Monitoring Stimulated Darkening from UV-C Light on Different Bean Genotypes by NMR Spectroscopy
Abstract
:1. Introduction
2. Experimental
2.1. Prolonged and Accelerated Darkening Study
2.2. NMR Analysis
2.2.1. TD-NMR Analysis (CPMG and ROSE)
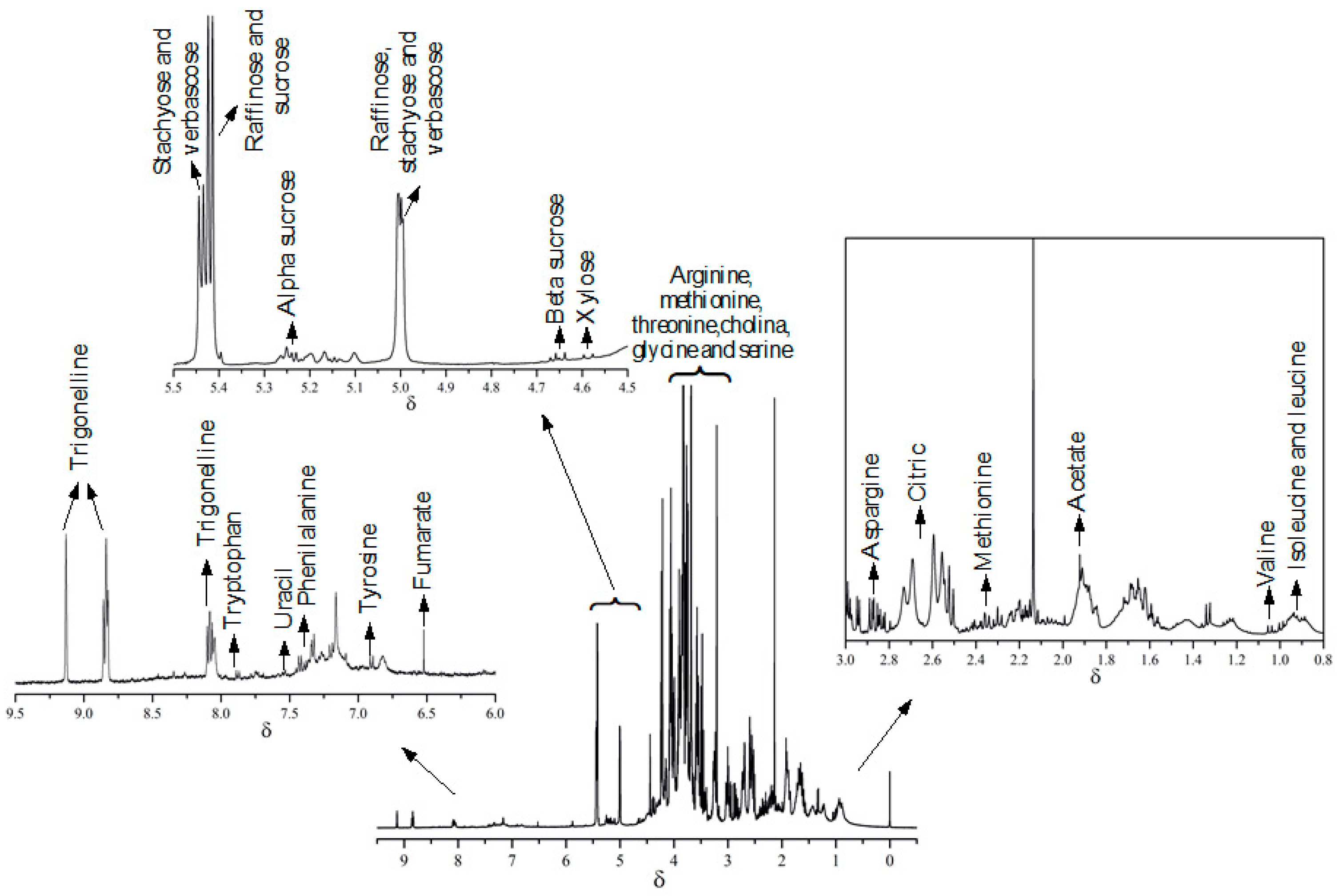
2.2.2. High-Resolution NMR
2.3. Chemometric Analysis
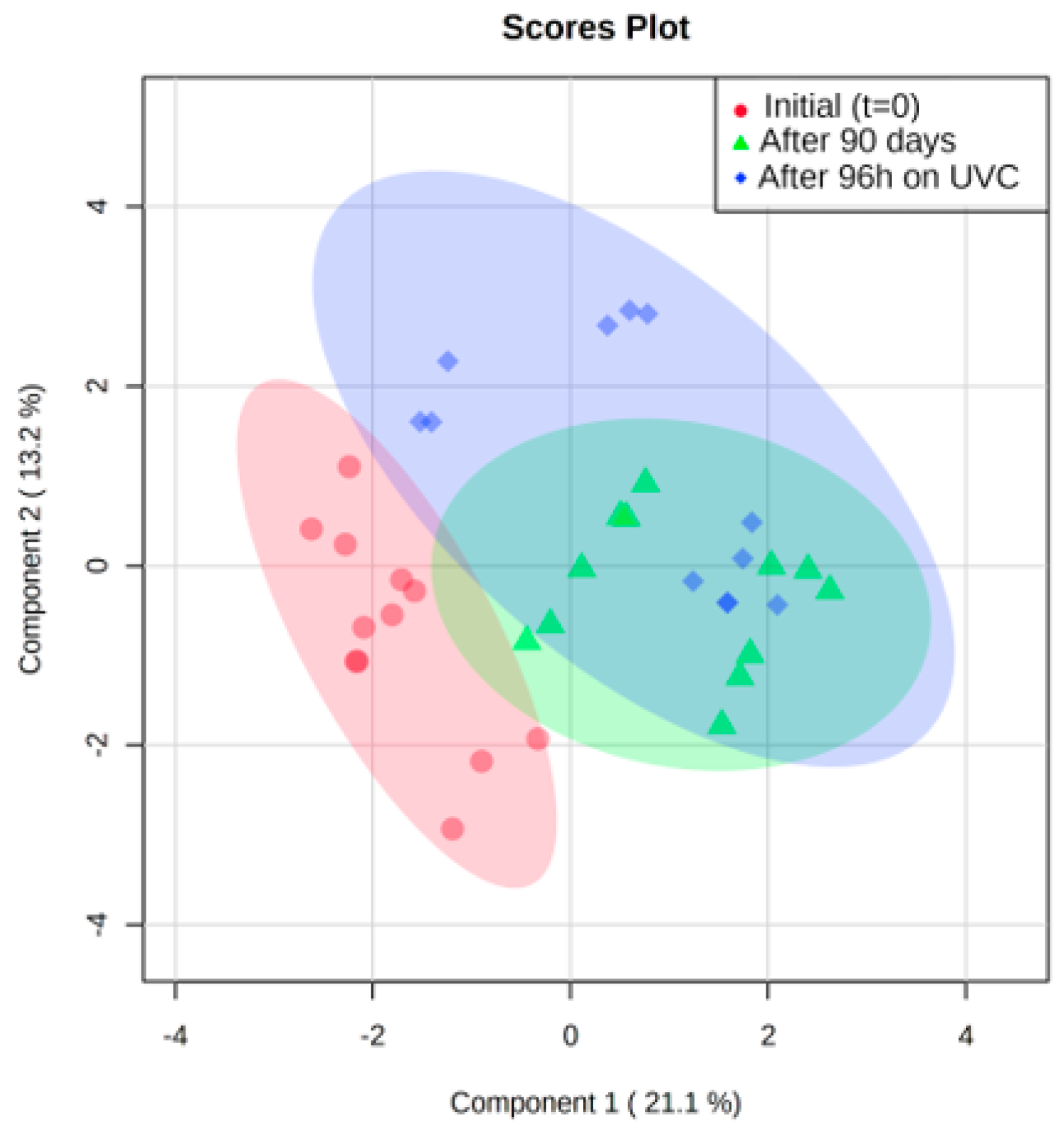
2.3.1. Multivariate Statistical Analysis
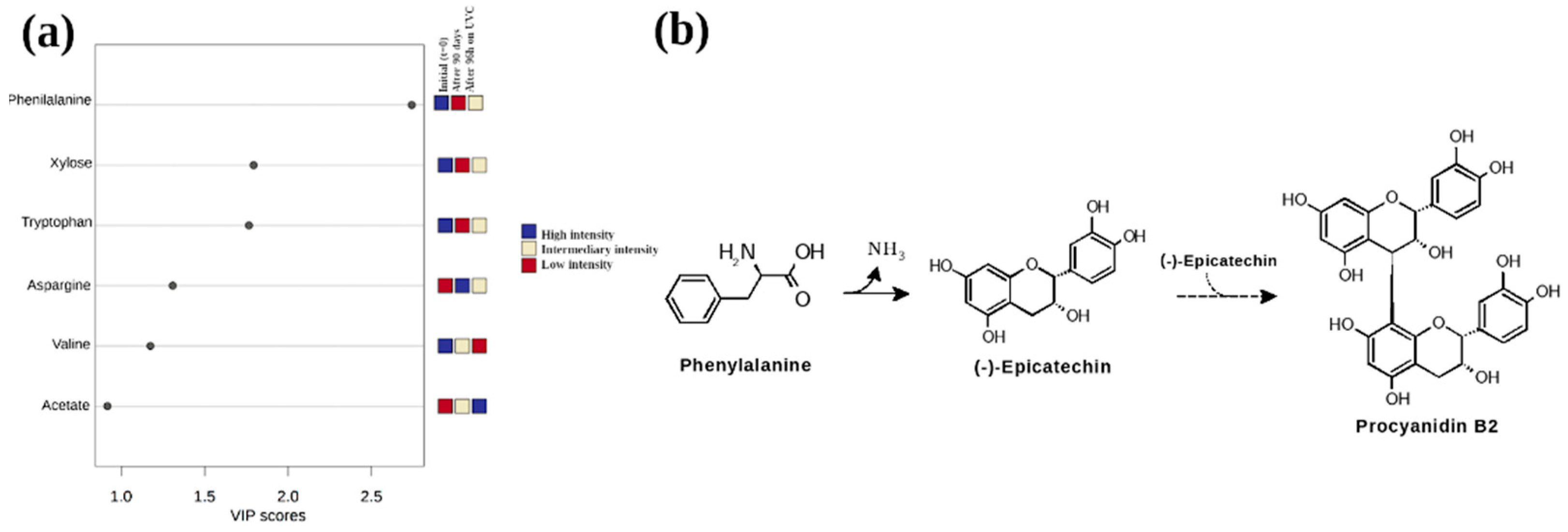
2.3.2. Quantitative Analysis of Variation in Selected Metabolites
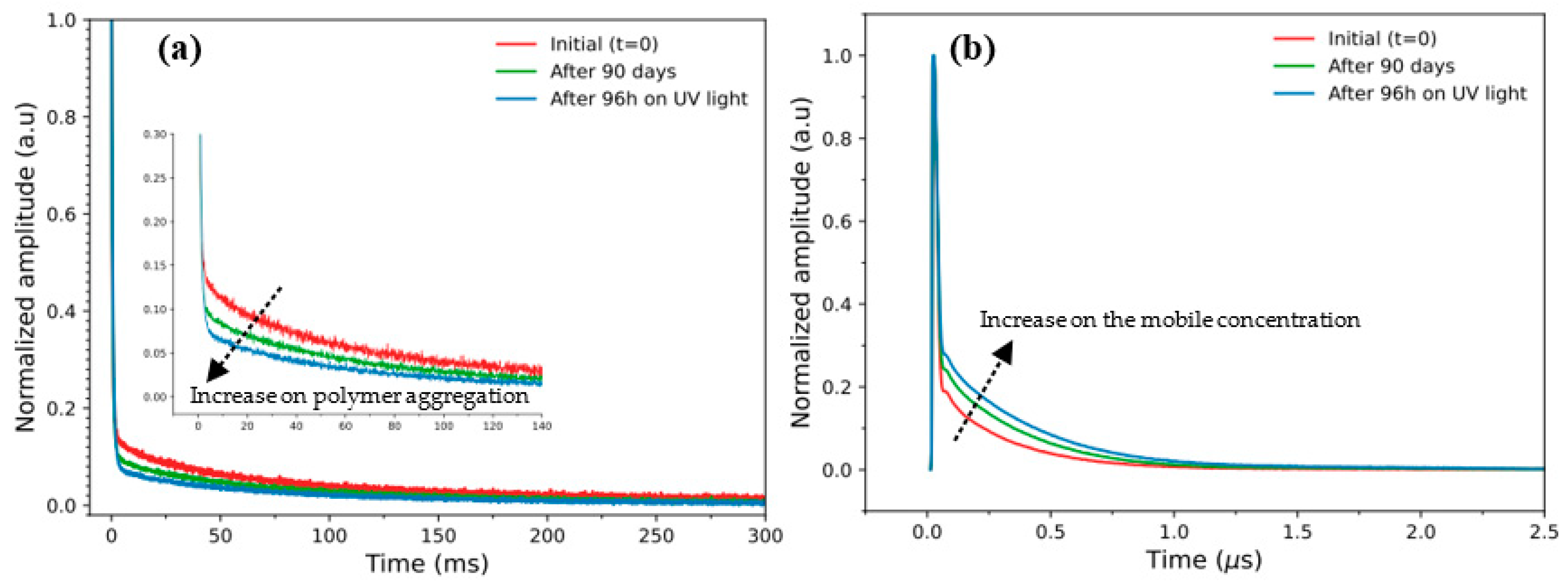
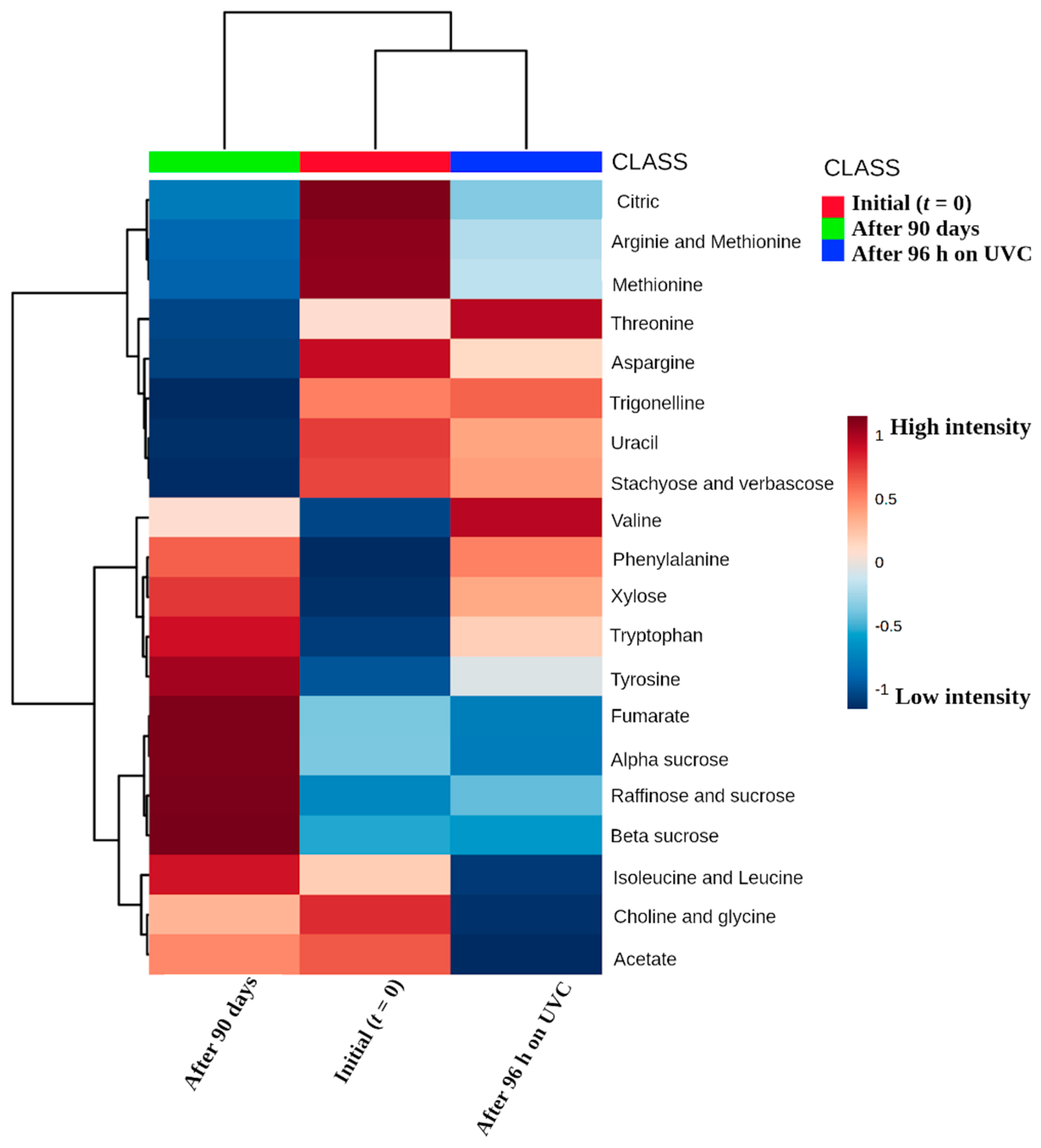
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Carneiro, J.E.; de Paula Júnior, T.; Borém, A. Feijão Do Plantio à Colheita, 1st ed.; Editora UFV: Viçosa, Brazil, 2014. [Google Scholar]
- Nasar-Abbas, S.M.; Plummer, J.A.; Siddique, K.H.M.; White, P.; Harris, D.; Dods, K. Cooking Quality of Faba Bean after Storage at High Temperature and the Role of Lignins and Other Phenolics in Bean Hardening. LWT-Food Sci. Technol. 2008, 41, 1260–1267. [Google Scholar] [CrossRef]
