225Ac-rHDL Nanoparticles: A Potential Agent for Targeted Alpha-Particle Therapy of Tumors Overexpressing SR-BI Proteins
Abstract
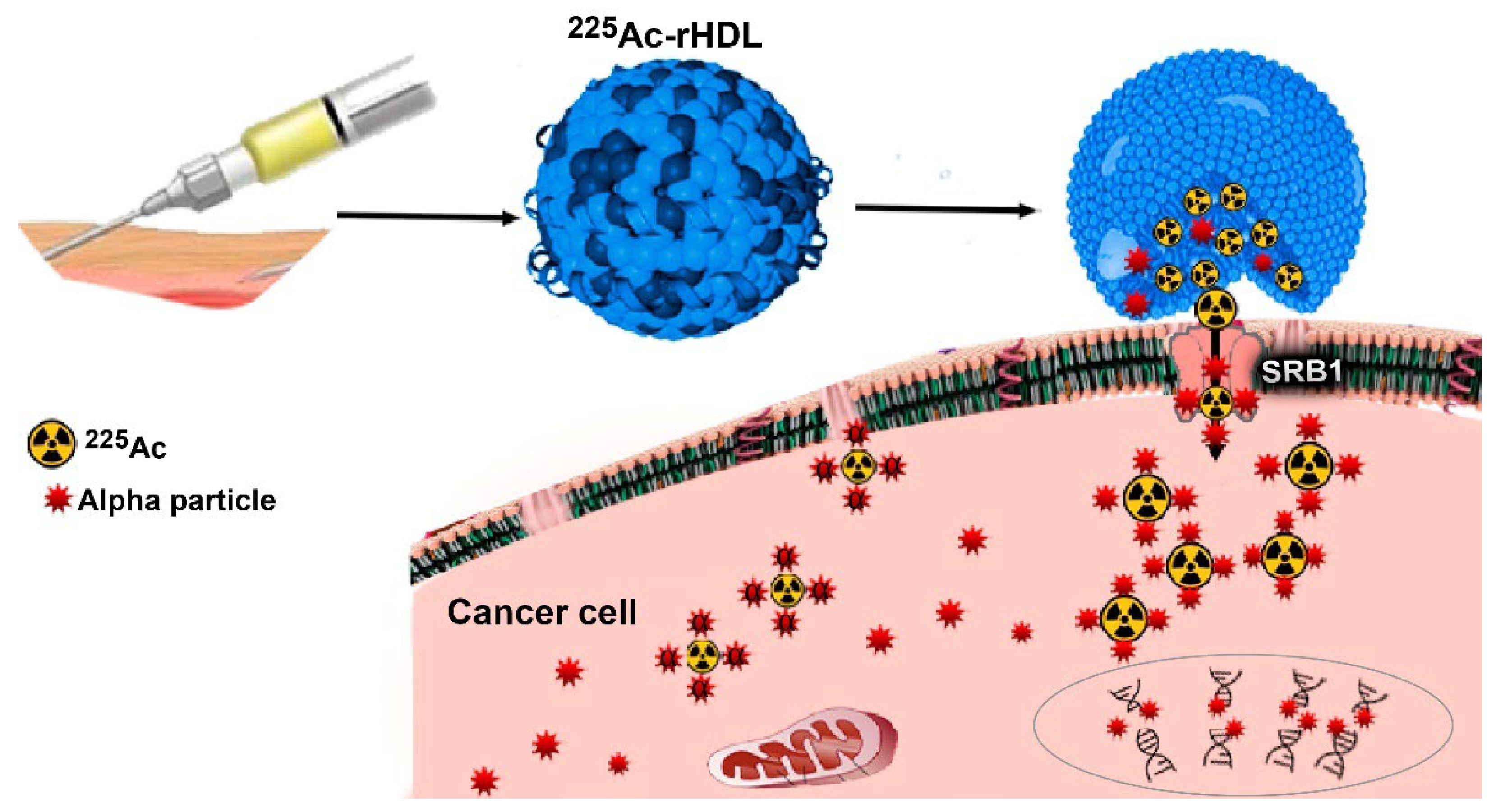
:1. Introduction
2. Results and Discussion
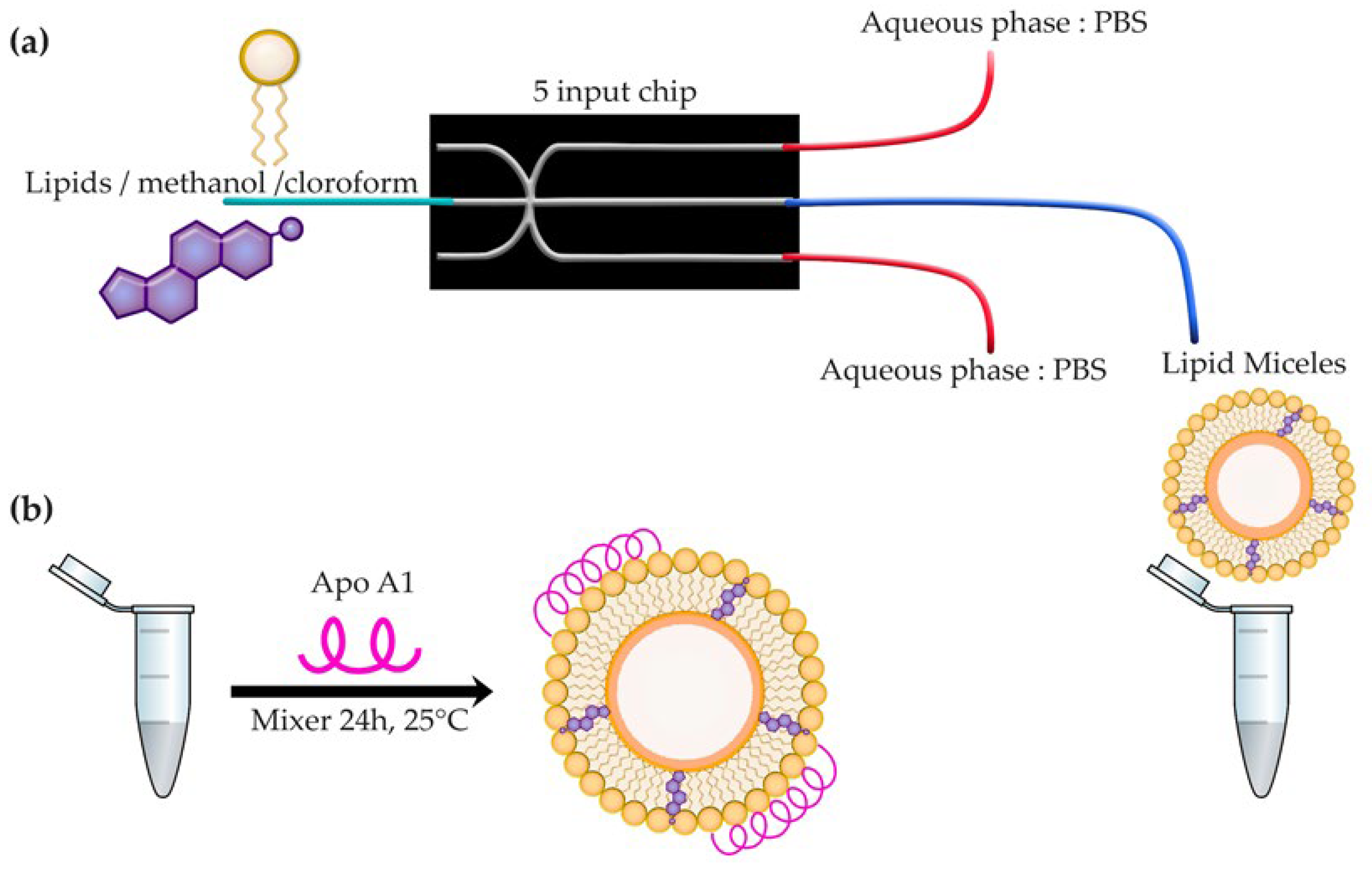
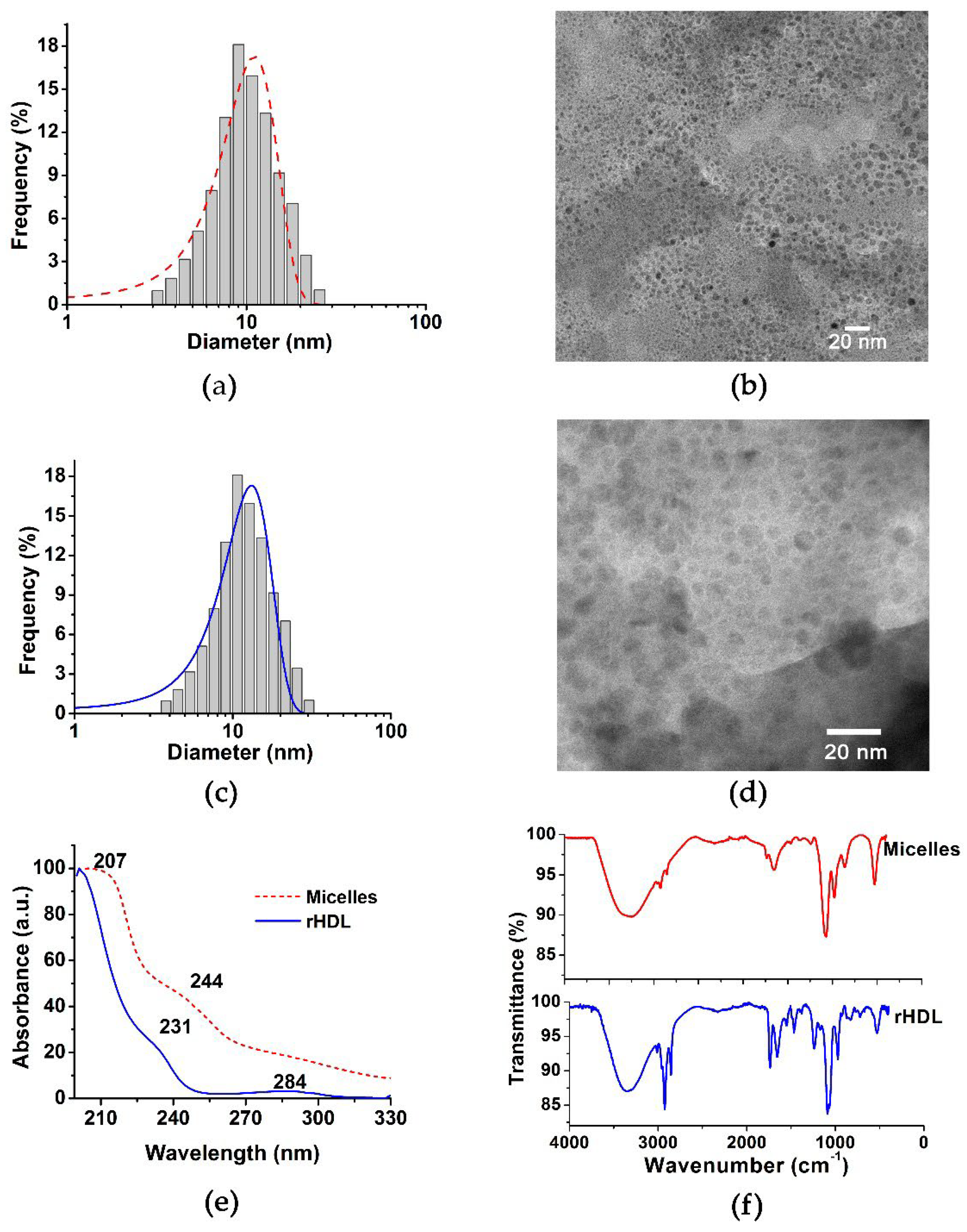
2.1. rHDL Assembly and Chemical Characterization
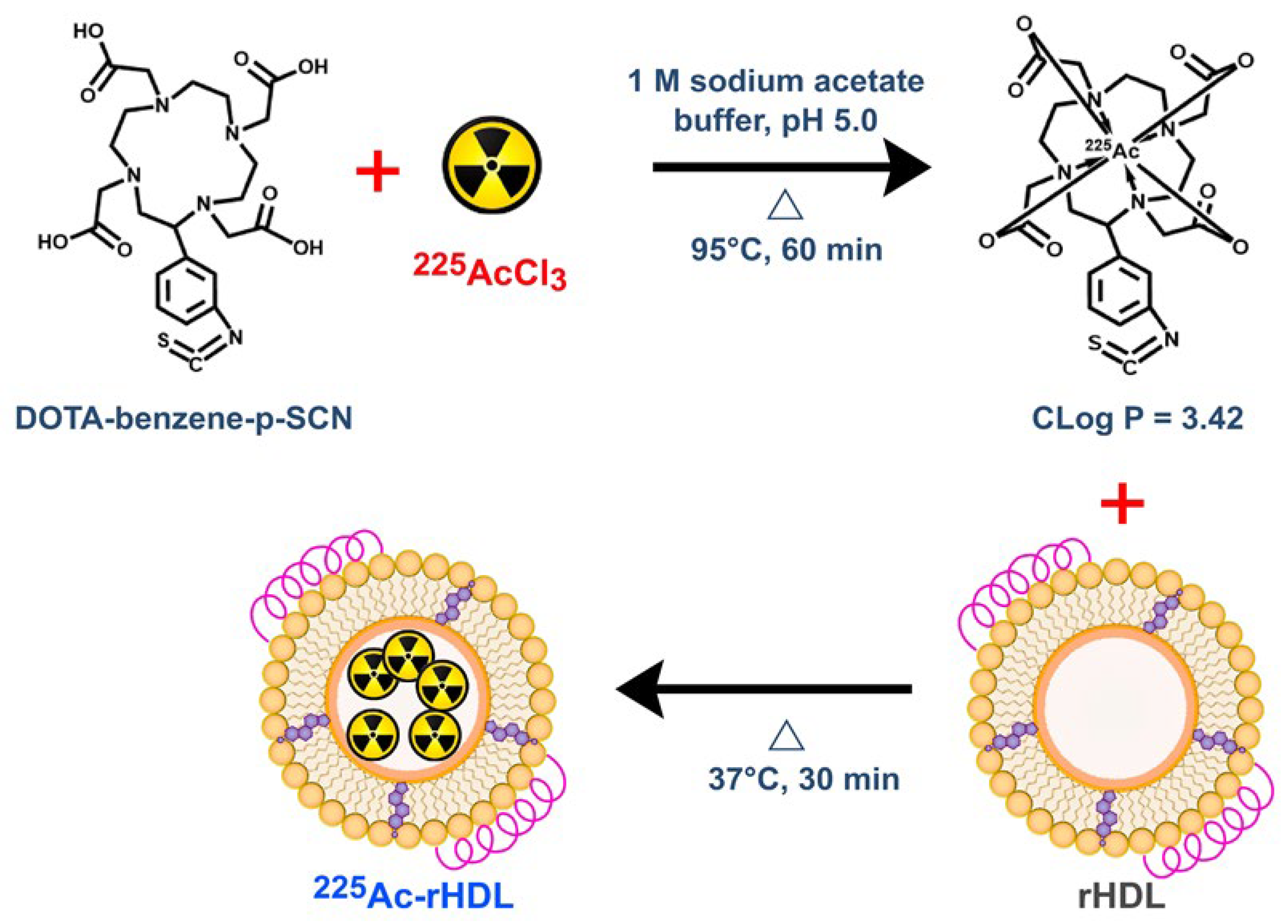
2.2. The 225Ac-rHDL
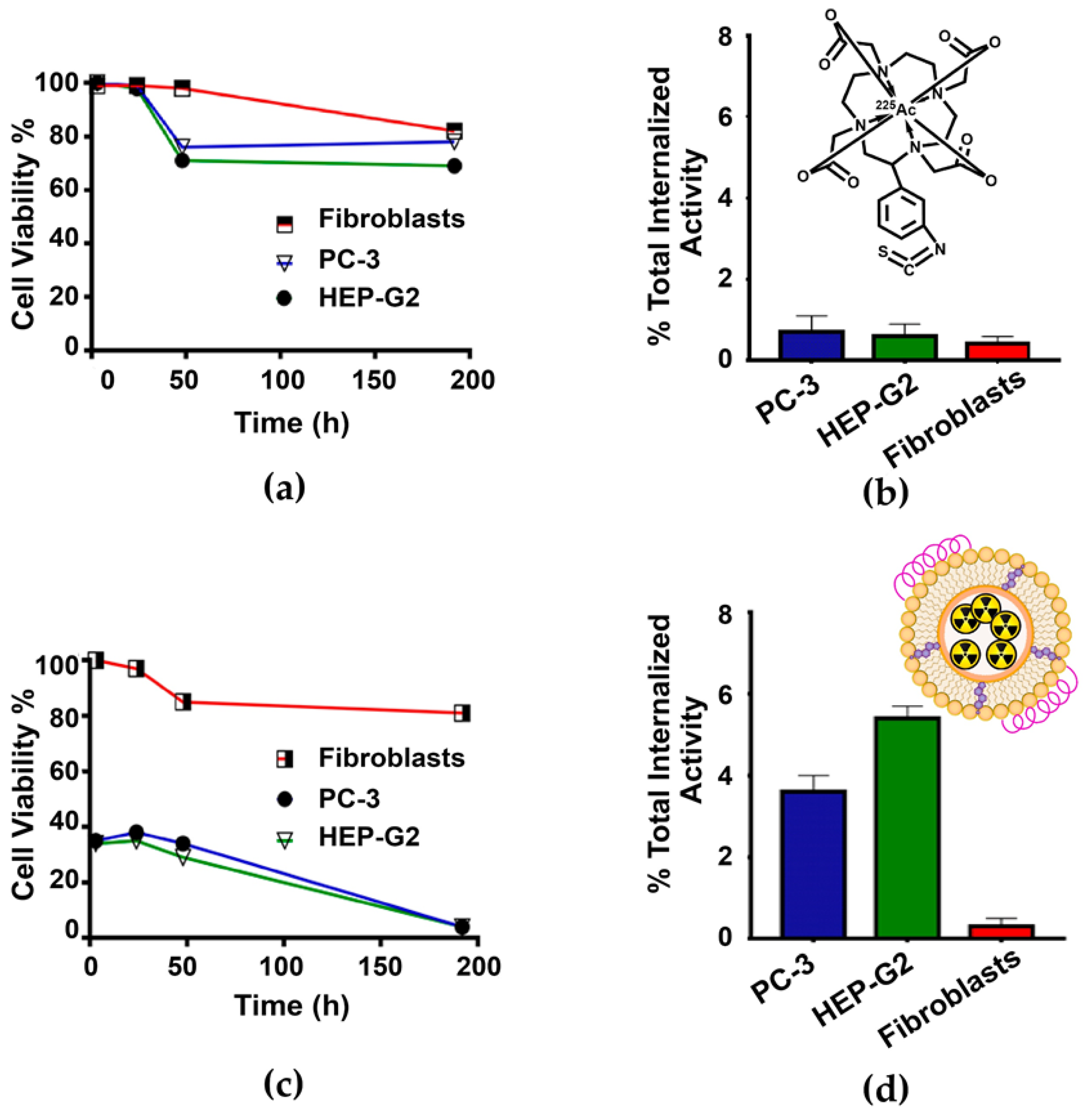
2.3. In Vitro Studies
2.3.1. Serum Stability of the 225Ac-rHDL System
2.3.2. Cell Viability Assay and Dose to the Nucleus
2.4. Biodistribution Assay
3. Materials and Methods
3.1. Preparation of Lipid Micelles and rHDL
3.2. Physicochemical Characterization of rHDL
3.2.1. Nanoparticle Size
3.2.2. Protein Content
3.2.3. UV-Vis Spectra
3.2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
3.3. Preparation of 225Ac-DOTA-Benzene-p-SCN
3.4. Preparation of 225Ac-rHDL
3.5. Serum Stability of the 225Ac-rHDL System
3.6. Cell Internalization
3.7. Cell Viability Assay
3.8. Radiation-Absorbed Dose Calculation
- = radiation-absorbed dose (Gy) to the cell nucleus.
- = branching fraction for daughter k.
- = absorbed dose to the cell nucleus from emission per nuclear transformation (Gy/Bq·s) from decay of daughter radionuclide “k” originated in the cytoplasm.
- = absorbed dose to the cell nucleus from electron (e) and photon (ph) emission per nuclear transformation (Gy/Bq·s) from decay of daughter radionuclide “k” originated in the cytoplasm.
- = total number of nuclear transformations from each daughter radionuclide “k” in the cytoplasm.
