The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening
Abstract
:1. Introduction
2. Results
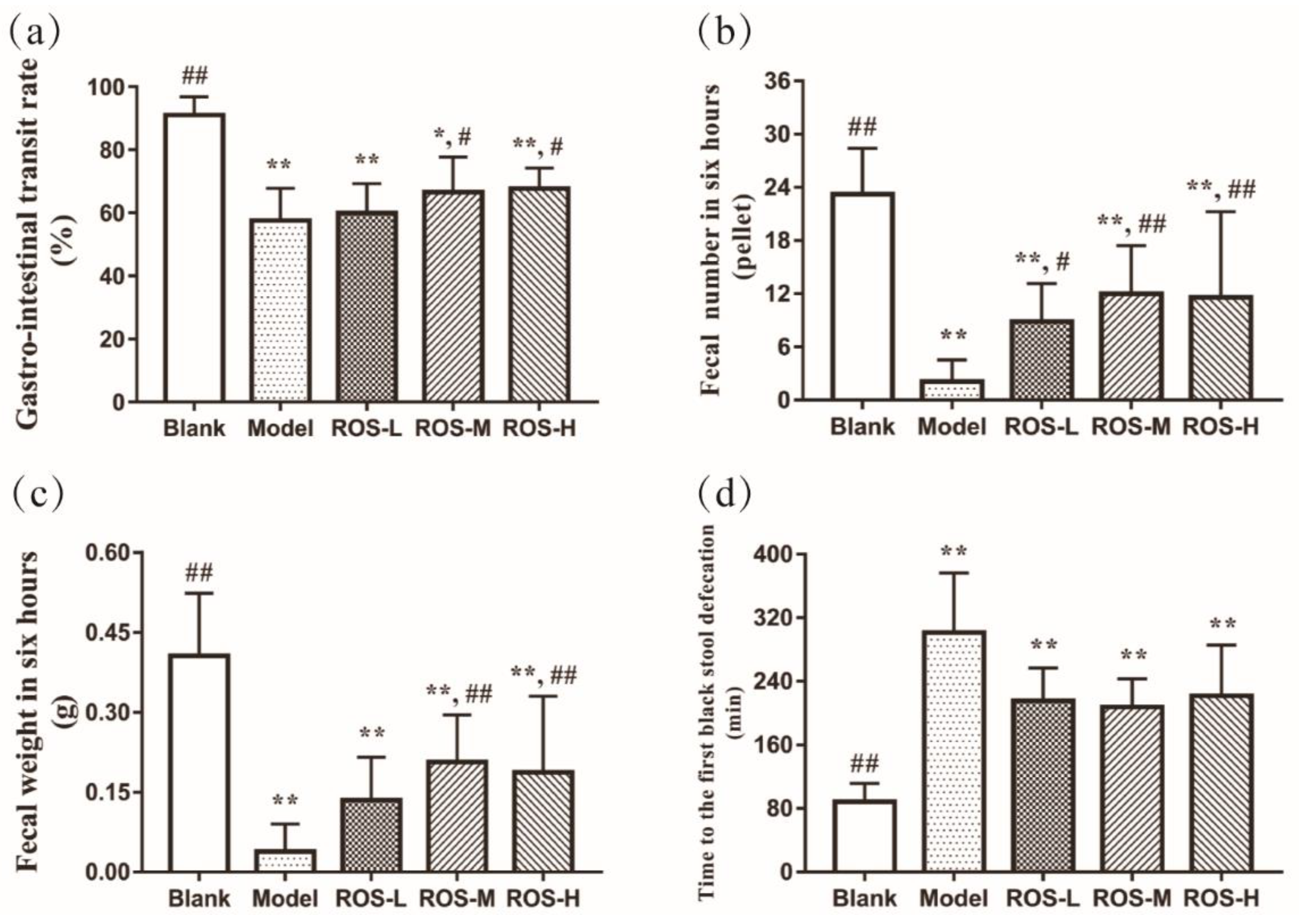
2.1. Effects of ROS on the Gastro-Intestinal Transit Rate and Defecation
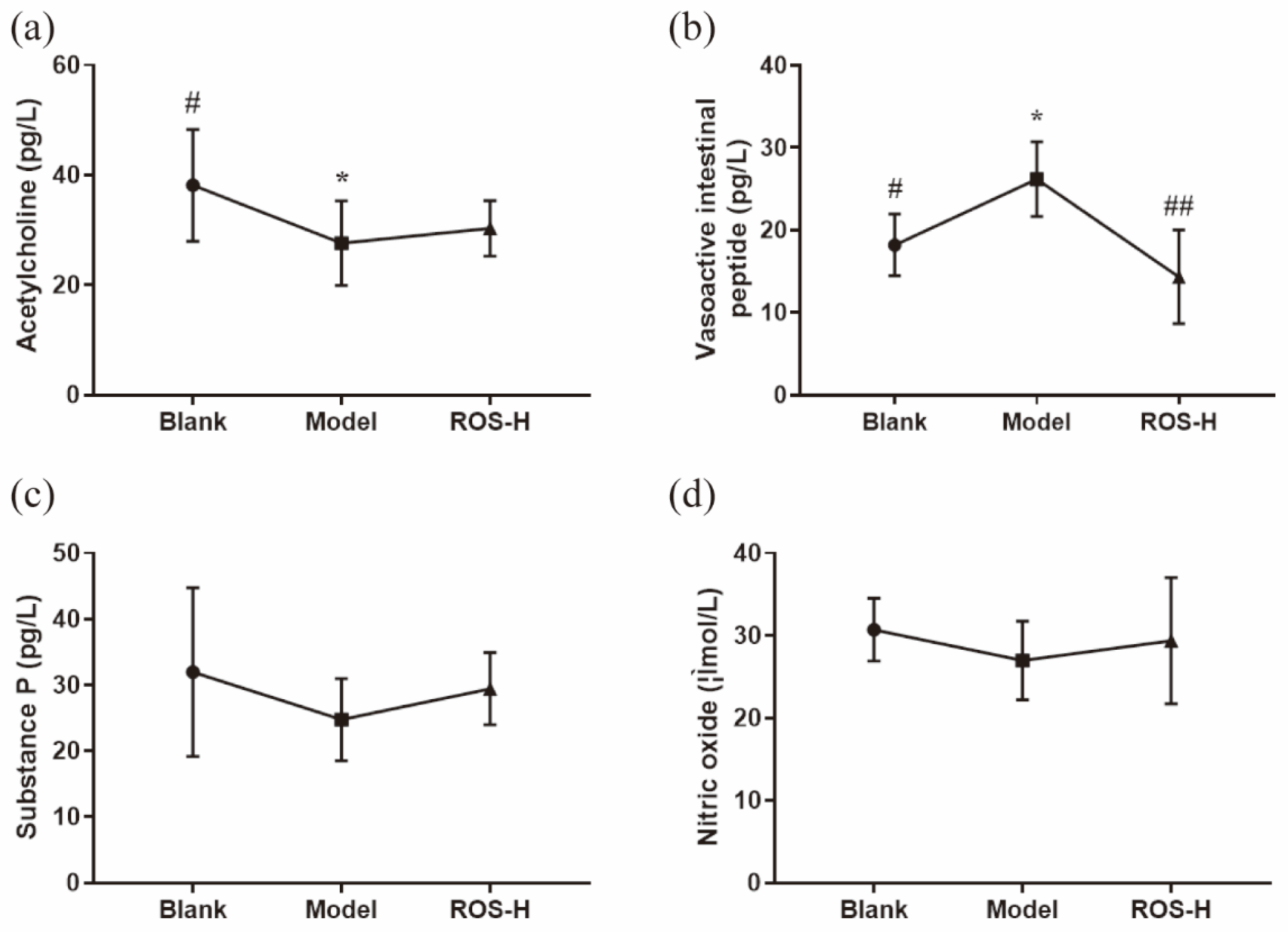
2.2. Effects of ROS on Serum Neurotransmitter
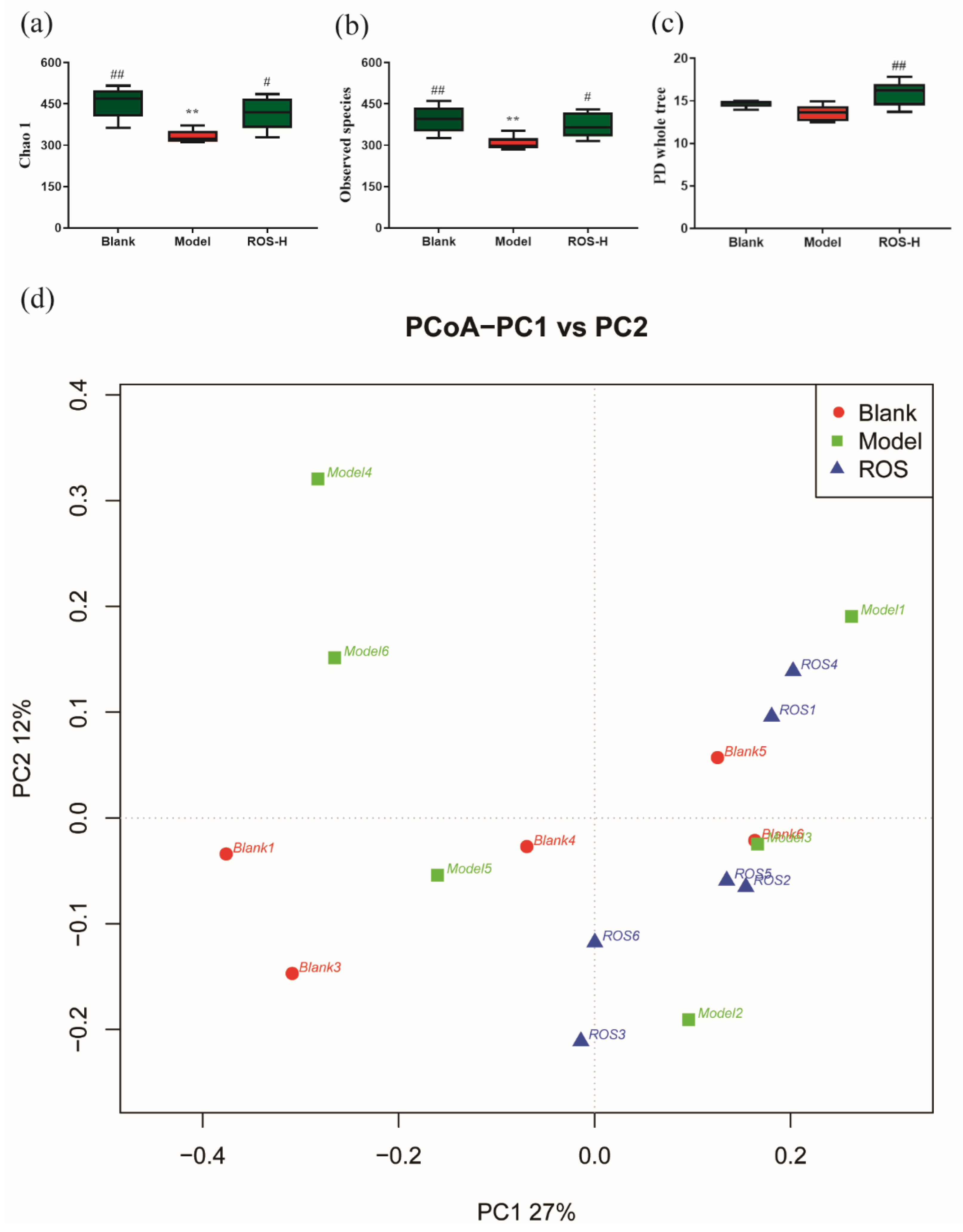
2.3. Effect of ROS on the Diversity of the Rectal Microbiota
2.4. Effect of ROS on the Level of the Rectal Microbiota
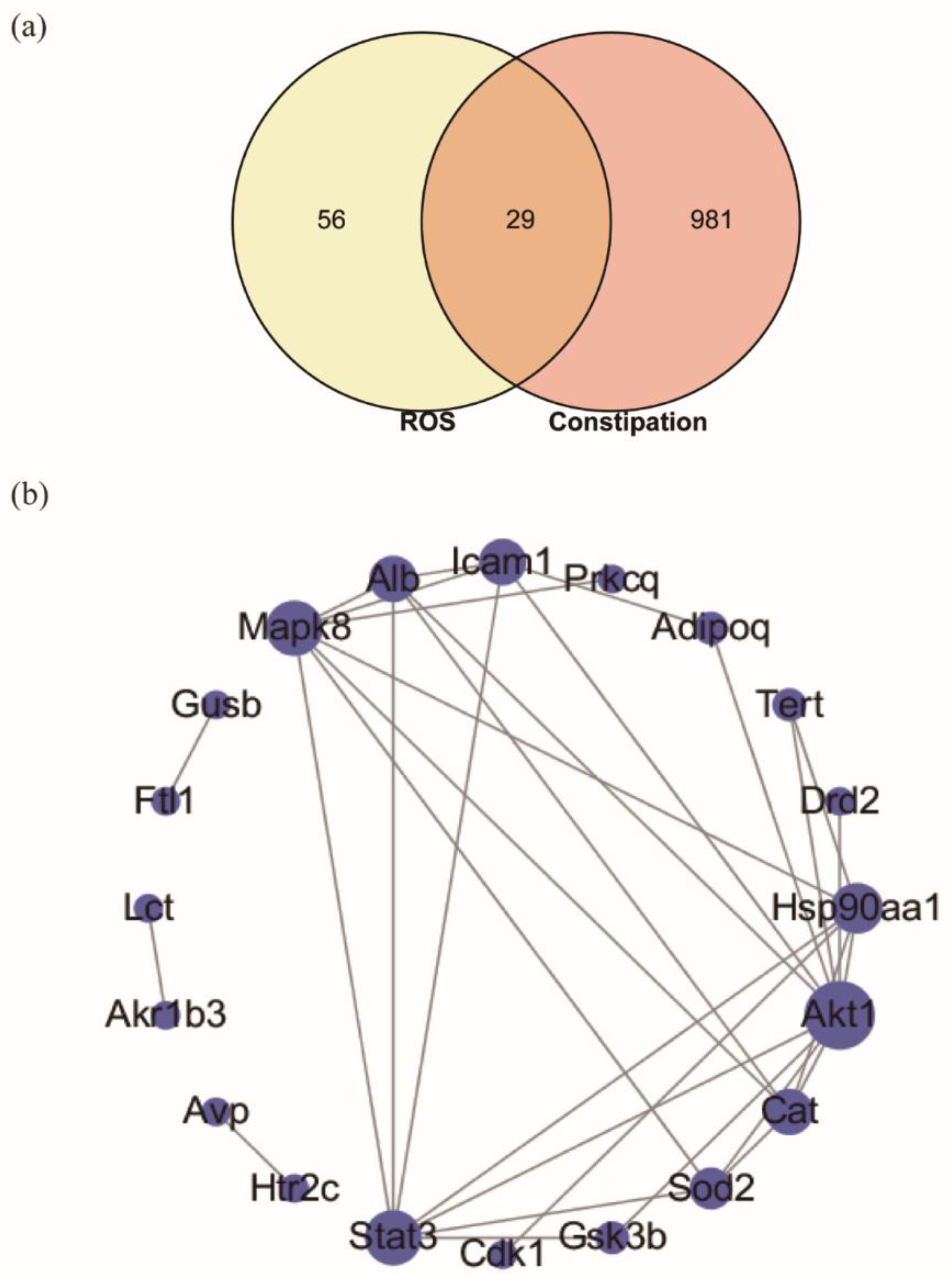
2.5. Targeted Screening and Analysis of Protein–Protein Interaction Network
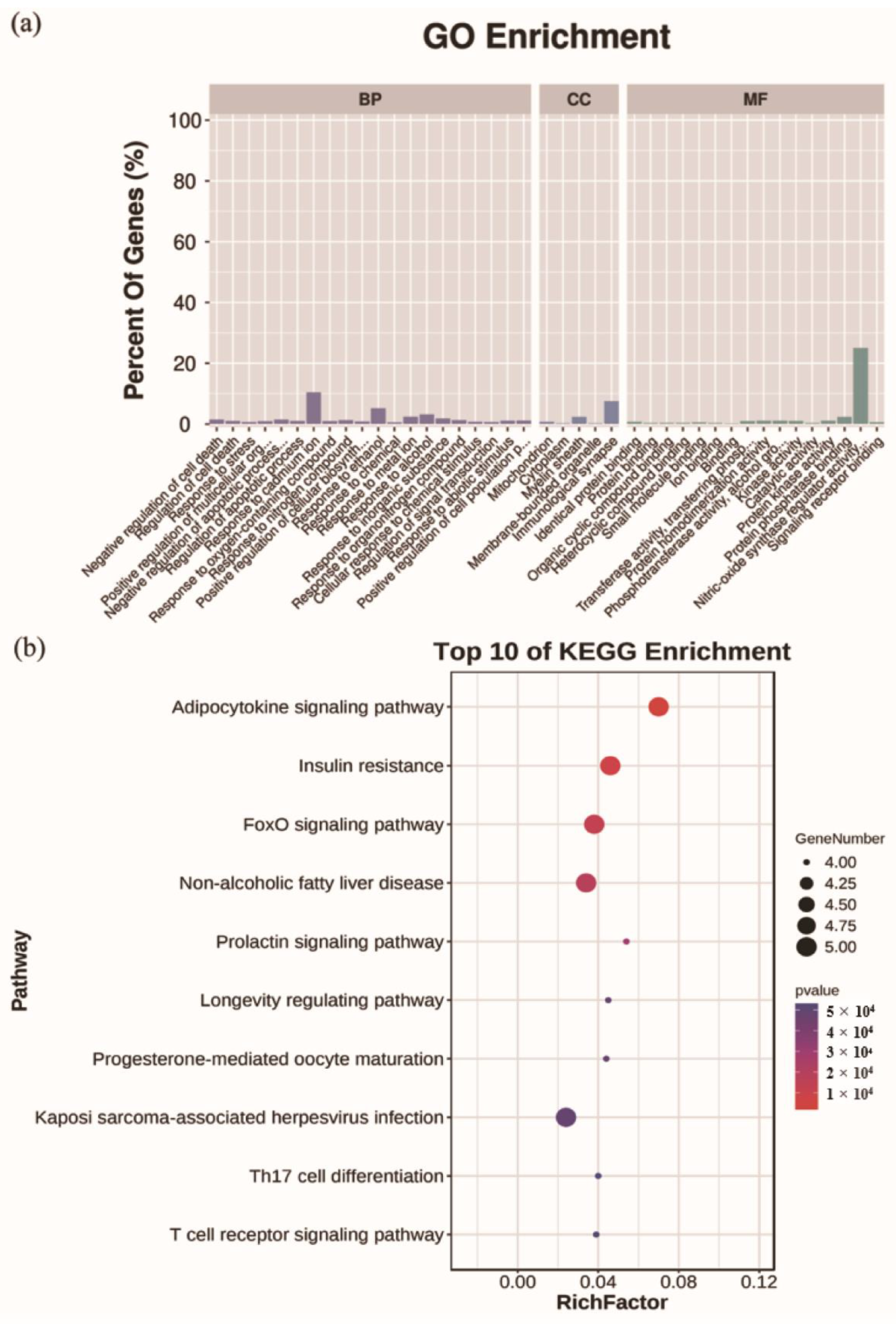
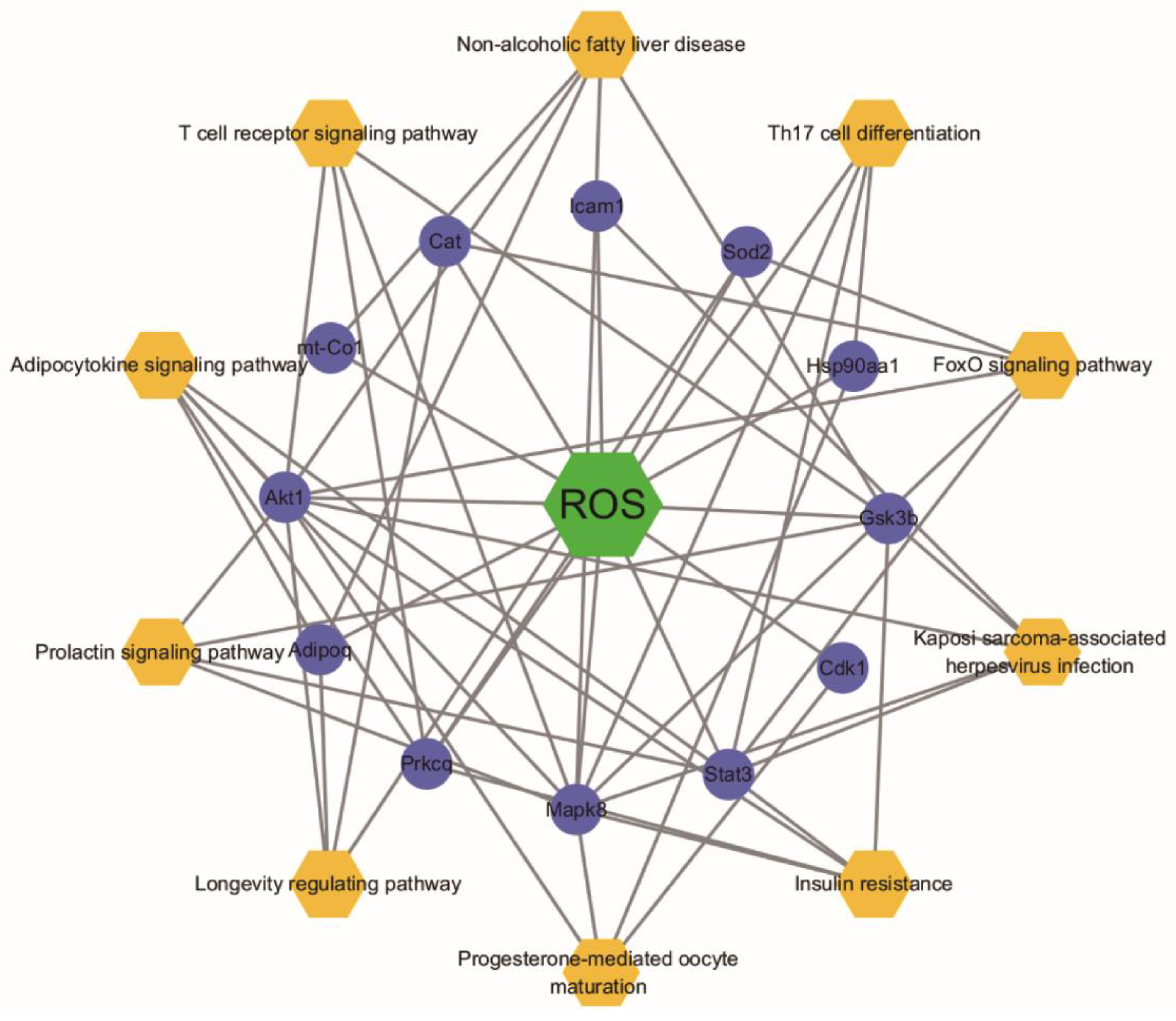
2.6. Analysis for Functional Enrichment and ROS-Target-Pathway Network
2.7. Homology Modeling and Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Arrangement of Animal Experiments
4.2.1. Dosage Regimen
4.2.2. GI Experiment
4.2.3. Defecation Experiment
4.2.4. Arrangement of Slow-Transit Constipation Experiment
4.3. 16S rDNA Sequencing
4.4. Determination of Neurotransmitters in Serum
4.5. Collection of Candidate Target and Construction of the Network
4.6. Enrichment Analysis and Cytoscape Network Construction
4.7. Preparation of Protein Structure
4.8. Molecular Docking
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, L.; Hu, L.; Xu, Q.; Yin, B.; Fang, D.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium adolescentis Exerts Strain-Specific Effects on Constipation Induced by Loperamide in BALB/c Mice. Int. J. Mol. Sci. 2017, 18, 318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, L.; Xu, Q.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct. 2017, 8, 3587–3600. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Q.; Li, D.-W.; Chen, Y.-Y.; Tao, H.-J.; Pu, Z.-J.; Zhang, J.; Tan, Y.-J.; Shi, X.-Q.; Yue, S.-J.; Zhou, G.