Isolation of a Melanoblast Stimulator from Dimocarpus longan, Its Structural Modification, and Structure–Activity Relationships for Vitiligo
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of HMF
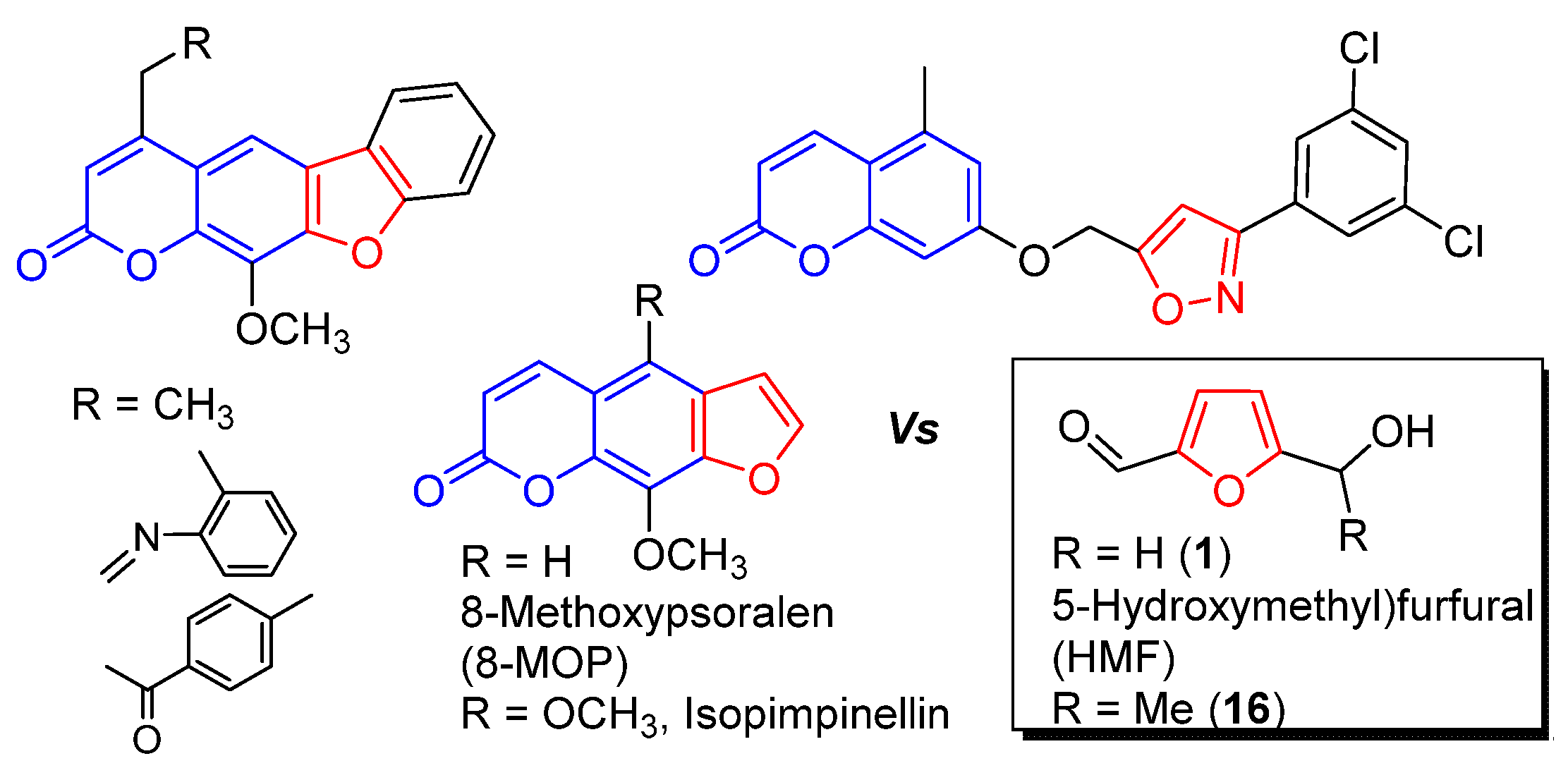
2.2. SAR Study
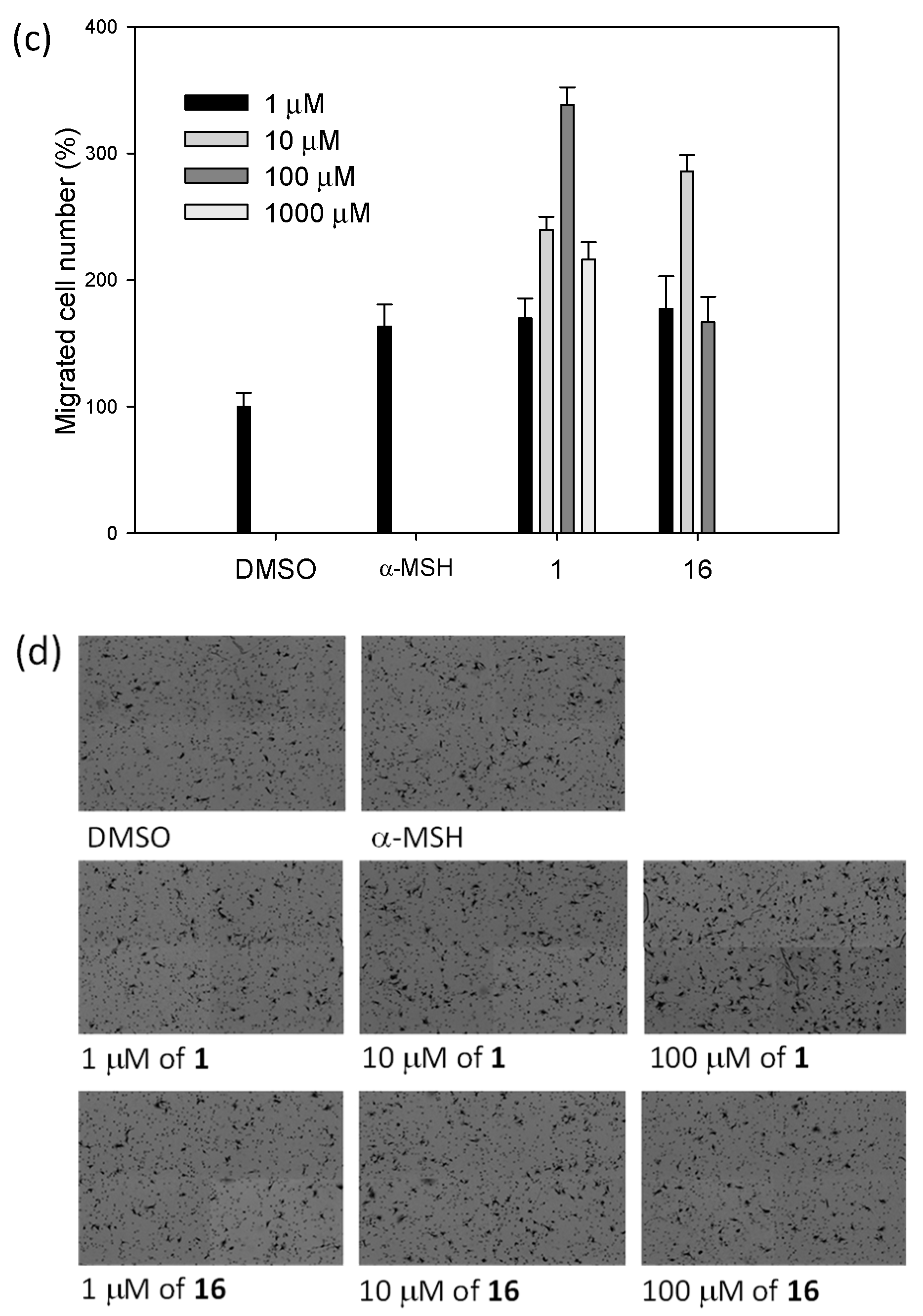
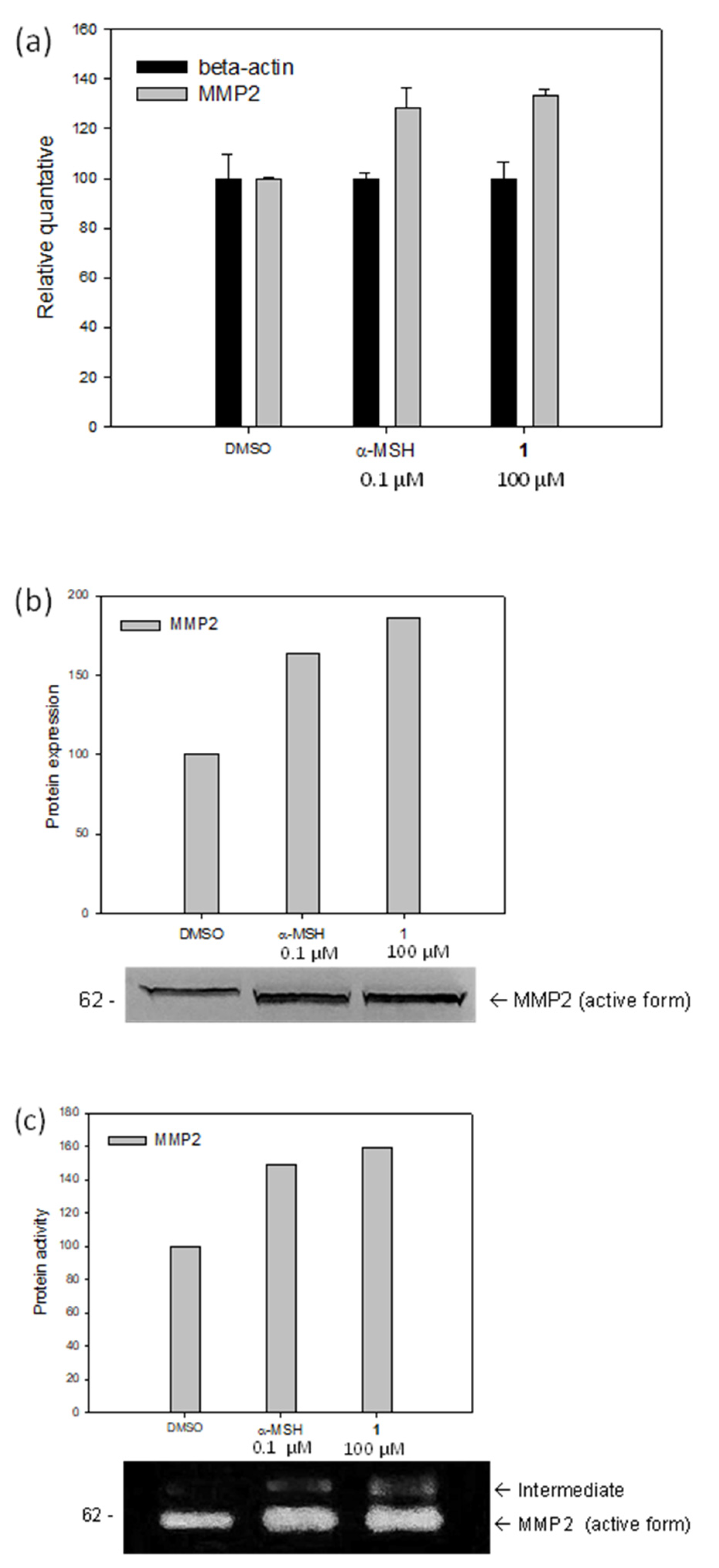
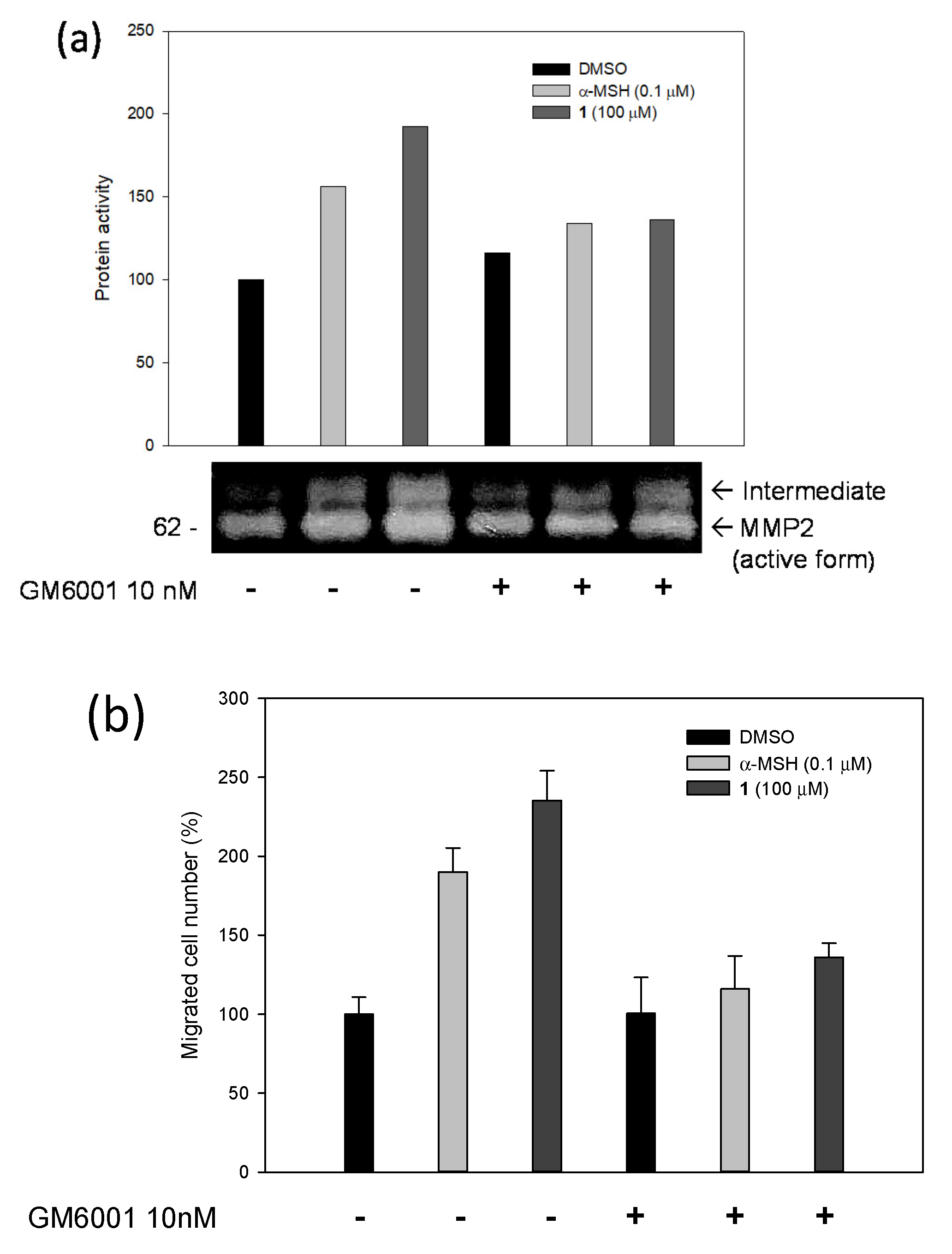
2.3. Biological Studies
3. Materials and Methods
3.1. Isolation of Stimulator (1)
3.2. Materials and Synthesis
3.3. Plant Material
3.4. Biological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ezzedine, K.; Eleftheriadou, V.; Whitton, M.; van Geel, N. Vitiligo. Lancet 2015, 386, 74–84. [Google Scholar] [CrossRef]
- Picardo, M.; Dell’Anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Grimes, P.E.; Halder, R.M.; Jones, C.; Chakrabarti, S.G.; Enterline, J.; Minus, H.R.; Kenney, J.A., Jr. Autoantibodies and Their Clinical Significance in a Black Vitiligo Population. Arch. Dermatol. 1983, 119, 300–303. [Google Scholar] [CrossRef] [PubMed]
- Koga, M. Vitiligo: A new classification and therapy. Br. J. Dermatol. 1977, 97, 255–261. [Google Scholar] [CrossRef]
- Laddha, N.C.; Dwivedi, M.; Mansuri, M.S.; Gani, A.R.; Ansarullah, M.; Ramachandran, A.V.; Dalai, S.; Begum, R. Vitiligo: Interplay between oxidative stress and immune system. Exp. Dermatol. 2013, 22, 245–250. [Google Scholar] [CrossRef]
