Yields of Photo-Proton Reactions on Nuclei of Nickel and Separation of Cobalt Isotopes from Irradiated Targets
Abstract
1. Introduction
2. Results and Discussion
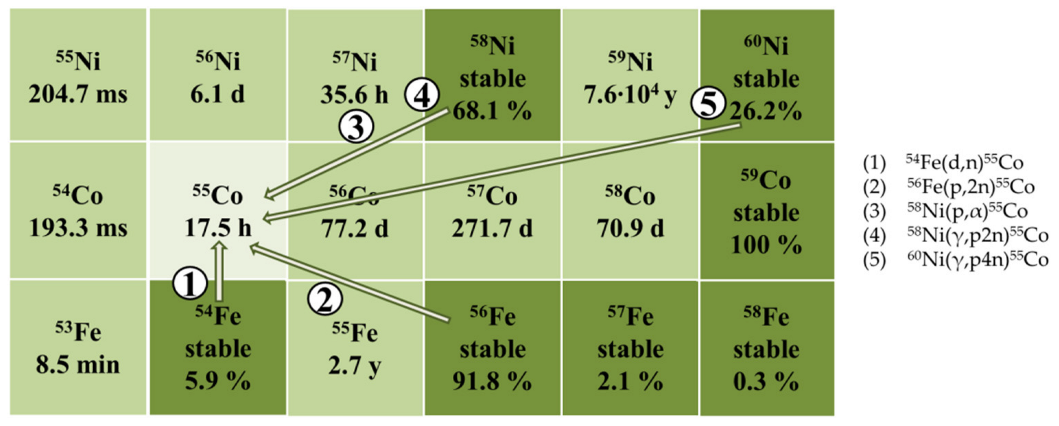
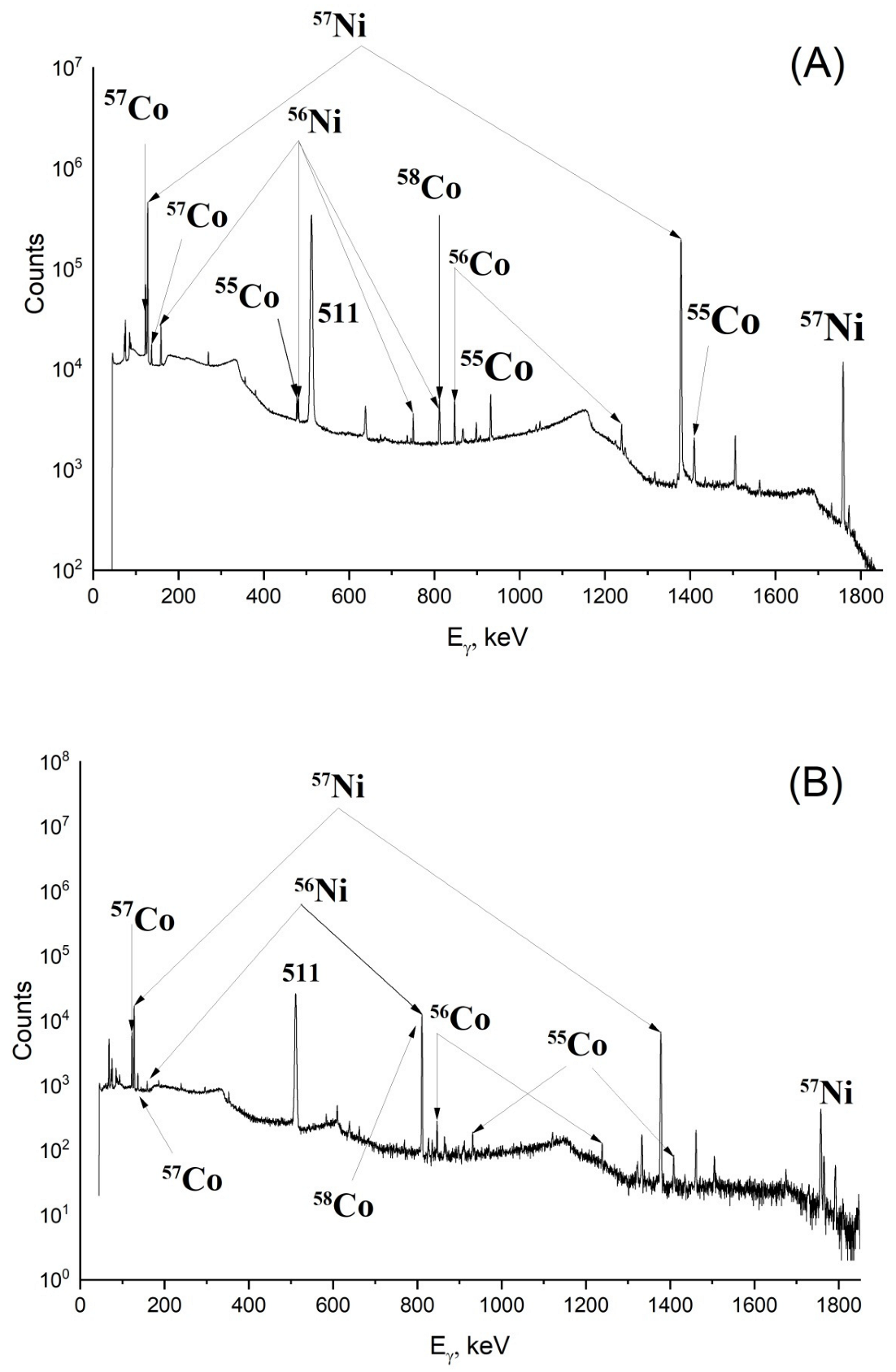
2.1. Radionuclide Composition of Irradiated Targets and Yields of Nuclear Reactions
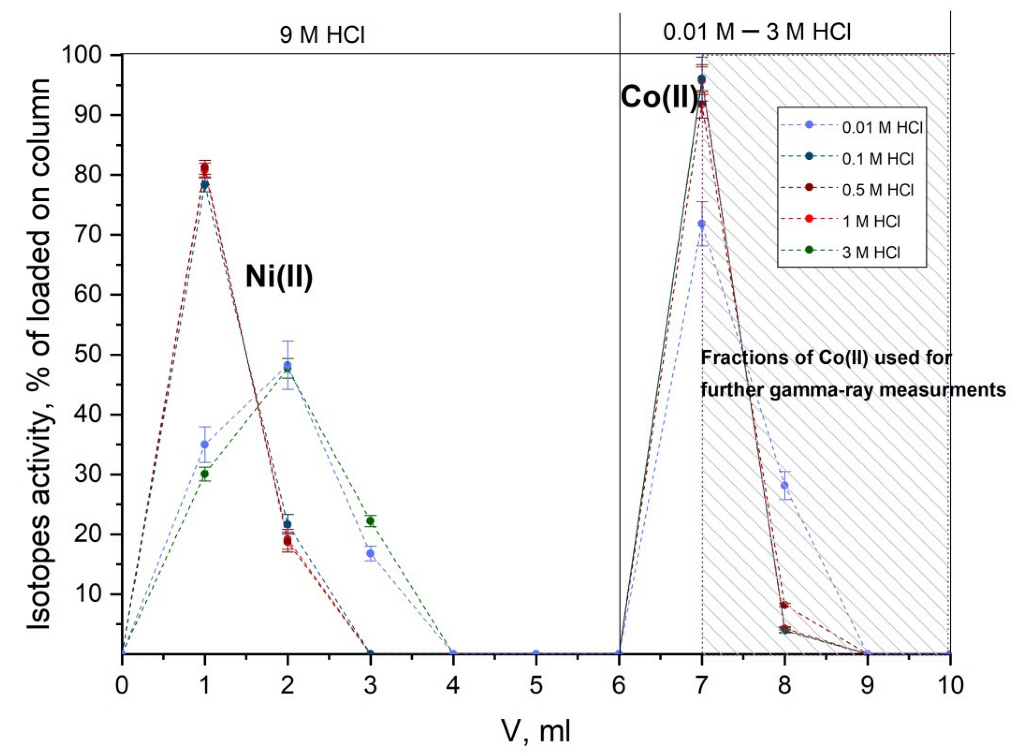
2.2. Separation of Co(II) Isotopes without a Carrier Using Extraction Chromatography
3. Materials and Methods
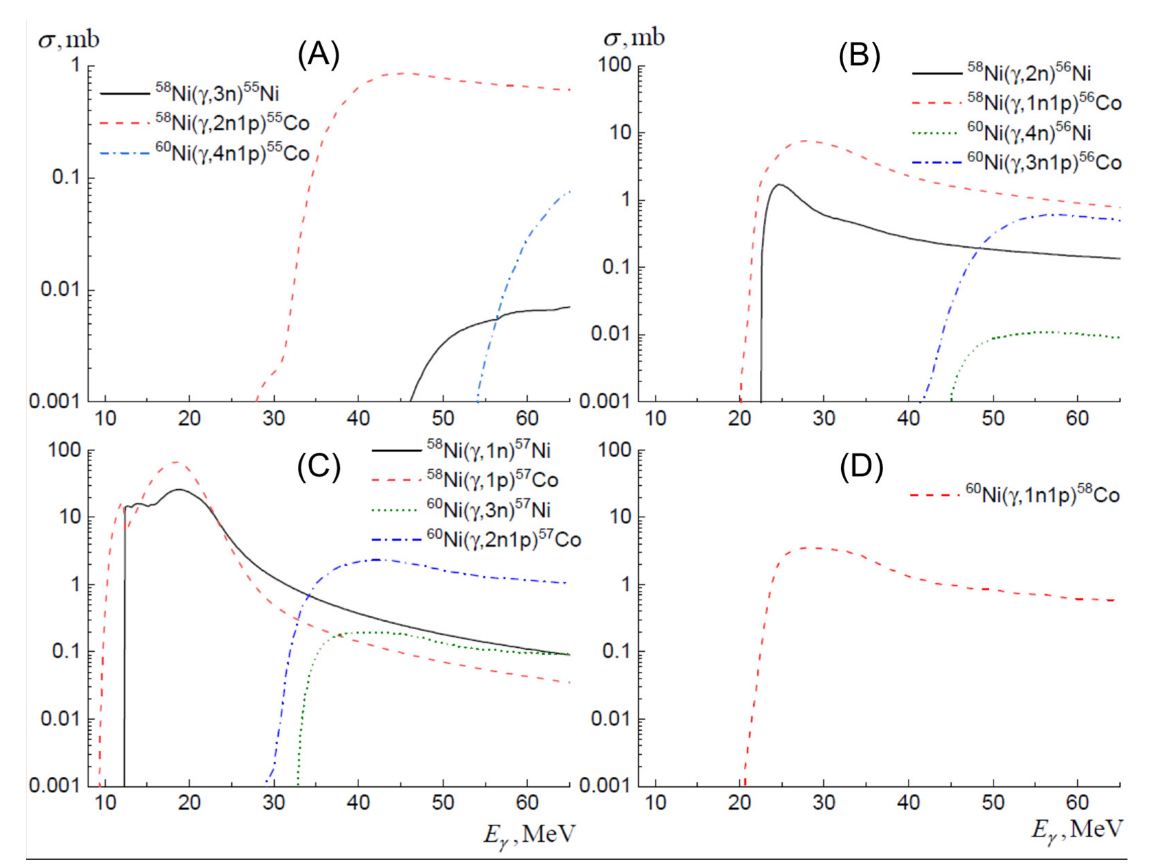
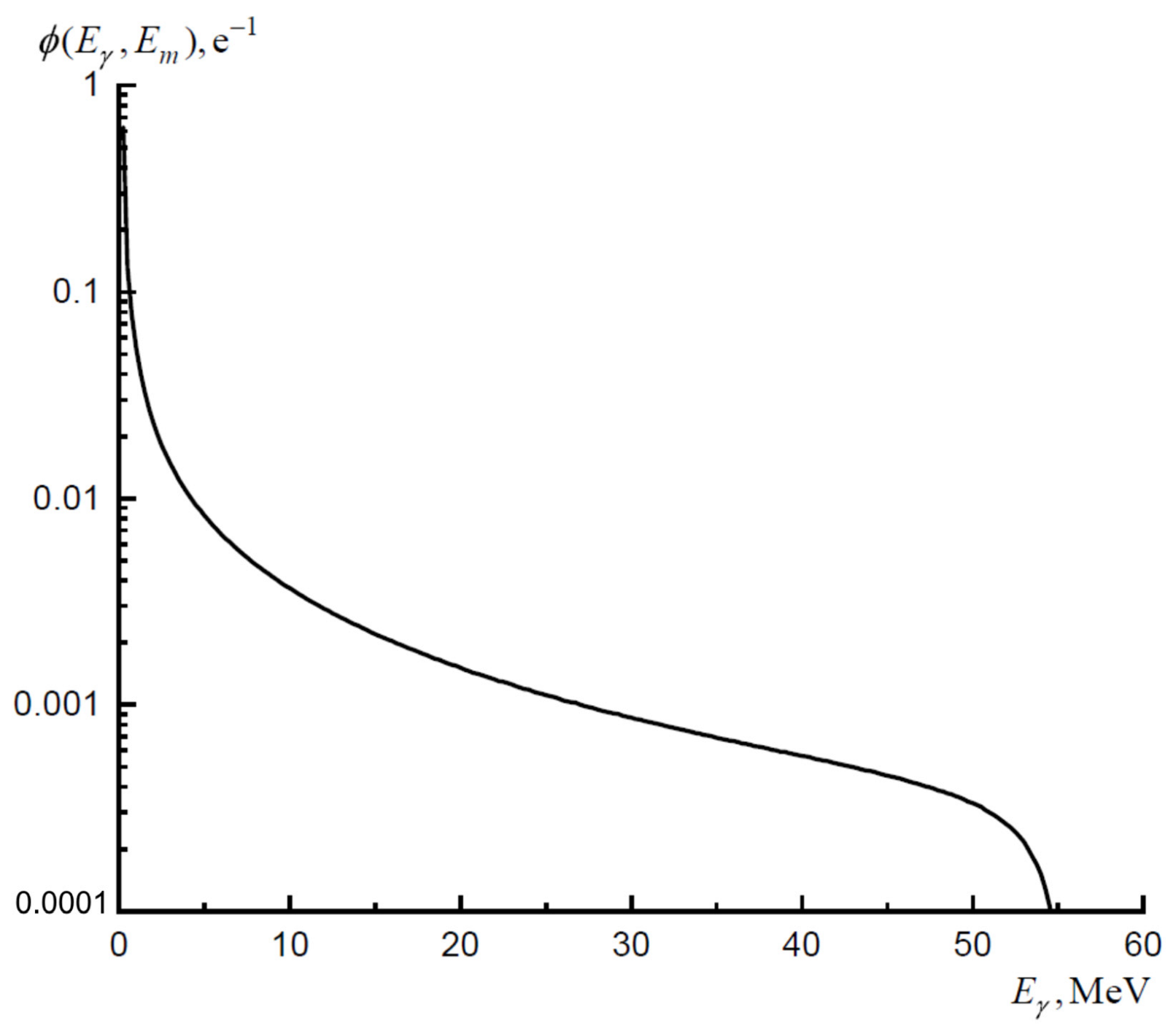
3.1. Theoretical Calculations of Cross-Sections and Yields of Photonuclear Reactions
3.2. Irradiation of Targets and Determination of Yields of Photonuclear Reactions
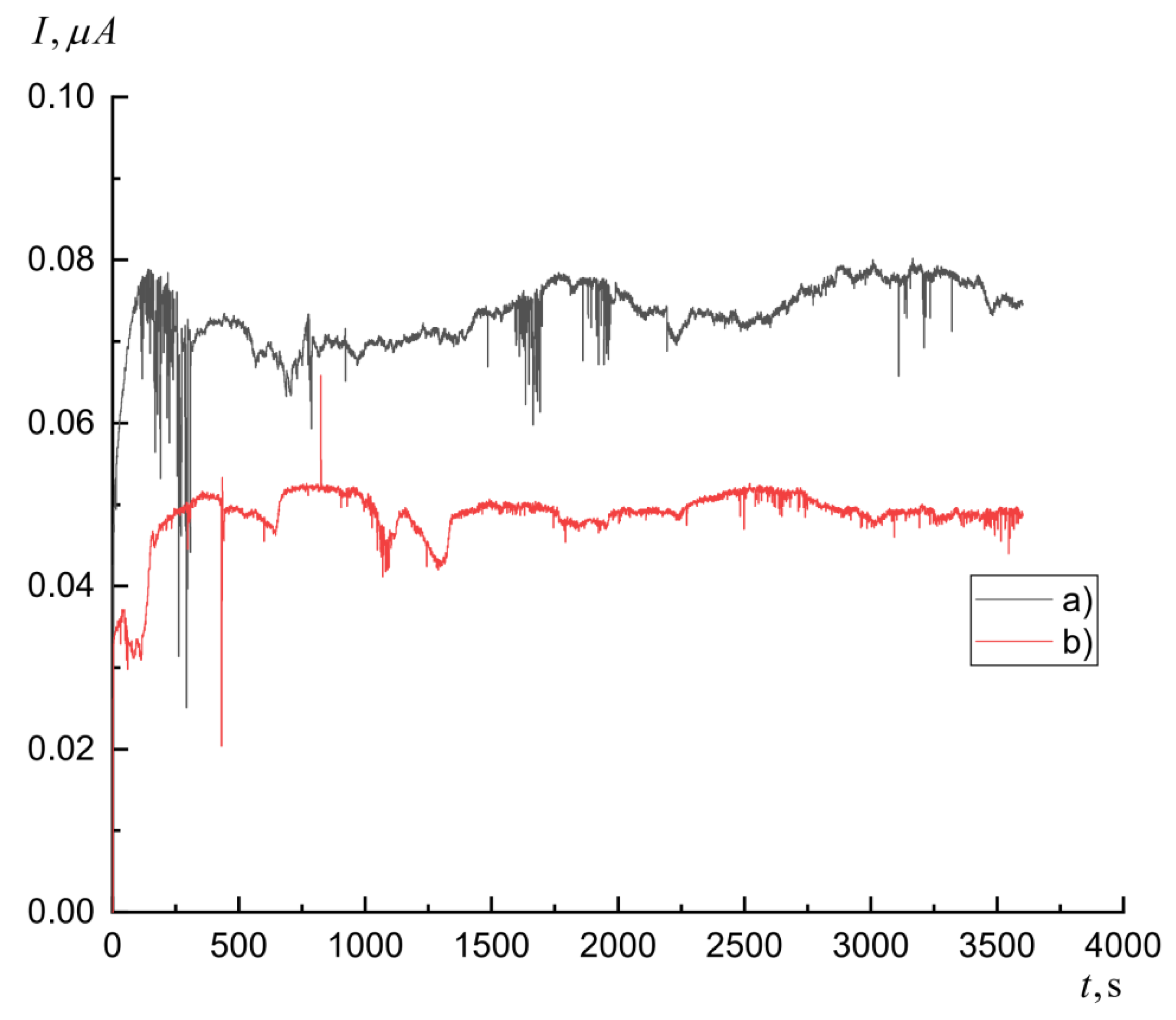
3.3. Separation of Co(II) Isotopes from Irradiated Nickel Target
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazakov, A.G.; Ekatova, T.Y.; Babenya, J.S. Photonuclear production of medical radiometals: A review of experimental studies. J. Radioanal. Nucl. Chem. 2021, 328, 493–505. [Google Scholar] [CrossRef]