- Elsadr, H.T.; Wright, L.C.; Peter Pauls, K.; Bett, K.E. Characterization of Seed Coat Post Harvest Darkening in Common Bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2011, 123, 1467–1472. [Google Scholar] [CrossRef]
- Siqueira, B.S.; Pereira, W.J.; Batista, K.A.; Oomah, B.D.; Fernandes, K.F.; Bassinello, P.Z. Influence of Storage on Darkening and Hardening of Slow- and Regular-Darkening Carioca Bean (Phaseolus vulgaris L.) Genotypes. J. Agric. Stud. 2014, 2, 87. [Google Scholar] [CrossRef]
- Freixas Coutin, J.A.; Munholland, S.; Silva, A.; Subedi, S.; Lukens, L.; Crosby, W.L.; Pauls, K.P.; Bozzo, G.G. Proanthocyanidin Accumulation and Transcriptional Responses in the Seed Coat of Cranberry Beans (Phaseolus vulgaris L.) with Different Susceptibility to Postharvest Darkening. BMC Plant Biol. 2017, 17, 89. [Google Scholar] [CrossRef]
- Erfatpour, M.; Pauls, K.P. A R2R3-MYB Gene-Based Marker for the Non-Darkening Seed Coat Trait in Pinto and Cranberry Beans (Phaseolus vulgaris L.) Derived from ‘Wit-Rood Boontje’. Theor. Appl. Genet. 2020, 133, 1977–1994. [Google Scholar] [CrossRef] [Green Version]