- Lerner, A.B. On the etiology of vitiligo and gray hair. Am. J. Med. 1971, 51, 141–147. [Google Scholar] [CrossRef]
- Imamura, S.; Tagami, H. Treatment of Vitiligo with Oral Corticosteroids. Dermatology 1976, 153, 179–185. [Google Scholar] [CrossRef]
- Grimes, P.E.; Soriano, T.; Dytoc, M.T. Topical tacrolimus for repigmentation of vitiligo. J. Am. Acad. Dermatol. 2002, 47, 789–791. [Google Scholar] [CrossRef]
- Ortonne, J.P.; Schmitt, D.; Thivolet, J. PUVA-induced Repigmentation of Vitiligo: Scanning Electron Microscopy of Hair Follicles. J. Investig. Dermatol. 1980, 74, 40–42. [Google Scholar] [CrossRef] [Green Version]
- Taieb, A.; Alomar, A.; Böhm, M.; Dell’Anna, M.L.; De Pase, A.; Eleftheriadou, V.; Ezzedine, K.; Gauthier, Y.; Gawkrodger, D.J.; Jouary, T.; et al. Guidelines for the management of vitiligo: The European Dermatology Forum consensus. Br. J. Dermatol. 2013, 168, 5–19. [Google Scholar] [CrossRef]
- Alhowaish, A.K.; Dietrich, N.; Onder, M.; Fritz, K. Effectiveness of a 308-nm excimer laser in treatment of vitiligo: A review. Lasers Med. Sci. 2013, 28, 1035–1041. [Google Scholar] [CrossRef]
- Nishimura, E.K. Melanocyte stem cells: A melanocyte reservoir in hair follicles for hair and skin pigmentation. Pigment Cell Melanoma Res. 2011, 24, 401–410. [Google Scholar] [CrossRef]