-S.; et al. Elucidating dosage-effect relationship of different efficacy of rhubarb in constipation model rats by factor analysis. J. Ethnopharmacol. 2019, 238, 111868. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, S.; Wang, Q.; Guan, X.; Qian, L.; Li, J.; Zheng, Y.; Lin, B. Pediococcus pentosaceus B49 from human colostrum ameliorates constipation in mice. Food Funct. 2020, 11, 5607–5620. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, J.W.; Kang, M.J.; Choi, H.J.; Bae, S.J.; Choi, Y.S.; Lee, Y.J.; Lee, H.S.; Hong, J.T.; Hwang, D.Y. Anti-Inflammatory Response and Muscarinic Cholinergic Regulation during the Laxative Effect of in Loperamide-Induced Constipation of SD Rats. Int. J. Mol. Sci. 2019, 20, 946. [Google Scholar] [CrossRef] [Green Version]
- Buddington, R.K.; Kapadia, C.; Neumer, F.; Theis, S. Oligofructose Provides Laxation for Irregularity Associated with Low Fiber Intake. Nutrients 2017, 9, 1372. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Pan, M.; Li, D.; Yin, Y.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Metagenomic insights into the effects of oligosaccharides on the microbial composition of cecal contents in constipated mice. J. Funct. Foods 2017, 38, 486–496. [Google Scholar] [CrossRef]
- Rao, S.S.; Yu, S.; Fedewa, A. Systematic review: Dietary fibre and FODMAP-restricted diet in the management of constipation and irritable bowel syndrome. Aliment. Pharmacol. Ther. 2015, 41, 1256–1270. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Yan, S.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 2017, 8, 1966–1978. [Google Scholar] [CrossRef]
- Mao, B.; Tang, H.; Gu, J.; Li, D.; Cui, S.; Zhao, J.; Zhang, H.; Chen, W. In vitro fermentation of raffinose by the human gut bacteria. Food Funct. 2018, 9, 5824–5831. [Google Scholar] [CrossRef]
- Liu, X.; Chen, S.; Yan, Q.; Li, Y.; Jiang, Z. Effect of Konjac mannan oligosaccharides on diphenoxylate-induced constipation in mice. J. Funct. Foods 2019, 57, 399–407. [Google Scholar] [CrossRef]
