Hydrogen-Bond-Assisted Diels–Alder Kinetics or Self-Healing in Reversible Polymer Networks? A Combined Experimental and Theoretical Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Microcalorimetry
2.2.2. Kinetic Calculations
2.2.3. Density Functional Theory Calculations
3. Results and Discussion
3.1. Experimental Study
3.1.1. Model Series of Reversible Networks: Effect of Concentrations of Hydroxyl and Ether Functional Groups on Initial Reaction Rate and Forward DA Rate Constant
3.1.2. Extended Series: Effect of Hydroxyl-free Systems and Increased Concentration of Hydroxyl Groups on Initial Reaction Rate and Forward DA Rate Constant
3.2. Preliminary DFT Study
3.3. Influence of Hydrogen Bonding on Diels–Alder Energetics of Substituted Furan and Maleimide
3.3.1. Influence of Hydrogen Bonding of Additive on Diels–Alder Energetics of Substituted Furan and Maleimide
3.3.2. Influence of Ester Substitution on Diels–Alder Energetics of Substituted Furan and Maleimide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Fringuelli, F.; Taticchi, A. The Diels-Alder Reaction; John Wiley: New York, NY, USA, 2002. [Google Scholar]
- Adzima, B.J.; Aguirre, H.A.; Kloxin, C.J.; Scott, T.F.; Bowman, C.N. Rheological and Chemical Analysis of Reverse Gelation in a Covalently Cross-Linked Diels-Alder Polymer Network. Macromolecules 2008, 41, 9112–9117. [Google Scholar] [CrossRef] [Green Version]
- Scheltjens, G.; Diaz, M.M.; Brancart, J.; Van Assche, G.; Van Mele, B. A self-healing polymer network based on reversible covalent bonding. React. Funct. Polym. 2013, 73, 413–420. [Google Scholar] [CrossRef]