- Lagunas-Solar, M.C.; Jungerman, J.A. Cyclotron production of carrier-free cobalt-55, a new positron-emitting label for bleomycin. Int. J. Appl. Radiat. Isot. 1979, 30, 25–32. [Google Scholar] [CrossRef]
- Nieweg, O.E.; Beekhuis, H.; Paans, A.M.J.; Piers, D.A.; Vaalburg, W.; Welleweerd, J.; Wiegman, I.; Woldring, M.G. Detection of lung cancer with 55Co-bleomycin using a positron camera. A comparison with 57Co-bleomycin and 55Co-bleomycin single photon scintigraphy. Eur. J. Nucl. Med. 1982, 7, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Zweit, J.; Smith, A.M.; Downey, S. Production of cobalt-55, a short-lived, positron emitting radiolabel for bleomycin. Int. J. Radiat. Appl. Instrumentation. Part A 1986, 37, 105–109. [Google Scholar] [CrossRef]
- Goethals, P.; Volkaert, A.; Vandewielle, C.; Dierckx, R.; Lameire, N. 55Co-EDTA for renal imaging using positron emission tomography (PET): A feasibility study. Nucl. Med. Biol. 2000, 27, 77–81. [Google Scholar] [CrossRef]
- Andersen, T.L.; Baun, C.; Olsen, B.B.; Dam, J.H.; Thisgaard, H. Improving contrast and detectability: Imaging with [55Co]Co-DOTATATE in comparison with [64Cu]Cu-DOTATATE and [68Ga]Ga-DOTATATE. J. Nucl. Med. 2020, 61, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Dam, J.H.; Olsen, B.B.; Baun, C.; Høilund-Carlsen, P.F.; Thisgaard, H. A PSMA ligand labeled with cobalt-55 for PET imaging of prostate cancer. Mol. Imaging Biol. 2017, 19, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Baidoo, K.E.; Brechbiel, M.W. Mapping biological behaviors by application of longer-lived positron emitting radionuclides. Adv. Drug Deliv. Rev. 2013, 65, 1098–1111. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.J. A nuclear chocolate box: The periodic table of nuclear medicine. Dalt. Trans. 2015, 44, 4819–4844. [Google Scholar] [CrossRef]
- Radford, L.L.; Fernandez, S.; Beacham, R.; El Sayed, R.; Farkas, R.; Benešová, M.; Müller, C.; Lapi, S.E. New 55Co-labeled albumin-binding folate derivatives as potential PET agents for folate receptor imaging. Pharmaceuticals 2019, 12, 166. [Google Scholar] [CrossRef]
- Jansen, H.M.L.; Knollema, S.; Van Der Duin, L.V.; Willemsen, A.T.M.; Wiersma, A.; Franssen, E.J.F.; Russel, F.G.M.; Korf, J.; Paans, A.M.J. Pharmacokinetics and dosimetry of cobalt-55 and cobalt-57. J. Nucl. Med. 1996, 37, 2082–2086. [Google Scholar]