3.9. Biodistribution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Simonsen, J.B. Schwendeman, Evaluation of reconstituted high-density lipoprotein (rHDL) as a drug delivery platform—A detailed survey of rHDL particles ranging from biophysical properties to clinical implications. Nanomedicine 2016, 12, 2161–2179. [Google Scholar] [CrossRef]
- Mooberry, L.K.; Sabnis, N.A.; Panchoo, M.; Nagarajan, B.; Lacko, A.G. Targeting the SR-B1 Receptor as a Gateway for Cancer Therapy and Imaging. Front. Pharmacol. 2016, 7, 466. [Google Scholar] [CrossRef]
- Shah, S.; Chib, R.; Raut, S.; Bermudez, J.; Sabnis, N.; Duggal, D.; Kimball, J.; Lacko, A.G.; Gryczynski, Z.; Gryczynski, I. Photophysical Characterization of Anticancer Drug Valrubicin in rHDL Nanoparticles and Its Use as an Imaging Agent. J. Photochem. Photobiol. B Biol. 2016, 155, 60–65. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Medina, C.; Tang, J.; Abdel-Atti, D.; Hogstad, B.; Merad, M.; Fisher, E.A.; Fayad, Z.A.; Lewis, J.; Mulder, W.J.; Reiner, T. Pet Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J. Nucl. Med. 2015, 56, 1272–1277. [Google Scholar] [CrossRef] [Green Version]
- Isaac-Olive, K.; Ocampo-García, B.E.; Aranda-Lara, L.; Santos-Cuevas, C.L.; Jiménez-Mancilla, N.P.; Luna-Gutiérrez, M.A.; Medina, L.A.; Nagarajan, B.; Sabnis, N.; Raut, S.; et al. [99mTc-Hynic-N-Dodecylamide]: A New Hydrophobic Tracer for Labelling Reconstituted High-Density Lipoproteins (Rhdl) for Radioimaging. Nanoscale 2018, 11, 541–551. [Google Scholar] [CrossRef]