- Mineo, P.; Barbera, V.; Romeo, G.; Ghezzo, F.; Scamporrino, E.; Spitaleri, F.; Chiacchio, U. Thermally reversible highly cross-linked polymeric materials based on furan/maleimide Diels-Alder adducts. J. Appl. Polym. Sci. 2015, 132, 42314. [Google Scholar] [CrossRef]
- Lyon, G.B.; Baranek, A.; Bowman, C.N. Scaffolded Thermally Remendable Hybrid Polymer Networks. Adv. Funct. Mater. 2016, 26, 1477–1485. [Google Scholar] [CrossRef]
- Diaz, M.M.; Van Assche, G.; Maurer, F.H.J.; Van Mele, B. Thermophysical characterization of a reversible dynamic polymer network based on kinetics and equilibrium of an amorphous furan-maleimide Diels-Alder cycloaddition. Polymer 2017, 120, 176–188. [Google Scholar] [CrossRef]
- Diaz, M.M.; Brancart, J.; Van Assche, G.; Van Mele, B. Room-temperature versus heating-mediated healing of a Diels-Alder crosslinked polymer network. Polymer 2018, 153, 453–463. [Google Scholar] [CrossRef]
- Cuvellier, A.; Verhelle, R.; Brancart, J.; Vanderborght, B.; Van Assche, G.; Rahier, H. The influence of stereochemistry on the reactivity of the Diels-Alder cycloaddition and the implications for reversible network polymerization. Polym. Chem. 2019, 10, 473–485. [Google Scholar] [CrossRef]
- Mangialetto, J.; Cuvellier, A.; Verhelle, R.; Brancart, J.; Rahier, H.; Van Assche, G.; Van den Brande, N.; Van Mele, B. Diffusion- and Mobility-Controlled Self-Healing Polymer Networks with Dynamic Covalent Bonding. Macromolecules 2019, 52, 8440–8452. [Google Scholar] [CrossRef]
- Ehrhardt, D.; Mangialetto, J.; Bertouille, J.; Van Durme, K.; Van Mele, B.; Van den Brande, N. Self-healing in mobility-restricted conditions maintaining mechanical robustness: Furan-maleimide Diels-Alder cycloadditions in polymer networks for ambient applications. Polymers 2020, 12, 2543. [Google Scholar] [CrossRef]