- Stevens, H.; Jansen, H.M.L.; De Reuck, J.; Lemmerling, M.; Strijckmans, K.; Goethals, P.; Lemahieu, I.; De Jong, B.M.; Willemsen, A.T.M.; Korf, J. 55Cobalt (Co) as a PET-tracer in stroke, compared with blood flow, oxygen metabolism, blood volume and gadolinium-MRI. J. Neurol. Sci. 1999, 171, 11–18. [Google Scholar] [CrossRef]
- Jansen, H.M.L.; Van Der Naalt, J.; Van Zomeren, A.H.; Paans, A.M.J.; Veenma-van Der Duin, L.; Hew, J.M.; Pruim, J.; Minderhoud, J.M.; Korf, J. Cobalt-55 positron emission tomography in traumatic brain injury: A pilot study. J. Neurol. Neurosurg. Psychiatry 1996, 60, 221–224. [Google Scholar] [CrossRef] [PubMed]
- Heppeler, A.; André, J.P.; Buschmann, I.; Wang, X.; Reubi, J.C.; Hennig, M.; Kaden, T.A.; Maecke, H.R. Metal-ion-dependent biological properties of a chelator-derived somatostatin analogue for tumour targeting. Chem. A Eur. J. 2008, 14, 3026–3034. [Google Scholar] [CrossRef]
- Amjed, N.; Hussain, M.; Aslam, M.N.; Tárkányi, F.; Qaim, S.M. Evaluation of nuclear reaction cross sections for optimization of production of the emerging diagnostic radionuclide 55Co. Appl. Radiat. Isot. 2016, 108, 38–48. [Google Scholar] [CrossRef]