- Kwon, J.I.; Park, Y.; Noh, D.O.; Suh, H.J.; Han, S.H. Complex-oligosaccharide composed of galacto-oligosaccharide and lactulose ameliorates loperamide-induced constipation in rats. Food Sci. Biotechnol. 2018, 27, 781–788. [Google Scholar] [CrossRef] [PubMed]
- Dinoto, A.; Marques, T.M.; Sakamoto, K.; Fukiya, S.; Watanabe, J.; Ito, S.; Yokota, A. Population dynamics of Bifidobacterium species in human feces during raffinose administration monitored by fluorescence in situ hybridization-flow cytometry. Appl. Environ. Microbiol. 2006, 72, 7739–7747. [Google Scholar] [CrossRef] [Green Version]
- Andersen, J.M.; Barrangou, R.; Abou Hachem, M.; Lahtinen, S.J.; Goh, Y.J.; Svensson, B.; Klaenhammer, T.R. Transcriptional analysis of oligosaccharide utilization by Bifidobacterium lactis Bl-04. BMC Genom. 2013, 14, 312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimaya, S.; Shimoyama, T.; Fukuda, S.; Matsuzaka, M.; Takahashi, I.; Umeda, T.; Chinda, D.; Saito, D.; Sakamoto, J.; Nagura, T.; et al. The recovery rate at the human terminal ileum of an orally administered non-digestive oligosaccharide (raffinose). Int. J. Food Sci. Nutr. 2009, 60, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Sonoyama, K.; Watanabe, J.; Yamaguchi, N.; Kikuchi, H.; Nagura, T.; Aritsuka, T.; Fukumoto, K.; Kasai, T. Reduction of allergic airway eosinophilia by dietary raffinose in Brown Norway rats. Br. J. Nutr. 2004, 92, 247–255. [Google Scholar] [CrossRef]
- Tateyama, I.; Hashii, K.; Johno, I.; Iino, T.; Hirai, K.; Suwa, Y.; Kiso, Y. Effect of xylooligosaccharide intake on severe constipation in pregnant women. J. Nutr. Sci. Vitaminol. 2005, 51, 445–448. [Google Scholar] [CrossRef] [Green Version]