- Mangialetto, J.; Verhelle, R.; Van Assche, G.; Van den Brande, N.; Van Mele, B. Time-Temperature-Transformation, Temperature-Conversion-Transformation, and Continuous-Heating-Transformation Diagrams of Reversible Covalent Polymer Networks. Macromolecules 2021, 54, 412–425. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Scheltjens, G.; Brancart, J.; De Graeve, I.; Van Mele, B.; Terryn, H.; Van Assche, G. Self-healing property characterization of reversible thermoset coatings. J. Therm. Anal. Calorim. 2011, 105, 805–809. [Google Scholar] [CrossRef]
- Ehrhardt, D.; Van Durme, K.; Jansen, J.F.G.A.; Van Mele, B.; Van den Brande, N. Self-healing UV-curable polymer network with reversible Diels-Alder bonds for applications in ambient conditions. Polymer 2020, 203, 122762. [Google Scholar] [CrossRef]
- Ehrhardt, D.; Mangialetto, J.; Van Durme, K.; Van Mele, B.; Van den Brande, N. From Slow to Fast Self-Healing at Ambient Temperature of High-Modulus Reversible Poly(methacrylate) Networks. Single- and Dual-Dynamics and the Effect of Phase-Separation. Macromolecules 2021, 54, 9960–9977. [Google Scholar] [CrossRef]
- Terryn, S.; Brancart, J.; Roels, E.; Van Assche, G.; Vanderborght, B. Room Temperature Self-Healing in Soft Pneumatic Robotics: Autonomous Self-Healing in a Diels-Alder Polymer Network. IEEE Robot. Autom. Mag. 2020, 27, 44–55. [Google Scholar] [CrossRef]