- Paus, R. Migrating melanocyte stem cells: Masters of disaster? Nat. Med. 2013, 19, 818–819. [Google Scholar] [CrossRef]
- Babitha, S.; Nguyen, D.H.; Park, S.-J.; Shin, J.-H.; Reyes, G.A.; Caburian, A.; Kim, E.-K. Potential of Cassia alata leaf extract in inducing differentiation and migration of mouse melanoblasts. Biotechnol. Bioprocess Eng. 2010, 15, 1071–1076. [Google Scholar] [CrossRef]
- Babitha, S.; Shin, J.-H.; Nguyen, D.H.; Park, S.-J.; Reyes, G.A.; Caburian, A.; Kim, E.K. A stimulatory effect of Cassia occidentalis on melanoblast differentiation and migration. Arch. Dermatol. 2011, 303, 211–216. [Google Scholar] [CrossRef]
- Niu, C.; Aisa, H.A. Upregulation of Melanogenesis and Tyrosinase Activity: Potential Agents for Vitiligo. Molecules 2017, 22, 1303. [Google Scholar] [CrossRef] [Green Version]
- Lei, T.C.; Vieira, W.D.; Hearing, V.J. In Vitro Migration of Melanoblasts Requires Matrix Metalloproteinase-2: Implications to Vitiligo Therapy by Photochemotherapy. Pigment Cell Res. 2002, 15, 426–432. [Google Scholar] [CrossRef]
- Fitzsimons, C.P.; Long, J.; MacKie, R.M. Synergistic carcinogenic potential of methotrexate and PUVA in psoriasis. Lancet 1983, 1, 235–236. [Google Scholar] [CrossRef]
- Marx, J.L.; Auerbach, R.; Possick, P.; Myrow, R.; Gladstein, A.H.; Kopf, A.W. Malignant melanoma in situ in two patients treated with psoralens and ultraviolet A. J. Am. Acad. Dermatol. 1983, 9, 904–911. [Google Scholar] [CrossRef]