- Beninger, C.W.; Gu, L.; Prior, R.L.; Junk, D.C.; Vandenberg, A.; Bett, K.E. Changes in Polyphenols of the Seed Coat during the After-Darkening Process in Pinto Beans (Phaseolus vulgaris L.). J. Agric. Food Chem. 2005, 53, 7777–7782. [Google Scholar] [CrossRef]
- Alvares, R.C.; Stonehouse, R.; Souza, T.L.P.O.; Melo, P.G.S.; Miklas, P.N.; Bett, K.E.; Melo, L.C.; Rodrigues, L.A.; Souza, L.L.; Pereira, H.S. Generation and Validation of Genetic Markers for the Selection of Carioca Dry Bean Genotypes with the Slow-Darkening Seed Coat Trait. Euphytica 2019, 215, 141. [Google Scholar] [CrossRef]
- Demito, A.; Ziegler, V.; Goebel, J.T.S.; Konopatzki, E.A.; Coelho, S.R.M.; Elias, M.C. Effects of Refrigeration on Biochemical, Digestibility, and Technological Parameters of Carioca Beans during Storage. J. Food Biochem. 2019, 43, e12900. [Google Scholar] [CrossRef]
- Espín, J.C.; Varón, R.; Fenoll, L.G.; Gilabert, M.A.; García-Ruíz, P.A.; Tudela, J.; García-Cánovas, F. Kinetic Characterization of the Substrate Specificity and Mechanism of Mushroom Tyrosinase. Eur. J. Biochem. 2000, 267, 1270–1279. [Google Scholar] [CrossRef] [Green Version]
- Park, D.; Maga, J.A. Dry Bean (Phaseolus vulgarjs) Color Stability as Influenced by Time and Moistijre Content. J. Food Process. Preserv. 1999, 23, 515–522. [Google Scholar] [CrossRef]