- Uerlings, J.; Schroyen, M.; Willems, E.; Tanghe, S.; Bruggeman, G.; Bindelle, J.; Everaert, N. Differential effects of inulin or its fermentation metabolites on gut barrier and immune function of porcine intestinal epithelial cells. J. Funct. Foods 2020, 67, 103855. [Google Scholar] [CrossRef]
- Liang, Y.X.; Wen, P.; Wang, Y.; OuYang, D.M.; Wang, D.; Chen, Y.Z.; Song, Y.; Deng, J.; Sun, Y.M.; Wang, H. The Constipation-Relieving Property of d-Tagatose by Modulating the Composition of Gut Microbiota. Int. J. Mol. Sci. 2019, 20, 5721. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Zhu, L.; Chen, J.; Tang, X.; Pan, M.; Yuan, W.; Wang, H. Efficacy of Probiotic Compounds in Relieving Constipation and Their Colonization in Gut Microbiota. Molecules 2022, 27, 666. [Google Scholar] [CrossRef]
- Włodarczyk, J.; Waśniewska, A.; Fichna, J.; Dziki, A.; Dziki, Ł.; Włodarczyk, M. Current Overview on Clinical Management of Chronic Constipation. J. Clin. Med. 2021, 10, 1738. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, E.P.; Burini, R.C. The impact of physical exercise on the gastrointestinal tract. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.G.; Wen, P.; Fu, H.Z.; Lin, G.Y.; Liao, S.T.; Zou, Y.X. Protective effect of mulberry (Morus atropurpurea) fruit against diphenoxylate-induced constipation in mice through the modulation of gut microbiota. Food Funct. 2019, 10, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.Q.; Li, X.P.; Ou-Yang, J.; Jiang, R.G.; Huang, F.F.; Wen, B.B.; Zhang, X.N.; Huang, J.A.; Liu, Z.H. The protective effects of yellow tea extract against loperamide-induced constipation in mice. Food Funct. 2021, 12, 5621–5636. [Google Scholar] [CrossRef]
- Iwasaki, M.; Akiba, Y.; Kaunitz, J.D. Recent advances in vasoactive intestinal peptide physiology and pathophysiology: Focus on the gastrointestinal system. F1000Reseach 2019, 8, F1000 Faculty Rev-1629. [Google Scholar] [CrossRef] [Green Version]