- Stern, R.S.; Laird, N.; Melski, J.; Parrish, J.A.; Fitzpatrick, T.B.; Bleich, H.L. Cutaneous squamous-cell carcinoma in patients treated with PUVA. N. Engl. J. Med. 1984, 310, 1156–1161. [Google Scholar] [CrossRef]
- Carbone, A.; Montalbano, A.; Spanò, V.; Musante, I.; Galietta, L.J.V.; Barraja, P. Furocoumarins as multi-target agents in the treatment of cystic fibrosis. Eur. J. Med. Chem. 2019, 180, 283–290. [Google Scholar] [CrossRef]
- Matsuda, H.; Hirata, N.; Kawaguchi, Y.; Yamazaki, M.; Naruto, S.; Shibano, M.; Taniguchi, M.; Baba, K.; Kubo, M. Melanogenesis Stimulation in Murine B16 Melanoma Cells by Umberiferae Plant Extracts and Their Coumarin Constituents. Biol. Pharm. Bull. 2005, 28, 1229–1233. [Google Scholar] [CrossRef] [Green Version]
- Niu, C.; Pang, G.X.; Li, G.; Dou, J.; Nie, L.F.; Himit, H.; Kabas, M.; Aisa, H.A. Synthesis and biological evaluation of furocoumarin derivatives on melanin synthesis in murine B16 cells for the treatment of vitiligo. Bioorg. Med. Chem. 2016, 24, 5960–5968. [Google Scholar] [CrossRef]
- Pang, G.X.; Niu, C.; Mamat, N.; Aisa, H.A. Synthesis and in vitro biological evaluation of novel coumarin derivatives containing isoxazole moieties on melanin synthesis in B16 cells and inhibition on bacteria. Bioorg. Med. Chem. Lett. 2017, 27, 2674–2677. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]