- de Almeida, C.P.; Santos, I.L.; de Carvalho Paulino, J.F.; Barbosa, C.C.F.; Pereira, C.C.A.; Carvalho, C.R.L.; de Moraes Cunha Gonçalves, G.; Song, Q.; Carbonell, S.A.M.; Chiorato, A.F.; et al. Genome-Wide Association Mapping Reveals New Loci Associated with Light-Colored Seed Coat at Harvest and Slow Darkening in Carioca Beans. BMC Plant Biol. 2021, 21, 343. [Google Scholar] [CrossRef]
- Chiorato, A.F.; Carbonell, S.A.M. O Melhoramento Genético Do Feijoeiro No Instituto Agronômico IAC (1932 a 2014). Camp. O Agron. 2014, 64–66, 6–13. [Google Scholar]
- Tormena, C.D.; Campos, R.C.S.; Marcheafave, G.G.; Edward Bruns, R.; Scarminio, I.S.; Pauli, E.D. Authentication of Carioca Common Bean Cultivars (Phaseolus vulgaris L.) Using Digital Image Processing and Chemometric Tools. Food Chem. 2021, 364, 130349. [Google Scholar] [CrossRef]
- Berrios, J.D.J.; Swanson, B.G.; Adeline Cheong, W. Physico-Chemical Characterization of Stored Black Beans (Phaseolus vulgaris L.). Food Res. Int. 1999, 32, 669–676. [Google Scholar] [CrossRef]