- Han, S.H.; Hong, K.B.; Kim, E.Y.; Ahn, S.H.; Suh, H.J. Effect of dual-type oligosaccharides on constipation in loperamide-treated rats. Nutr. Res. Pract. 2016, 10, 583–589. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Jiao, S.; Wang, Z.A.; Du, Y. Exploring Effects of Chitosan Oligosaccharides on Mice Gut Microbiota in Fermentation and Animal Model. Front. Microbiol. 2018, 9, 2388. [Google Scholar] [CrossRef] [Green Version]
- Soret, R.; Chevalier, J.; De Coppet, P.; Poupeau, G.; Derkinderen, P.; Segain, J.P.; Neunlist, M. Short-Chain Fatty Acids Regulate the Enteric Neurons and Control Gastrointestinal Motility in Rats. Gastroenterology 2010, 138, 1772–1782. [Google Scholar] [CrossRef]
- Zhuang, M.; Shang, W.T.; Ma, Q.C.; Strappe, P.; Zhou, Z.K. Abundance of Probiotics and Butyrate-Production Microbiome Manages Constipation via Short-Chain Fatty Acids Production and Hormones Secretion. Mol. Nutr. Food Res. 2019, 63, 13. [Google Scholar] [CrossRef]
- Huang, L.S.; Kong, C.; Gao, R.Y.; Yan, X.B.; Yu, H.J.; Wen, B.; Zhu, Q.; Shen, T.Y.; Sun, Z.L.; Qin, H.L. Analysis of fecal microbiota in patients with functional constipation undergoing treatment with synbiotics. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 555–563. [Google Scholar] [CrossRef]
- Chai, M.; Wang, L.; Li, X.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Different Bifidobacterium bifidum strains change the intestinal flora composition of mice via different mechanisms to alleviate loperamide-induced constipation. Food Funct. 2021, 12, 6058–6069. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, C.; Cui, S.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Adhesive Bifidobacterium Induced Changes in Cecal Microbiome Alleviated Constipation in Mice. Front. Microbiol. 