- Terryn, S.; Langenbach, J.; Roels, E.; Brancart, J.; Bakkali-Hassani, C.; Poutrel, Q.A.; Georgopoulou, A.; George Thuruthel, T.; Safaei, A.; Ferrentino, P.; et al. A review on self-healing polymers for soft robotics. Mater. Today 2021, 47, 187–205. [Google Scholar] [CrossRef]
- Roels, E.; Terryn, S.; Brancart, J.; Verhelle, R.; Van Assche, G.; Vanderborght, B. Additive Manufacturing for Self-Healing Soft Robots. Soft Robot. 2020, 7, 711–723. [Google Scholar] [CrossRef]
- Rawal, V.H.; Thadani, A.N.; Unni, A.K.; Huang, Y. Single enantiomers from a chiral-alcohol catalyst. Nature 2003, 424, 146. [Google Scholar]
- Wittkopp, A.; Schreiner, P.R. Metal-free, noncovalent catalysis of Diels-Alder reactions by Neutral hydrogen bond donors in organic solvents and in water. Chem.-A Eur. J. 2003, 9, 407–414. [Google Scholar] [CrossRef]
- Tan, B.; Hernández-Torres, G.; Barbas, C.F. Highly efficient hydrogen-bonding catalysis of the diels-alder reaction of 3-vinylindoles and methyleneindolinones provides carbazolespirooxindole skeletons. J. Am. Chem. Soc. 2011, 133, 12354–12357. [Google Scholar] [CrossRef]
- Yan, C.X.; Yang, F.; Yang, X.; Zhou, D.G.; Zhou, P.P. Insights into the Diels-Alder Reaction between 3-Vinylindoles and Methyleneindolinone without and with the Assistance of Hydrogen-Bonding Catalyst Bisthiourea: Mechanism, Origin of Stereoselectivity, and Role of Catalyst. J. Org. Chem. 2017, 82, 3046–3061. [Google Scholar] [CrossRef] [PubMed]
- Vermeeren, P.; Hamlin, T.A.; Bickelhaupt, F.M.; Fernández, I. Bifunctional Hydrogen Bond Donor-Catalyzed Diels–Alder Reactions: Origin of Stereoselectivity and Rate Enhancement. Chem.-A Eur. J. 2021, 27, 5180–5190. [Google Scholar] [CrossRef] [PubMed]