- Sitarz, M.; Cussonneau, J.P.; Matulewicz, T.; Haddad, F. Radionuclide candidates for β+γ coincidence PET: An overview. Appl. Radiat. Isot. 2020, 155, 108898. [Google Scholar] [CrossRef]
- Zaman, M.; Kim, G.; Naik, H.; Kim, K.; Shahid, M.; Nadeem, M.; Shin, S.G.; Cho, M.H. Flux weighted average cross-sections of natNi(γ,x) reactions with the bremsstrahlung end-point energies of 55, 59, 61 and 65 MeV. Nucl. Phys. A 2018, 978, 173–186. [Google Scholar] [CrossRef]
- Naik, H.; Kim, G.; Nguyen, T.H.; Kim, K.; Shin, S.G.; Kye, Y.C.M. Measurement of natNi(γ,xn)57,56Ni and natNi(γ,pxn)58−55Co reaction cross sections in bremsstrahlung with end-point energies of 65 and 75 MeV. J. Radioanal. Nucl. Chem. 2020, 324, 837–846. [Google Scholar] [CrossRef]
- Maziére, B.; Stulzaft, O.; Verret, J.; Comar, D.; Syrota, A. [55Co]- and [64Cu]-DTPA: New radiopharmaceuticals for quantitative tornocisternography. Int. J. Appl. Radiat. Isot. 1983, 34, 595–601. [Google Scholar] [CrossRef]
- Spellerberg, S.; Reimer, P.; Blessing, G.; Coenen, H.H.; Qaim, S.M. Production of 55Co and 57Co via proton induced reactions on highly enriched 58Ni. Appl. Radiat. Isot. 1998, 49, 1519–1522. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Rowshanfarzad, P.; Akhlaghi, M.; Sabet, M.; Kamali-Dehghan, M.; Pouladi, M. Preparation and biological evaluation of a [55Co]-2-acetylpyridine thiosemicarbazone. Sci. Pharm. 2009, 77, 567–578. [Google Scholar] [CrossRef]
- Jalilian, A.R.; Rowshanfarzad, P.; Yari-Kamrani, Y.; Sabet, M.; Majdabadi, A. Preparation and evaluation of [55Co](II)-DTPA for blood cell labeling. Open Inorg. Chem. J. 2009, 3, 21–25. [Google Scholar] [CrossRef]
- Valdovinos, H.F.; Graves, S.; Barnhart, T.; Nickles, R.J. 55Co separation from proton irradiated metallic nickel. AIP Conf. Proc. 2014, 1626, 217–220. [Google Scholar] [CrossRef]
- Reimer, P.; Qaim, Q.S. Excitation functions of proton induced reactions on highly enriched 58Ni with special relevance to the production of 55Co and 57Co. Radiochim. Acta 1998, 80, 113–120. [Google Scholar] [CrossRef]
- Mastren, T.; Marquez, B.V.; Sultan, D.E.; Bollinger, E.; Eisenbeis, P.; Voller, T.; Lapi, S.E. Cyclotron production of high-specific activity 55Co and in vivo evaluation of the stability of 55Co metal-chelate-peptide complexes. Mol. Imaging 2015, 14, 526–532. [Google Scholar] [CrossRef]
