1.21 | 60.83 ± 1.09 | 53.98 ± 1.23 | 53.38 ± 1.19 | 50.47 ± 0.12 |
| S2G2 | 30.23 ± 1.15 | 43.65 ± 1.09 | 53.14 ± 1.02 | 57.24 ± 0.92 | 53.20 ± 1.29 | 62.98 ± 1.10 | 54.99 ± 0.84 | 54.39 ± 1.36 | 55.69 ± 1.12 | 50.33 ± 0.12 |
| DG1 | 31.96 ± 1.02 | 43.06 ± 1.11 | 57.78 ± 1.11 | 57.67 ± 0.99 | 52.36 ± 1.04 | 64.01 ± 1.05 | 61.82 ± 1.11 | 54.54 ± 1.20 | 53.88 ± 1.23 | 50.00 ± 0.14 |
| DG2 | 30.07 ± 1.01 | 43.26 ± 1.02 | 53.70 ± 1.21 | 58.36 ± 0.87 | 53.36 ± 1.01 | 63.07 ± 1.04 | 55.62 ± 0.94 | 54.58 ± 1.21 | 55.58 ± 1.31 | 50.00 ± 0.19 |
| System | R1G1 | R1G2 | S1G1 | S1G2 | R2G1 | R2G2 | S2G1 | S2G2 | DG1 | DG2 |
|---|---|---|---|---|---|---|---|---|---|---|
| St. dev. | 7.4 | 4.3 | 7.3 | 4.0 | 7.4 | 3.9 | 6.5 | 4.1 | 4.6 | 3.8 |
| D2D [m2/s] × 10−11 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| System | R1G1 | R1G2 | S1G1 | S1G2 | R2G1 | R2G2 | S2G1 | S2G2 | DG1 | DG2 |
| Cholesterol | 3.0 | 3.4 | 2.6 | 2.4 | 3.0 | 3.5 | 3.1 | 3.5 | 3.7 | 3.3 |
| DOPC | 1.2 | 2.0 | 0.8 | 3.8 | 2.1 | 2.7 | 1.7 | 3.6 | 3.0 | 3.6 |
| System | R1G1 | R1G2 | S1G1 | S1G2 | R2G1 | R2G2 | S2G1 | S2G2 | DG1 | DG2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Average minimum distance | 0.18 | 0.17 | 0.19 | 0.20 | 0.18 | 0.19 | 0.18 | 0.19 | 0.19 | 0.19 |
| Number of residues in close contact | 169 | 176 | 149 | 151 | 155 | 158 | 155 | 160 | 123 | 134 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żuk, J.; Bartuzi, D.; Miszta, P.; Kaczor, A.A. The Role of Lipids in Allosteric Modulation of Dopamine D2 Receptor—In Silico Study. Molecules 2022, 27, 1335. https://doi.org/10.3390/molecules27041335
Żuk J, Bartuzi D, Miszta P, Kaczor AA. The Role of Lipids in Allosteric Modulation of Dopamine D2 Receptor—In Silico Study. Molecules. 2022; 27(4):1335. https://doi.org/10.3390/molecules27041335
Chicago/Turabian StyleŻuk, Justyna, Damian Bartuzi, Przemysław Miszta, and Agnieszka A. Kaczor. 2022. "The Role of Lipids in Allosteric Modulation of Dopamine D2 Receptor—In Silico Study" Molecules 27, no. 4: 1335. https://doi.org/10.3390/molecules27041335
APA StyleŻuk, J., Bartuzi, D., Miszta, P., & Kaczor, A. A. (2022). The Role of Lipids in Allosteric Modulation of Dopamine D2 Receptor—In Silico Study. Molecules, 27(4), 1335. https://doi.org/10.3390/molecules27041335