- Houk, K.N. Frontier molecular orbital theory of cycloaddition reactions. Acc. Chem. Res. 1975, 8, 361–369. [Google Scholar] [CrossRef]
- Fleming, J. Frontier Orbitals and Organic Chemical Reactions; Wiley: New York, NY, USA, 1976. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision A.03; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dunning, T.H. Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys. 1989, 90, 1007–1023. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.Y.; Shi, J.; Guo, Q.X. Accurate prediction of rate constants of Diels-Alder reactions and application to design of Diels-Alder ligation. Org. Biomol. Chem. 2012, 10, 2673–2682. [Google Scholar] [CrossRef]
- Levandowski, B.J.; Houk, K.N. Theoretical analysis of reactivity patterns in Diels-Alder reactions of cyclopentadiene, cyclohexadiene, and cycloheptadiene with symmetrical and unsymmetrical dienophiles. J. Org. Chem. 2015, 80, 3530–3537. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Liang, Y.; Liu, F.; Houk, K.N. Diels-Alder Reactivities of Benzene, Pyridine, and Di-, Tri-, and Tetrazines: The Roles of Geometrical Distortions and Orbital Interactions. J. Am. Chem. Soc. 2016, 138, 1660–1667. [Google Scholar] [CrossRef]
- Zhang, C.; Jiao, H.; Jia, W. Theoretical predication of Diels-Alder reactions of highly strained dienophiles. Comput. Theor. Chem. 2020, 1175, 112734. [Google Scholar] [CrossRef]
- Jungbauer, S.H.; Walter, S.M.; Schindler, S.; Rout, L.; Kniep, F.; Huber, S.M. Activation of a carbonyl compound by halogen bonding. Chem. Commun. 2014, 50, 6281–6284. [Google Scholar] [CrossRef]