2019, 10, 1721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, G.; Yang, S.; Sun, S.; Si, Q.; Wang, L.; Zhang, Q.; Wu, G.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus rhamnosus Strains Relieve Loperamide-Induced Constipation via Different Pathways Independent of Short-Chain Fatty Acids. Front. Cell Infect. Microbiol. 2020, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, A.L. Network pharmacology: The next paradigm in drug discovery. Nat. Chem. Biol. 2008, 4, 682–690. [Google Scholar] [CrossRef]
- Hopkins, A.L. Network pharmacology. Nat. Biotechnol. 2007, 25, 1110–1111. [Google Scholar] [CrossRef]
- Raposa, B.; Pónusz, R.; Gerencsér, G.; Budán, F.; Gyöngyi, Z.; Tibold, A.; Hegyi, D.; Kiss, I.; Koller, Á.; Varjas, T.J.P.I. Food additives: Sodium benzoate, potassium sorbate, azorubine, and tartrazine modify the expression of NFκB, GADD45α, and MAPK8 genes. Physiol. Int. 2016, 103, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Zhao, S.; Chen, Q.; Kang, X.; Kong, B.; Wang, Z. Aberrantly Expressed Genes and miRNAs in Slow Transit Constipation Based on RNA-Seq Analysis. BioMed Res. Int. 2018, 2018, 2617432. [Google Scholar] [CrossRef] [Green Version]
- Linan-Rico, A.; Turco, F.; Ochoa-Cortes, F.; Harzman, A.; Needleman, B.J.; Arsenescu, R.; Abdel-Rasoul, M.; Fadda, P.; Grants, I.; Whitaker, E.; et al. Molecular Signaling and Dysfunction of the Human Reactive Enteric Glial Cell Phenotype: Implications for GI Infection, IBD, POI, Neurological, Motility, and GI Disorders. Inflamm. Bowel. Dis. 2016, 22, 1812–1834. [Google Scholar] [CrossRef]
- Takiishi, T.; Fenero, C.I.M.; Câmara, N.O.S. Intestinal barrier and gut microbiota: Shaping our immune responses throughout life. Tissue Barriers 2017, 5, e1373208. [Google Scholar] [CrossRef]