- Allen, B. Systemic Targeted Alpha Radiotherapy for Cancer. J. Biomed. Phys. Eng. 2013, 3, 67–80. [Google Scholar] [CrossRef] [Green Version]
- Lacoeuille, F.; Arlicot, N.; Faivre-Chauvet, A. Targeted Alpha and Beta Radiotherapy: An Overview of Radiopharmaceutical and Clinical Aspects. Méd. Nucl. 2018, 42, 32–44. [Google Scholar] [CrossRef]
- Gupta, U.; Singh, V.K.; Kumar, V.; Khajuria, Y. Spectroscopic Studies of Cholesterol: Fourier Transform Infra-Red and Vibrational Frequency Analysis. Mater. Focus 2014, 3, 211–217. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Near-Infrared Spectroscopy in Bio-Applications. Molecules 2020, 25, 2948. [Google Scholar] [CrossRef]
- Peng, Y.; Akmentin, W.; Connelly, M.A.; Lund-Katz, S.; Phillips, M.C.; Williams, D.L. Scavenger Receptor BI (SR-BI) Clustered on Microvillar Extensions Suggests that This Plasma Membrane Domain Is a Way Station for Cholesterol Trafficking between Cells and High-Density Lipoprotein. Mol. Biol. Cell 2004, 15, 384–396. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-Y.; Seideman, J.; Sofou, S. Enhanced Loading Efficiency and Retention of 225Ac in Rigid Liposomes for Potential Targeted Therapy of Micrometastases. Bioconjug. Chem. 2008, 19, 1274–1282. [Google Scholar] [CrossRef]
- McDevitt, M.R.; Ma, D.; Simon, J.; Frank, R.; Scheinberg, D.A. Design and synthesis of 225Ac radioimmunopharmaceuticals. Appl. Radiat. Isot. 2002, 57, 841–847. [Google Scholar] [CrossRef]