- Kee, C.W.; Wong, M.W. In Silico Design of Halogen-Bonding-Based Organocatalyst for Diels-Alder Reaction, Claisen Rearrangement, and Cope-Type Hydroamination. J. Org. Chem. 2016, 81, 7459–7470. [Google Scholar] [CrossRef] [PubMed]
- Nziko, V.D.P.N.; Scheiner, S. Catalysis of the Aza-Diels-Alder Reaction by Hydrogen and Halogen Bonds. J. Org. Chem. 2016, 81, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feller, D. Application of systematic sequences of wave functions to the water dimer. J. Chem. Phys. 1992, 96, 6104–6114. [Google Scholar] [CrossRef]
- Feller, D. The use of systematic sequences of wave functions for estimating the complete basis set, full configuration interaction limit in water. J. Chem. Phys. 1993, 98, 7059–7071. [Google Scholar] [CrossRef]
- Johnson, E.R.; Mori-Sánchez, P.; Cohen, A.J.; Yang, W. Delocalization errors in density functionals and implications for main-group thermochemistry. J. Chem. Phys. 2008, 129. [Google Scholar] [CrossRef] [Green Version]
- Takeda, Y.; Hisakuni, D.; Lin, C.H.; Minakata, S. 2-Halogenoimidazolium Salt Catalyzed Aza-Diels-Alder Reaction Through Halogen-Bond Formation. Org. Lett. 2015, 17, 318–321. [Google Scholar] [CrossRef]
- Yaghoobi, F.; Sohrabi-Mahboub, M. Theoretical Study on the Aza-Diels-Alder Reaction Catalyzed by PHCl2 Lewis Acid via Pnicogen Bonding. J. Phys. Chem. A 2018, 122, 2781–2791. [Google Scholar] [CrossRef]
- Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S.M. Iodine(III) Derivatives as Halogen Bonding Organocatalysts. Angew. Chem. Int. Ed. 2018, 57, 3830–3833. [Google Scholar] [CrossRef]
- Vermeeren, P.; Hamlin, T.A.; Fernández, I.; Bickelhaupt, F.M. Origin of rate enhancement and asynchronicity in iminium catalyzed Diels-Alder reactions. Chem. Sci. 2020, 11, 8105–8112. [Google Scholar] [CrossRef]