- Granito, M.; Álvarez, G. Lactic Acid Fermentation of Black Beans (Phaseolus vulgaris): Microbiological and Chemical Characterization. J. Sci. Food Agric. 2006, 86, 1164–1171. [Google Scholar] [CrossRef]
- Encina, A.; Sevillano, J.M.; Acebes, J.L.; Alvarez, J. Cell Wall Modifications of Bean (Phaseolus vulgaris) Cell Suspensions during Habituation and Dehabituation to Dichlobenil. Physiol. Plant. 2002, 114, 182–191. [Google Scholar] [CrossRef]
- Deng, G.; Rodríguez-Espinosa, M.E.; Feng, X.; Guevara-Oquendo, V.H.; Lei, Y.; Yan, M.; Yang, J.-C.; Zhang, H.; Deng, H.; Zhang, W.; et al. Using Advanced Vibrational Molecular Spectroscopy (ATR-Ft/IRS) to Study Heating Process Induced Changes on Protein Molecular Structure of Biodegradation Residues in Cool-Climate Adapted Faba Bean Seeds: Relationship with Rumen and Intestinal Protein Digestion in Ruminant Systems. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 234, 118220. [Google Scholar] [CrossRef]
- Hacisalihoglu, G.; Larbi, B.; Settles, A.M. Near-Infrared Reflectance Spectroscopy Predicts Protein, Starch, and Seed Weight in Intact Seeds of Common Bean (Phaseolus vulgaris L.). J. Agric. Food Chem. 2010, 58, 702–706. [Google Scholar] [CrossRef]