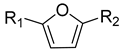 | |||||
|---|---|---|---|---|---|
| No. | R1 | R2 | Melanin Content a | Cell Migration a | Cell Viability a |
| 1 | CHO | CH2OH | 147 (4) b | 318 (17) b | 108 (4) b |
| 2 | CHO | CH2OCH2Ph | 82 (4) | 114 (19) | 104 (2) |
| 3 | CHO | 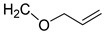 | 95 (8) | 134 (12) | 106 (1) |
| 4 | CHO | 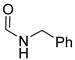 | 97 (4) | 115 (20) | 109 (2) |
| 5 | CHO | 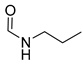 | 94 (2) | 229 (12) | 100 (1) |
| 6 | CHO | 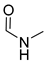 | 96 (3) | 139 (18) | 106 (3) |
| 7 | CHO | CO2CH2Ph | 134 (2) | 202 (62) | 100 (4) |
| 8 | CHO | 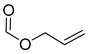 | 122 (5) | 178 (20) | 109 (2) |
| 9 | CHO | CO2CH3 | 136 (1) | 296 (19) | 110 (3) |
| 10 | CHO | CHO | 95 (6) | 117 (22) | 23 (6) |
| 11 | CH2OH | CO2CH2CH3 | 104 (5) | 111 (14) | 71 (4) |
| 12 | CH2OH | CO2CH3 | 145 (6) | 161 (29) | 80 (1) |
| 13 | CH2OH | CO2H | 158 (1) | 264 (8) | 93 (1) |
| 14 | CH2OH | CH2OH | 139 (2) | 173 (30) | 86 (2) |
| 15 | CH2OH |  | 139 (20) | 214 (14) | 97 (1) |
| 16 | CHO | 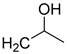 | 160 (1) | 356 (25) | 97 (1) |
| 17 | CHO | 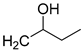 | 112 (2) | 261 (17) | 98 (1) |
| 18 | CHO | 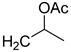 | 116 (3) | 230 (25) | 100 (1) |