- Vermeeren, P.; Hamlin, T.A.; Fernández, I.; Bickelhaupt, F.M. How Lewis Acids Catalyze Diels–Alder Reactions. Angew. Chemie Int. Ed. 2020, 59, 6201–6206. [Google Scholar] [CrossRef] [PubMed]
- Hajgató, B.; Deleuze, M.S.; Tozer, D.J.; De Proft, F. A benchmark theoretical study of the electron affinities of benzene and linear acenes. J. Chem. Phys. 2008, 129, 084308. [Google Scholar] [CrossRef] [PubMed]
- Nobeli, I.; Price, S.L.; Lommerse, J.P.M.; Taylor, R. Hydrogen bonding properties of oxygen and nitrogen acceptors in aromatic heterocycles. J. Comput. Chem. 1997, 18, 2060–2074. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing noncovalent interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [Green Version]
- Contreras-garcía, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef]
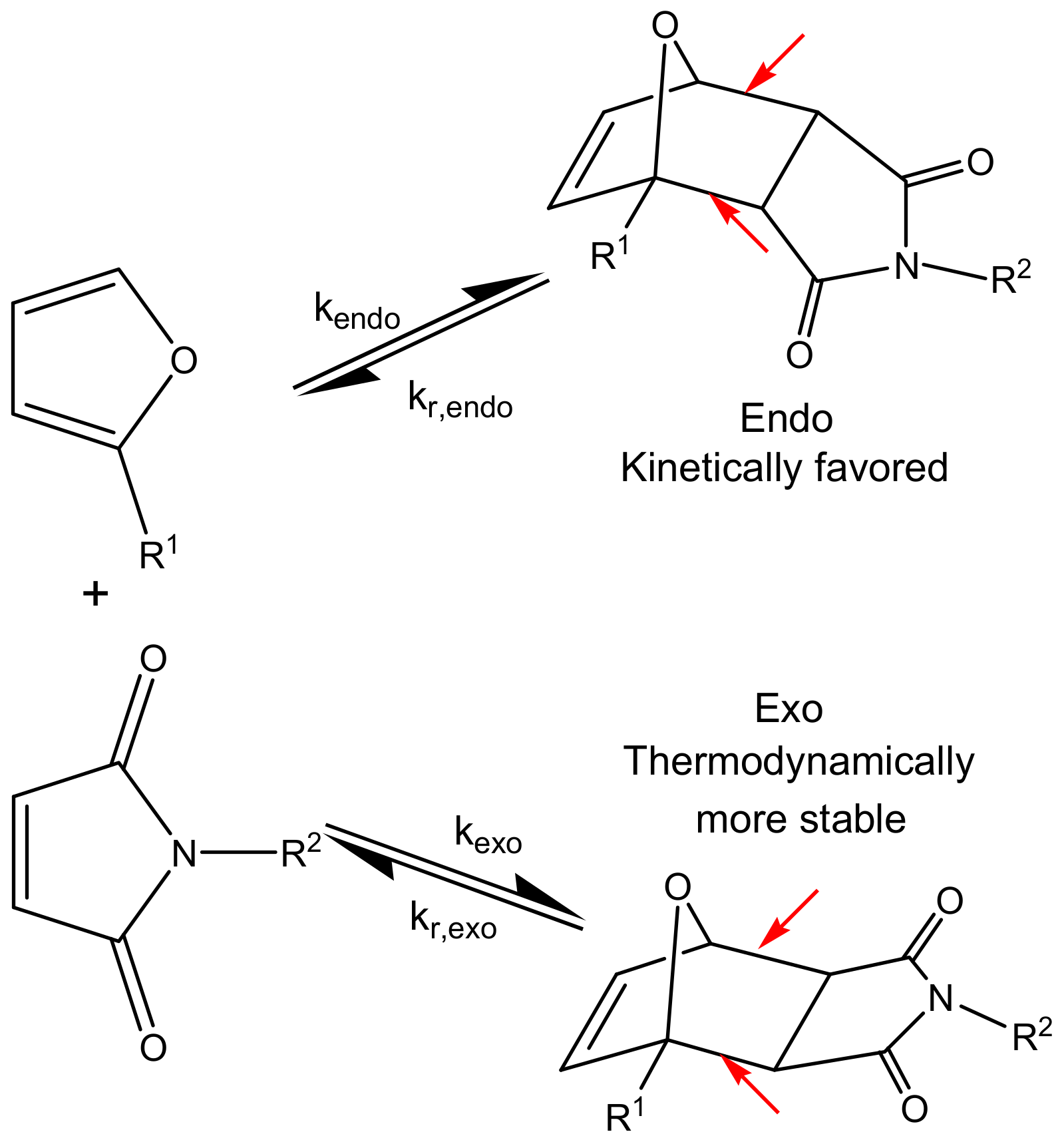
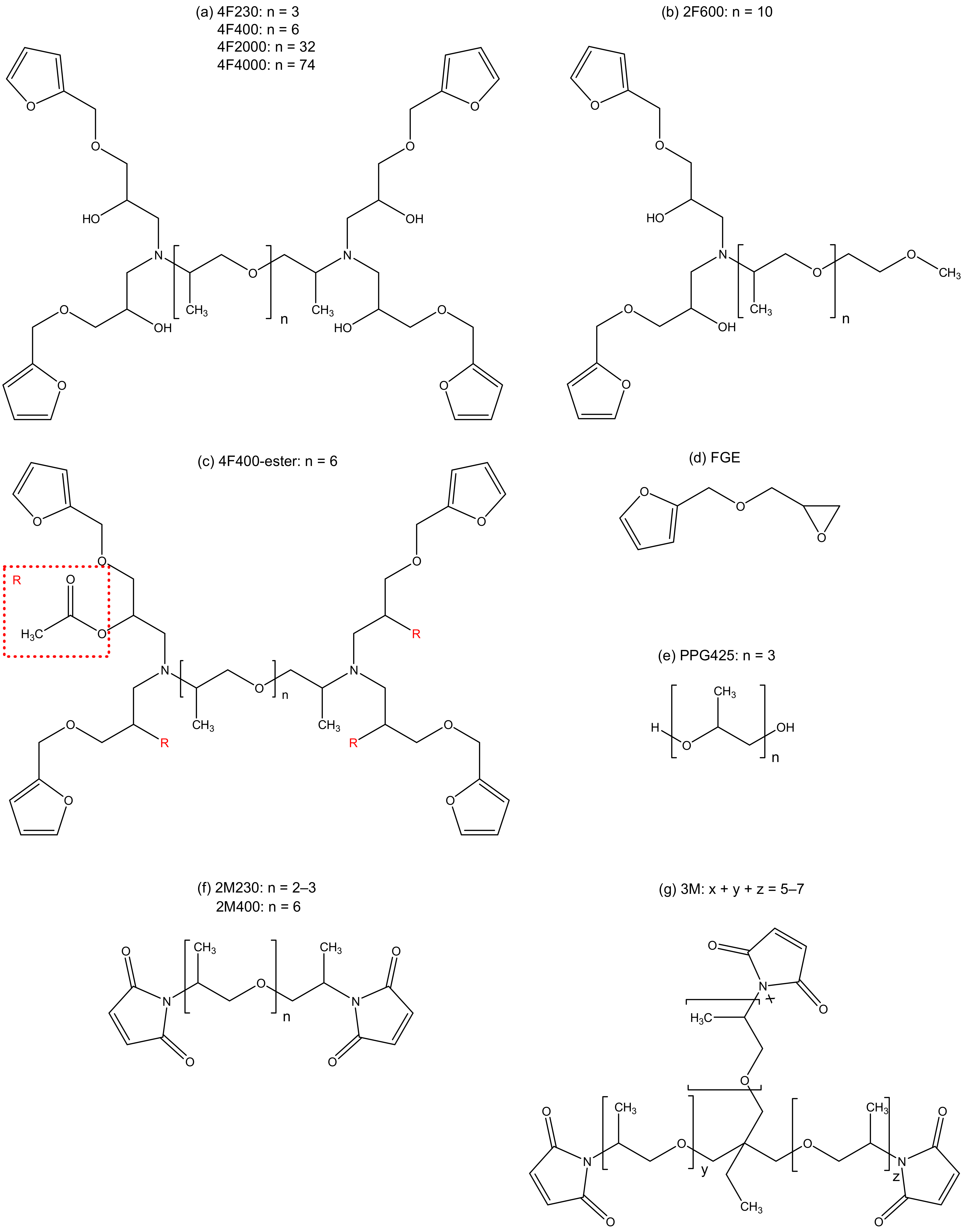
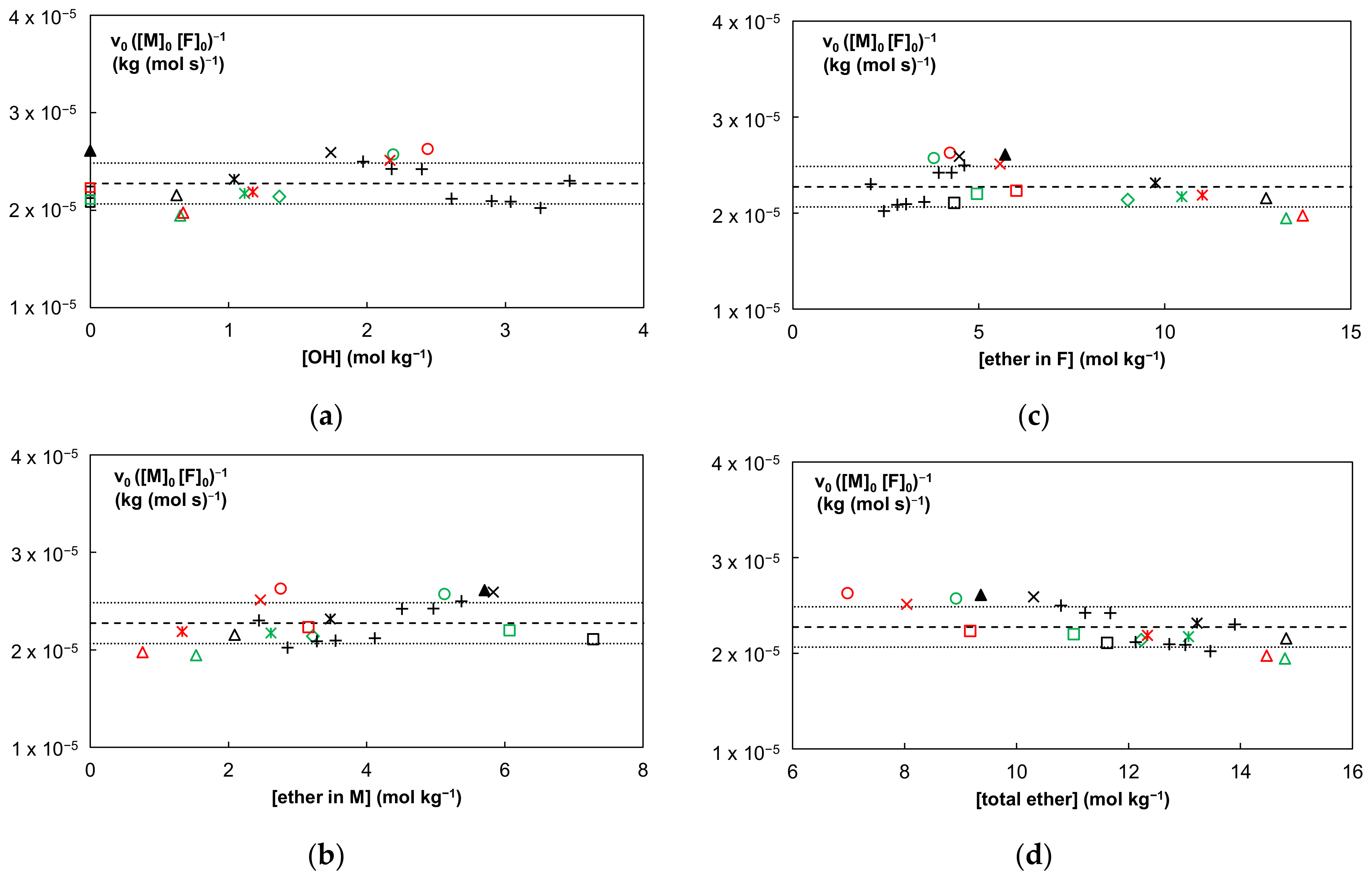
| Compound | 2M230 | 2M400 | 3M |
|---|---|---|---|
| FGE | □ | □ | □ |
| 2F600 | ◇ | ||
| 4F230 | ○ | ○ | |
| 4F400 | ✕ | ✕ | |
| 4F2000 | 🞵 | 🞵 | 🞵 |
| 4F4000 | △ | △ | △ |
| 4F400 + PPG425 | + | ||
| 4F400-ester | ▲ |
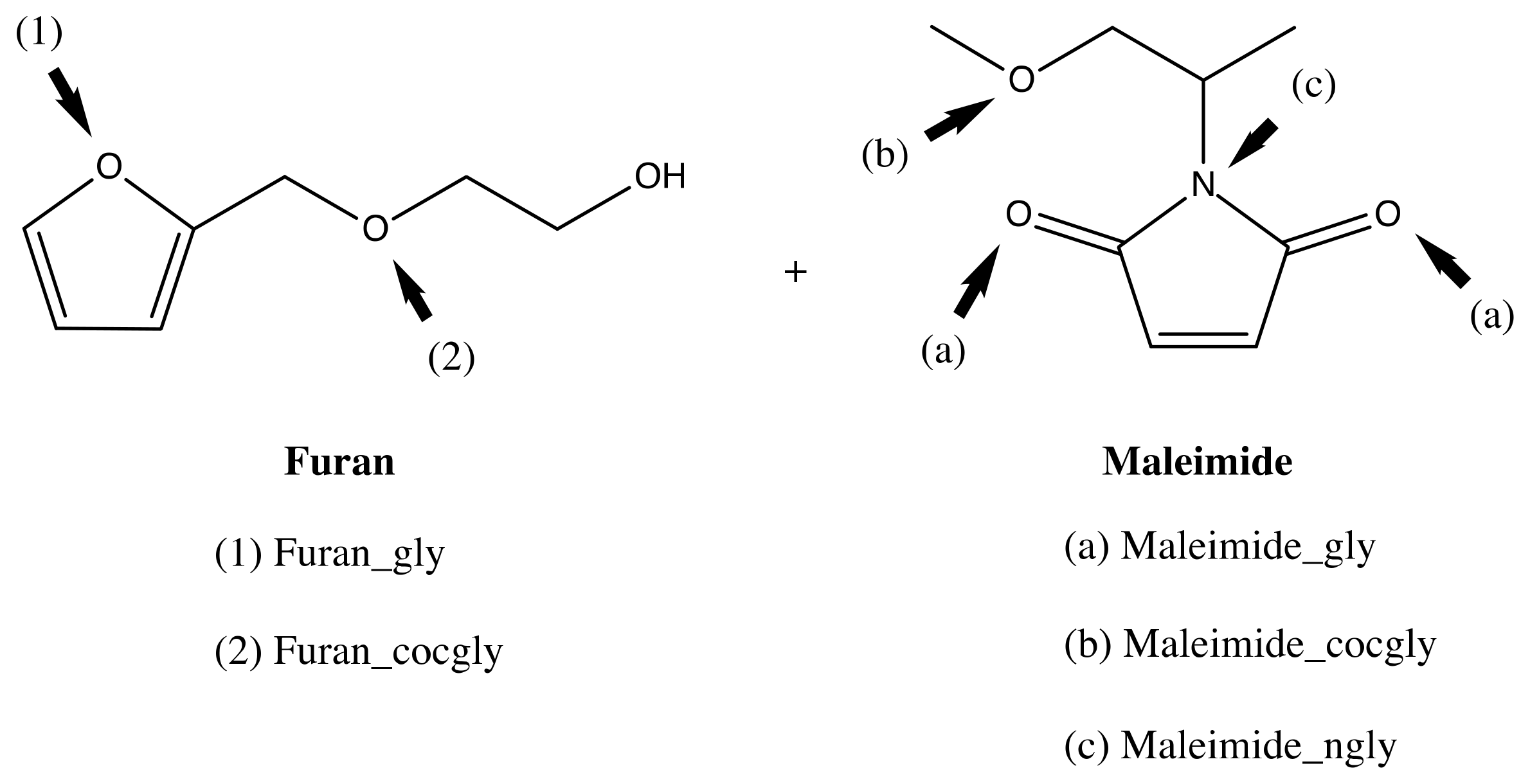
| Furan | Maleimide | Normal Demand | Inverse Demand |
|---|---|---|---|
| M(L) − F(H) | F(L) − M(H) | ||
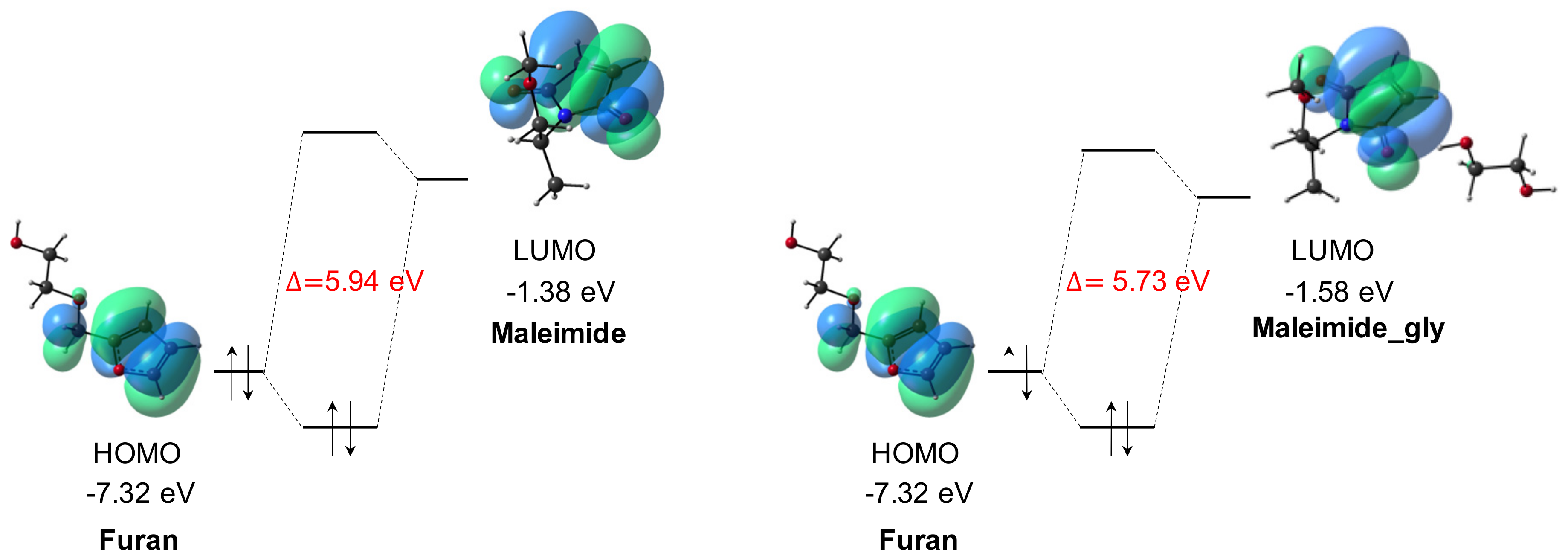
| Furan | Maleimide | 5.94 | 10.42 |
| Furan_IHB | Maleimide | 6.52 | 9.76 |
| Furan_gly | Maleimide | 6.22 | 10.06 |
| Furan_cocgly | Maleimide | 6.12 | 10.18 |
| Furan | Maleimide_gly | 5.73 | 10.84 |