- Bandekar, A.; Zhu, C.; Jindal, R.; Bruchertseifer, F.; Morgenstern, A.; Sofou, S. Anti–Prostate-Specific Membrane Antigen Liposomes Loaded with 225Ac for Potential Targeted Antivascular α-Particle Therapy of Cancer. J. Nucl. Med. 2014, 55, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; de Kruijff, R.; Rol, A.; Thijssen, L.; Mendes, E.; Morgenstern, A.; Bruchertseifer, F.; Stuart, M.; Wolterbeek, H.; Denkova, A. Retention Studies of Recoiling Daughter Nuclides of 225Ac in Polymer Vesicles. Appl. Radiat. Isot. 2014, 85, 45–53. [Google Scholar] [CrossRef]
- Biesbroeck, R.; Oram, J.F.; Albers, J.J.; Bierman, E.L. Specific High-Affinity Binding of High-Density Lipoproteins to Cultured Human Skin Fibroblasts and Arterial Smooth Muscle Cells. J. Clin. Investig. 1983, 71, 525–539. [Google Scholar] [CrossRef] [Green Version]
- Sticozzi, C.; Belmonte, G.; Pecorelli, A.; Cervellati, F.; Leoncini, S.; Signorini, C.; Ciccoli, L.; De Felice, C.; Hayek, J.; Valacchi, G. Scavenger Receptor B1 Post-Translational Modifications in Rett Syndrome. FEBS Lett. 2013, 587, 2199–2204. [Google Scholar] [CrossRef] [Green Version]
- Connelly, A.M.; Williams, D.L. Scavenger Receptor B1: A Scavenger Receptor with a Mission to Transport High Density Lipoprotein Lipids. Curr. Opin. Lipidol. 2004, 15, 287–295. [Google Scholar] [CrossRef]
- Tiggemann, M. MIRD Pamphlet No. 25: MIRDcell V2.0 Software Tool for Dosimetric Analysis of Biologic Response of Multicellular Populations. J. Nucl. Med. 2014, 55, 1557–1564. [Google Scholar] [CrossRef]