- Mastren, T.; Sultan, D.; Lapi, S.E. Production and separation of 55Co via the 58Ni(p,α)55Co reaction. AIP Conf. Proc. 2012, 1509, 96–100. [Google Scholar] [CrossRef]
- Valdovinos, H.F.; Hernandez, R.; Graves, S.; Ellison, P.A.; Barnhart, T.E.; Theuer, C.P.; Engle, J.W.; Cai, W.; Nickles, R.J. Cyclotron production and radiochemical separation of 55Co and 58mCo from 54Fe, 58Ni and 57Fe targets. Appl. Radiat. Isot. 2017, 130, 90–101. [Google Scholar] [CrossRef]
- Valdovinos, H.F.; Graves, S.; Barnhart, T.; Nickles, R.J. Simplified and reproducible radiochemical separations for the production of high specific activity 61Cu, 64Cu, 86Y and 55Co. AIP Conf. Proc. 2017, 1845, 020021. [Google Scholar] [CrossRef]
- Koning, A.J.; Duijvestijn, M.C.; Hilarie, S. Talys 1.0. In Proceedings of the International Conference on Nuclear Data for Science and Technology, Nice, France, 17 June 2007; pp. 211–214. [Google Scholar]
- Belgya, T.; Bersillon, O.; Capote, R.; Fukahori, T.; Zhigang, G.; Goriely, S.; Herman, M.; Ignatyuk, A.V.; Kailas, S.; Koning, A.; et al. Handbook for Calculations of Nuclear Reaction Data, RIPL-2; IAEA-TECDOC-1506; IAEA: Vienna, Austria, 2006; Available online: http://www-nds.iaea.org/RIPL-2/ (accessed on 20 January 2022).