- Plans, M.; Simó, J.; Casañas, F.; Sabaté, J. Near-Infrared Spectroscopy Analysis of Seed Coats of Common Beans (Phaseolus vulgaris L.): A Potential Tool for Breeding and Quality Evaluation. J. Agric. Food Chem. 2012, 60, 706–712. [Google Scholar] [CrossRef]
- Plans, M.; Simó, J.; Casañas, F.; del Castillo, R.R.; Rodriguez-Saona, L.E.; Sabaté, J. Estimating Sensory Properties of Common Beans (Phaseolus vulgaris L.) by near Infrared Spectroscopy. Food Res. Int. 2014, 56, 55–62. [Google Scholar] [CrossRef]
- Alonso-Simón, A.; Encina, A.E.; García-Angulo, P.; Álvarez, J.M.; Acebes, J.L. FTIR Spectroscopy Monitoring of Cell Wall Modifications during the Habituation of Bean (Phaseolus vulgaris L.) Callus Cultures to Dichlobenil. Plant Sci. 2004, 167, 1273–1281. [Google Scholar] [CrossRef]
- Santiago-Ramos, D.; de Dios Figueroa-Cárdenas, J.; Véles-Medina, J.J.; Salazar, R. Physicochemical Properties of Nixtamalized Black Bean (Phaseolus vulgaris L.) Flours. Food Chem. 2018, 240, 456–462. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Arráez-Román, D.; Lozano-Sánchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phytochemical Characterisation of Green Beans (Phaseolus vulgaris L.) by Using High-Performance Liquid Chromatography Coupled with Time-of-Flight Mass Spectrometry. Phytochem. Anal. 2013, 24, 105–116. [Google Scholar] [CrossRef]
- Sánchez-Mata, M.C.; Cámara-Hurtado, M.; Díez-Marqués, C.; Torija-Isasa, M.E. Comparison of High-Performance Liquid Chromatography and Spectrofluorimetry for Vitamin C Analysis of Green Beans (Phaseolus vulgaris L.). Eur. Food Res. Technol. 2000, 210, 220–225. [Google Scholar] [CrossRef]
- Garcia, R.H.S.; Filgueiras, J.G.; DeAzevedo, E.R.; Colnago, L.A. Power-Optimized, Time-Reversal Pulse Sequence for a Robust Recovery of Signals from Rigid Segments Using Time Domain NMR. Solid State Nucl. Magn. Reson. 2019, 104, 101619. [Google Scholar] [CrossRef]
- FooDB. Food Data Base. Available online: https://foodb.ca/ (accessed on 4 February 2022).
- HMDB. Human Metabolome Database. Available online: https://hmdb.ca/ (accessed on 20 January 2022).
- Chenomx. Chenomx Inc.|Metabolite Discovery and Measurement. Available online: https://www.chenomx.com/ (accessed on 4 February 2022).