- Ulluwishewa, D.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Wells, J.M.; Roy, N.C. Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 2011, 141, 769–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdu, E.F.; Bercik, P.; Collins, S.M. Effect of probiotics on gastrointestinal function: Evidence from animal models. Ther. Adv. Gastroenterol. 2009, 2, 31–35. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Yi, R.; Qian, Y.; Park, K.Y. Lactobacillus plantarum YS-3 Prevents Activated Carbon-Induced Constipation in Mice. J. Med. Food 2018, 21, 575–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.J.; Tang, X.D.; Yu, J.; Zhang, H.Y.; Li, X.R. Gut microbiota alterations from different Lactobacillus probiotic-fermented yoghurt treatments in slow-transit constipation. J. Funct. Foods 2017, 38, 110–118. [Google Scholar] [CrossRef]
- Zhu, F.; Xu, S.; Zhang, Y.; Chen, F.; Ji, J.; Xie, G. Total Glucosides of Paeony Promote Intestinal Motility in Slow Transit Constipation Rats through Amelioration of Interstitial Cells of Cajal. PLoS ONE 2016, 11, e0160398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef] [Green Version]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Zhang, F.; Yang, K.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Hu, H.; Gao, K.; Wang, W.; et al. SymMap: An integrative database of traditional Chinese medicine enhanced by symptom mapping. Nucleic Acids Res. 2019, 47, D1110–D1117. [Google Scholar] [CrossRef] [Green Version]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards Suite: From Gene Data Mining to Disease Genome Sequence Analyses. Curr. Protoc. Bioinform. 2016, 54, 1–30. [Google Scholar] [CrossRef]
- Piñero, J.; Ramírez-Anguita, J.M.; Saüch-Pitarch, J.; Ronzano, F.; Centeno, E.; Sanz, F.; Furlong, L.I. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Res. 2020, 48, D845–D855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Ke, H.; Qiu, Y.; Cai, M.; Qu, J.; Leng, A. Systematically Characterizing Chemical Profile and Potential Mechanisms of Qingre Lidan Decoction Acting on Cholelithiasis by Integrating UHPLC-QTOF-MS and Network Target Analysis. Evid.