| 19 | CHO | 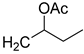 | 115 (3) | 260 (17) | 101 (2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.-W.; Choi, S.; Kim, G.; Lee, H.B.; Rao, P.S.; Shin, J.; Kim, E.K.; Cho, D.-G. Isolation of a Melanoblast Stimulator from Dimocarpus longan, Its Structural Modification, and Structure–Activity Relationships for Vitiligo. Molecules 2022, 27, 2135. https://doi.org/10.3390/molecules27072135
Song J-W, Choi S, Kim G, Lee HB, Rao PS, Shin J, Kim EK, Cho D-G. Isolation of a Melanoblast Stimulator from Dimocarpus longan, Its Structural Modification, and Structure–Activity Relationships for Vitiligo. Molecules. 2022; 27(7):2135. https://doi.org/10.3390/molecules27072135
Chicago/Turabian StyleSong, Jae-Won, Sunju Choi, Gayeong Kim, Hyang Bok Lee, P. Sankara Rao, Jeonghyun Shin, Eun Ki Kim, and Dong-Gyu Cho. 2022. "Isolation of a Melanoblast Stimulator from Dimocarpus longan, Its Structural Modification, and Structure–Activity Relationships for Vitiligo" Molecules 27, no. 7: 2135. https://doi.org/10.3390/molecules27072135
APA StyleSong, J.-W., Choi, S., Kim, G., Lee, H. B., Rao, P. S., Shin, J., Kim, E. K., & Cho, D.-G. (2022). Isolation of a Melanoblast Stimulator from Dimocarpus longan, Its Structural Modification, and Structure–Activity Relationships for Vitiligo. Molecules, 27(7), 2135. https://doi.org/10.3390/molecules27072135