- Bannik, K.; Madas, B.; Jarzombek, M.; Sutter, A.; Siemeister, G.; Mumberg, D.; Zitzmann-Kolbe, S. Radiobiological Effects of the Alpha Emitter Ra-223 on Tumor Cells. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hartman, T.; Lundqvist, H.; Westlin, J.-E.; Carlsson, J. Radiation Doses to the Cell Nucleus in Single Cells and Cells in Micrometastases in Targeted Therapy with 131I Labeled Ligands or Antibodies. Int. J. Radiat. Oncol. 2000, 46, 1025–1036. [Google Scholar] [CrossRef]
- Kuai, R.; Li, D.; Chen, Y.E.; Moon, J.J.; Schwendeman, A. High-Density Lipoproteins: Nature’s Multifunctional Nanoparticles. ACS Nano 2016, 10, 3015–3041. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Liu, C.; Ren, Y.; Guo, X.; Jiang, J.; Xie, Q.; Xia, L.; Wang, F.; Zhu, H.; Yang, Z. Development of an Albumin-Based PSMA Probe with Prolonged Half-Life. Front. Mol. Biosci. 2020, 7, 585024. [Google Scholar] [CrossRef]
- Beyer, G.J.; Bergmann, R.; Schomäcker, K.; Rösch, F.; Schäfer, G.; Kulikov, E.V.; Novgorodov, A.F. Comparison of the Biodistribution of 225Ac and Radio-Lanthanides as Citrate Complexes. Isot. Isot. Environ. Health Stud. 1990, 26, 111–114. [Google Scholar] [CrossRef]
- Gao, Y.; Grover, P.; Schreckenbach, G. Stabilization of Hydrated AcIII Cation: The Role of Superatom States in Actinium-Water Bonding. Chem. Sci. 2021, 12, 2655–2666. [Google Scholar] [CrossRef]
- Thiele, N.A.; Wilson, J.J. Actinium-225 for Targeted α Therapy: Coordination Chemistry and Current Chelation Approaches. Cancer Biother. Radiopharm. 2018, 33, 336–348. [Google Scholar] [CrossRef]
- Makvandi, M.; Dupis, E.; Engle, J.W.; Nortier, F.M.; Fassbender, M.; Simon, S.; Birnbaum, E.R.; Atcher, R.W.; John, K.D.; Rixe, O.; et al. Alpha-Emitters and Targeted Alpha Therapy in Oncology: From Basic Science to Clinical Investigations. Target. Oncol. 2018, 13, 189–203. [Google Scholar] [CrossRef]