- International Commission on Illumination. ISO—CIE—International Commission on Illumination. Available online: https://www.iso.org/committee/55238/x/catalogue/ (accessed on 4 February 2022).
- Junk-Knievel, D.C.; Vandenberg, A.; Bett, K.E. An Accelerated Postharvest Seed-Coat Darkening Protocol for Pinto Beans Grown across Different Environments. Crop Sci. 2007, 47, 694–700. [Google Scholar] [CrossRef]
- Bruker. TopSpin|NMR Data Analysis|Bruker. Available online: https://www.bruker.com/en/products-and-solutions/mr/nmr-software/topspin.html (accessed on 4 February 2022).
- MetaboAnalyst. MetaboAnalyst. Available online: https://www.metaboanalyst.ca/ (accessed on 4 February 2022).
- Xia, J.; Psychogios, N.; Young, N.; Wishart, D.S. MetaboAnalyst: A Web Server for Metabolomic Data Analysis and Interpretation. Nucleic Acids Res. 2009, 37, W652–W660. [Google Scholar] [CrossRef] [Green Version]
- Dixon, R.A.; Xie, D.Y.; Sharma, S.B. Proanthocyanidins—A Final Frontier in Flavonoid Research? New Phytol. 2005, 165, 9–28. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.X.; Bozzo, G.G.; Freixas-Coutin, J.A.; Marcone, M.F.; Pauls, P.K.; Tang, Y.; Zhang, B.; Liu, R.; Tsao, R. Free and Conjugated Phenolic Compounds and Their Antioxidant Activities in Regular and Non-Darkening Cranberry Bean (Phaseolus vulgaris L.) Seed Coats. J. Funct. Foods 2015, 18, 1047–1056. [Google Scholar] [CrossRef]
- Stein, O.; Granot, D. An Overview of Sucrose Synthases in Plants. Front. Plant Sci. 2019, 10, 95. [Google Scholar] [CrossRef] [Green Version]
- Eveland, A.L.; Jackson, D.P. Sugars, Signalling, and Plant Development. J. Exp. Bot. 2012, 63, 3367–3377. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador, M.V.; Kock, F.V.C.; Santos, I.L.; Paulino, J.F.C.; de Almeida, C.P.; dos Santos Garcia, R.H.; Benchimol-Reis, L.L.; Colnago, L.A.; Ferreira, A.G. Monitoring Stimulated Darkening from UV-C Light on Different Bean Genotypes by NMR Spectroscopy. Molecules 2022, 27, 2060. https://doi.org/10.3390/molecules27072060
Salvador MV, Kock FVC, Santos IL, Paulino JFC, de Almeida CP, dos Santos Garcia RH, Benchimol-Reis LL, Colnago LA, Ferreira AG. Monitoring Stimulated Darkening from UV-C Light on Different Bean Genotypes by NMR Spectroscopy. Molecules. 2022; 27(7):2060. https://doi.org/10.3390/molecules27072060
Chicago/Turabian StyleSalvador, Marília Vilela, Flávio Vinícius Crizóstomo Kock, Isabella Laporte Santos, Jean Fausto Carvalho Paulino, Caléo Panhoca de Almeida, Rodrigo Henrique dos Santos Garcia, Luciana Lasry Benchimol-Reis, Luiz Alberto Colnago, and Antonio Gilberto Ferreira. 2022. "Monitoring Stimulated Darkening from UV-C Light on Different Bean Genotypes by NMR Spectroscopy" Molecules 27, no. 7: 2060. https://doi.org/10.3390/molecules27072060
APA StyleSalvador, M. V., Kock, F. V. C., Santos, I. L., Paulino, J. F. C., de Almeida, C. P., dos Santos Garcia, R. H., Benchimol-Reis, L. L., Colnago, L. A., & Ferreira, A. G. (2022). Monitoring Stimulated Darkening from UV-C Light on Different Bean Genotypes by NMR Spectroscopy. Molecules, 27(7), 2060. https://doi.org/10.3390/molecules27072060