- Ermakov, A.N.; Ishkhanov, B.S.; Kamanin, A.N.; Pakhomov, N.I.; Khankin, V.V.; Shvedunov, V.I.; Shvedunov, N.V.; Zhuravlev, E.E.; Karev, A.I.; Sobenin, N.P. A multipurpose pulse race-track microtron with an energy of 55 MeV. Instrum. Exp. Tech. 2018, 61, 173–191. [Google Scholar] [CrossRef]
- Belyshev, S.S.; Stopani, K.A. Automatic data acquisition and analysis in activation experiments. Mosc. Univ. Phys. Bull. 2013, 68, 88–91. [Google Scholar] [CrossRef]
- Pourmand, A.; Dauphas, N. Distribution coefficients of 60 elements on TODGA resin: Application to Ca, Lu, Hf, U and Th isotope geochemistry. Talanta 2010, 81, 741–753. [Google Scholar] [CrossRef]
| Isotope | T1/2 | YEOB, kBq/(µA·h·g/cm2) | ||
|---|---|---|---|---|
| natNi | 60Ni | 58Ni | ||
| 56Ni | 6.08 d | 20.7 ± 0.3 (32.5) | 0.16 ± 0.01 (0.06) | 29.6 ± 0.3 (47.7) |
| 57Ni | 35.6 h | 3883 ± 76 (4404) | 48.3 ± 1.0 (21) | 5440 ± 93 (6460) |
| 55Co | 17.53 h | 60.2 ± 4.2 (72.9) | 1.1 ± 0.1 (0.02) | 82.4 ± 5.1 (107) |
| 56Co | 77.27 d | 16.5 ± 0.1 (17.4) | 0.48 ± 0.03 (0.16) | 21.6 ± 0.2 (25.4) |
| 57Co | 271.8 d | 66.1 ± 0.4 (46.5) | 2.75 ± 0.04 (1.35) | 81.8 ± 0.6 (67.8) |
| 58Co | 70.86 d | 10.3 ± 0.1 (3.6) | 39.6 ± 0.4 (13.6) | 0 |
| Isotope | Content, % |
|---|---|
| 58Ni | 0.31 |
| 60Ni | 99.6 ± 0.1 |
| 61Ni | 0.05 |
| 62Ni | 0.04 |
| 64Ni | <0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazakov, A.G.; Babenya, J.S.; Ekatova, T.Y.; Belyshev, S.S.; Khankin, V.V.; Kuznetsov, A.A.; Vinokurov, S.E.; Myasoedov, B.F. Yields of Photo-Proton Reactions on Nuclei of Nickel and Separation of Cobalt Isotopes from Irradiated Targets. Molecules 2022, 27, 1524. https://doi.org/10.3390/molecules27051524
Kazakov AG, Babenya JS, Ekatova TY, Belyshev SS, Khankin VV, Kuznetsov AA, Vinokurov SE, Myasoedov BF. Yields of Photo-Proton Reactions on Nuclei of Nickel and Separation of Cobalt Isotopes from Irradiated Targets. Molecules. 2022; 27(5):1524. https://doi.org/10.3390/molecules27051524
Chicago/Turabian StyleKazakov, Andrey G., Julia S. Babenya, Taisya Y. Ekatova, Sergey S. Belyshev, Vadim V. Khankin, Alexander A. Kuznetsov, Sergey E. Vinokurov, and Boris F. Myasoedov. 2022. "Yields of Photo-Proton Reactions on Nuclei of Nickel and Separation of Cobalt Isotopes from Irradiated Targets" Molecules 27, no. 5: 1524. https://doi.org/10.3390/molecules27051524
APA StyleKazakov, A. G., Babenya, J. S., Ekatova, T. Y., Belyshev, S. S., Khankin, V. V., Kuznetsov, A. A., Vinokurov, S. E., & Myasoedov, B. F. (2022). Yields of Photo-Proton Reactions on Nuclei of Nickel and Separation of Cobalt Isotopes from Irradiated Targets. Molecules, 27(5), 1524. https://doi.org/10.3390/molecules27051524