- Miederer, M.; Scheinberg, D.A.; McDevitt, M.R. Realizing the Potential of the Actinium-225 Radionuclide Generator in Targeted Alpha Particle Therapy Applications. Adv. Drug Deliv. Rev. 2008, 60, 1371–1382. [Google Scholar] [CrossRef] [Green Version]
- Tafreshi, N.K.; Doligalski, M.L.; Tichacek, C.J.; Pandya, D.N.; Budzevich, M.M.; El-Haddad, G.; Khushalani, N.I.; Moros, E.G.; McLaughlin, M.L.; Wadas, T.J.; et al. Development of Targeted Alpha Particle Therapy for Solid Tumors. Molecules 2019, 24, 4314. [Google Scholar] [CrossRef] [Green Version]
- Shahzad, M.M.; Mangala, L.S.; Han, H.D.; Lu, C.; Bottsford-Miller, J.; Nishimura, M.; Mora, E.M.; Lee, J.-W.; Stone, R.L.; Pecot, C.V.; et al. Targeted Delivery of Small Interfering RNA Using Reconstituted High-Density Lipoprotein Nanoparticles. Neoplasia 2011, 13, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Peltek, O.O.; Muslimov, A.R.; Zyuzin, M.V.; Timin, A.S. Current Outlook on Radionuclide Delivery Systems: From Design Consideration to Translation into Clinics. J. Nanobiotechnol. 2019, 17, 1–34. [Google Scholar] [CrossRef] [Green Version]
- Beattie, B.J.; Thorek, D.L.J.; Schmidtlein, C.R.; Pentlow, K.S.; Humm, J.L.; Hielscher, A.H. Quantitative Modeling of Cerenkov Light Production Efficiency from Medical Radionuclides. PLoS ONE 2012, 7, e31402. [Google Scholar] [CrossRef] [Green Version]

| Parameter | Lipid Micelles | rHDL |
|---|---|---|