-Based Complement. Altern. Med. 2019, 2019, 2675287. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [Green Version]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [Green Version]
- Guex, N.; Peitsch, M.C.; Schwede, T. Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 2009, 30, S162–S173. [Google Scholar] [CrossRef]


| Targets | Degree | Affinity (kcal/mol) |
|---|---|---|
| Akt1 | 10 | −8.4 |
| Stat3 | 7 | −6.7 |
| Mapk8 | 7 | −7.5 |
| Hsp90aa1 | 6 | −6.4 |
| Cat | 5 | −5.9 |
| Alb | 5 | −6.6 |
| Icam1 | 5 | −5.5 |
| Sod2 | 4 | −5.5 |
| Gsk3b | 2 | −6.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Y.; Wang, Y.; Wen, P.; Chen, Y.; Ouyang, D.; Wang, D.; Zhang, B.; Deng, J.; Chen, Y.; Sun, Y.; et al. The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening. Molecules 2022, 27, 2235. https://doi.org/10.3390/molecules27072235
Liang Y, Wang Y, Wen P, Chen Y, Ouyang D, Wang D, Zhang B, Deng J, Chen Y, Sun Y, et al. The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening. Molecules. 2022; 27(7):2235. https://doi.org/10.3390/molecules27072235
Chicago/Turabian StyleLiang, Yuxuan, Yu Wang, Peng Wen, Yongchun Chen, Dongmei Ouyang, Da Wang, Bin Zhang, Jie Deng, Yanhong Chen, Yuanming Sun, and et al. 2022. "The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening" Molecules 27, no. 7: 2235. https://doi.org/10.3390/molecules27072235
APA StyleLiang, Y., Wang, Y., Wen, P., Chen, Y., Ouyang, D., Wang, D., Zhang, B., Deng, J., Chen, Y., Sun, Y., & Wang, H. (2022). The Anti-Constipation Effects of Raffino-Oligosaccharide on Gut Function in Mice Using Neurotransmitter Analyses, 16S rRNA Sequencing and Targeted Screening. Molecules, 27(7), 2235. https://doi.org/10.3390/molecules27072235