| Particle diameter (nm), DLS | 11.03 ± 0.04 | 13.14 ± 0.20 |
| Particle diameter (nm), TEM | 10.20 ± 0.13 | 11.10 ± 0.17 |
| Polydispersity index | 0.121 | 0.176 |
| Protein concentration (mg/mL) | NA 1 | 0.131 |
| Time (h) | Fibroblast | PC-3 | HEP-G2 |
|---|---|---|---|
| Radiation Dose | Radiation Dose | Radiation Dose | |
| 3 | 0.5 | 14.0 | 20.7 |
| 24 | 3.9 | 107.3 | 161.4 |
| 48 | 8.1 | 208.2 | 312.2 |
| 192 | 23.8 | 682.5 | 1025.5 |
| Internalized Activity (Bq/cell) | 0.0005 | 0.0144 | 0.0216 |
| Tissue | Time (h) | ||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 3 | 24 | 48 | 96 | 192 | |
| Liver | 40.40 ± 2.12 | 31.87 ± 3.19 | 23.68 ± 1.13 | 18.27 ± 2.32 | 11.12 ± 2.21 | 8.42 ± 1.32 | 6.01 ± 1.07 |
| Spleen | 1.10 ± 0.20 | 1.30 ± 0.10 | 1.74 ± 0.24 | 1.61 ± 0.21 | 1.02 ± 0.09 | 0.92 ± 0.15 | 0.74 ± 0.08 |
| Lung | 1.02 ± 0.32 | 0.87 ± 0.21 | 0.54 ± 0.11 | 0.47 ± 0.10 | 0.21 ± 0.06 | 0.11 ± 0.08 | 0.04 ± 0.01 |
| Kidney | 4.85 ± 0.89 | 3.14 ± 0.47 | 2.63 ± 0.23 | 1.98 ± 0.82 | 1.23 ± 0.45 | 0.81 ± 0.28 | 0.32 ± 0.12 |
| Intestine | 2.88 ± 0.12 | 1.77 ± 0.92 | 1.25 ± 0.15 | 0.84 ± 0.31 | 0.81 ± 0.12 | 0.68 ± 0.10 | 0.42 ± 0.06 |
| Heart | 0.50 ± 0.14 | 0.34 ± 0.09 | 0.21 ± 0.05 | 0.17 ± 0.04 | 0.09 ± 0.02 | 0.05 ± 0.02 | 0.01 ± 0.01 |
| Organ | Biokinetic Model | Biological Residence Time (h) | Total Nuclear Transformations | Absorbed Dose (Gy) |
|---|---|---|---|---|
| Tumor | 6772 | 130 | 649.20 | |
| Liver | 0.138 | 0.928 | 2.17 | |
| Kidneys | 0.160 | 0.153 | 2.06 | |
| Spleen | 0.091 | 0.087 | 3.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernández-Jiménez, T.; Ferro-Flores, G.; Morales-Ávila, E.; Isaac-Olivé, K.; Ocampo-García, B.; Aranda-Lara, L.; Santos-Cuevas, C.; Luna-Gutiérrez, M.; De Nardo, L.; Rosato, A.; et al. 225Ac-rHDL Nanoparticles: A Potential Agent for Targeted Alpha-Particle Therapy of Tumors Overexpressing SR-BI Proteins. Molecules 2022, 27, 2156. https://doi.org/10.3390/molecules27072156
Hernández-Jiménez T, Ferro-Flores G, Morales-Ávila E, Isaac-Olivé K, Ocampo-García B, Aranda-Lara L, Santos-Cuevas C, Luna-Gutiérrez M, De Nardo L, Rosato A, et al. 225Ac-rHDL Nanoparticles: A Potential Agent for Targeted Alpha-Particle Therapy of Tumors Overexpressing SR-BI Proteins. Molecules. 2022; 27(7):2156. https://doi.org/10.3390/molecules27072156
Chicago/Turabian StyleHernández-Jiménez, Tania, Guillermina Ferro-Flores, Enrique Morales-Ávila, Keila Isaac-Olivé, Blanca Ocampo-García, Liliana Aranda-Lara, Clara Santos-Cuevas, Myrna Luna-Gutiérrez, Laura De Nardo, Antonio Rosato, and et al. 2022. "225Ac-rHDL Nanoparticles: A Potential Agent for Targeted Alpha-Particle Therapy of Tumors Overexpressing SR-BI Proteins" Molecules 27, no. 7: 2156. https://doi.org/10.3390/molecules27072156
APA StyleHernández-Jiménez, T., Ferro-Flores, G., Morales-Ávila, E., Isaac-Olivé, K., Ocampo-García, B., Aranda-Lara, L., Santos-Cuevas, C., Luna-Gutiérrez, M., De Nardo, L., Rosato, A., & Meléndez-Alafort, L. (2022). 225Ac-rHDL Nanoparticles: A Potential Agent for Targeted Alpha-Particle Therapy of Tumors Overexpressing SR-BI Proteins. Molecules, 27(7), 2156. https://doi.org/10.3390/molecules27072156