| Furan_IHB | Maleimide_gly | 6.31 | 10.18 |
| Furan | Maleimide_cocgly | 6.07 | 10.48 |
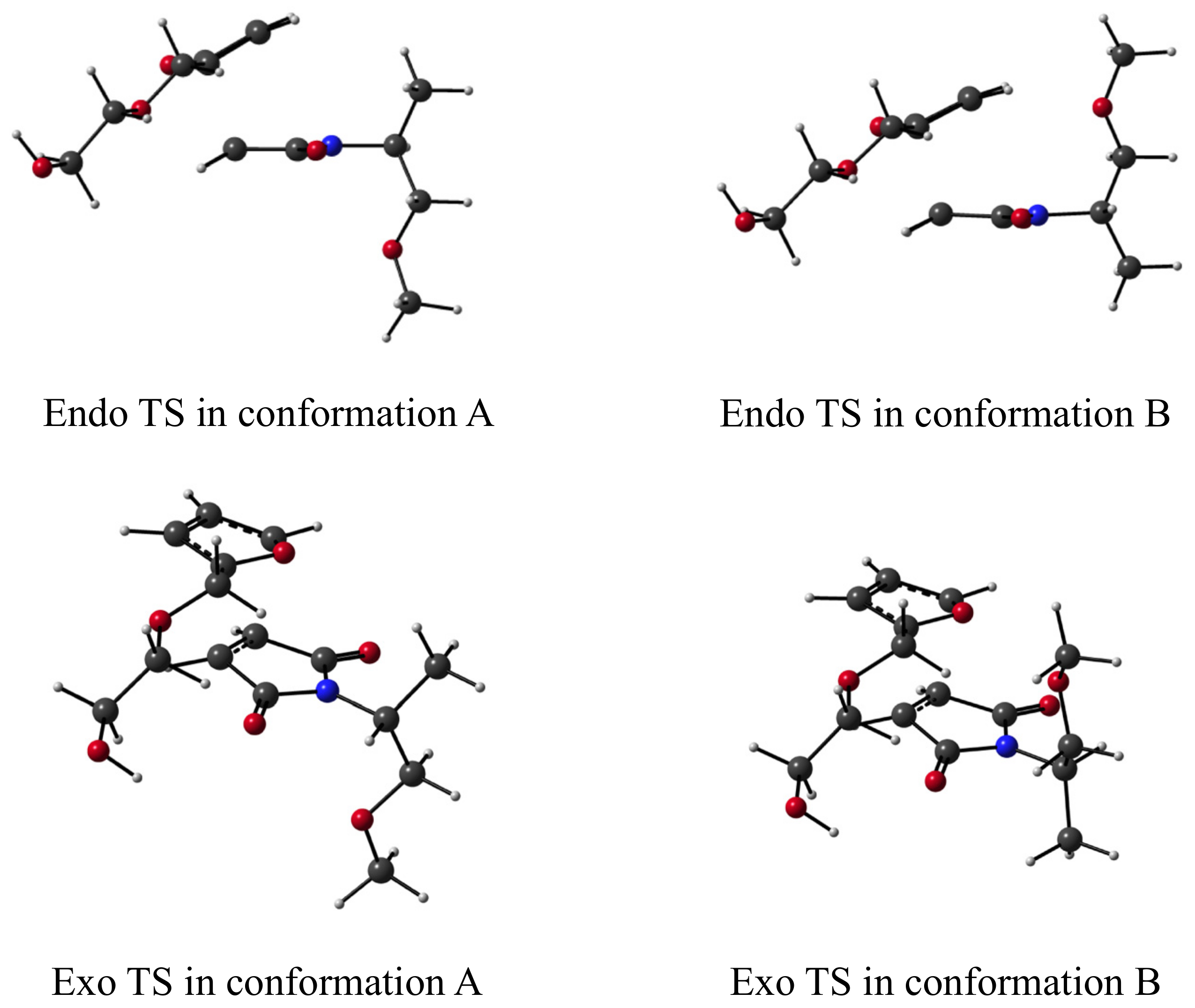
| HB? | Endo/Exo | R1_gly + R2 | RC | TS | P_gly | P (+ gly) | TS − RC | TS − P(_gly) |
|---|---|---|---|---|---|---|---|---|
| no | Endo a | - | −7.6 | 11.9 | - | −14.9 | 19.4 | 26.7 |
| Endo b | - | −8.4 | 10.3 | - | −15.3 | 18.6 | 25.6 | |
| Exo a | - | −8.4 | 11.6 | - | −17.0 | 20.0 | 28.6 | |
| Exo b | - | −9.6 | 10.2 | - | −16.9 | 19.8 | 27.1 | |
| M[C=O] + F[-OH] | Endo a | - | −11.8 | 6.5 | - | −18.8 | 18.4 | 25.3 |
| Endo b | - | −13.9 | 3.9 | - | −20.4 | 17.8 | 24.2 | |
| Exo a | - | −11.9 | 7.2 | - | −21.3 | 19.1 | 28.5 | |
| Exo b | - | −13.5 | 5.4 | - | −21.7 | 18.9 | 27.1 | |
| F[(O)] + glycol | Endo a | −7.8 d | −15.8 d | 7.7 | −20.7 | −14.5 | 23.6 d | 28.4 |
| (Furan_gly) | Endo b | −7.5 d | −17.0 d | 6.1 | −21.1 | −14.8 | 23.2 d | 27.2 |
| Exo a | −5.4 | −13.0 | 8.7 | −22.6 | −16.4 | 21.7 | 31.4 | |
| Exo b,c | −4.7 | −14.8 | 4.8 | −23.1 | −15.9 | 19.6 | 28.0 | |
| F[-O-] + glycol | Endo a | −5.9 | −13.9 | 5.6 | −21.9 | −14.9 | 19.5 | 27.5 |
| (Furan_cocgly) | Endo b | −5.9 | −16.5 | 3.8 | −22.7 | −15.3 | 20.4 | 26.5 |
| Exo a | −5.4 | −15.0 | 5.2 | −23.4 | −17.0 | 20.1 | 28.5 | |
| Exo b | −5.4 | −16.6 | 4.5 | −22.8 | −16.4 | 21.1 | 27.2 | |
| M[C=O] + glycol | Endo a | −6.7 | −15.2 | 2.7 | −22.3 | −14.9 | 18.0 | 25.0 |
| (Maleimide_gly) | Endo b | −5.8 | −15.8 | 1.4 | −22.5 | −15.3 | 17.1 | 23.9 |
| Exo a | −7.1 | −15.2 | 3.0 | −24.5 | −16.9 | 18.2 | 27.5 | |
| Exo b | −5.7 | −16.2 | 1.9 | −24.2 | −16.9 | 18.1 | 26.1 | |
| M[-O-] + glycol | Endo a | −8.0 | −15.5 | 3.7 | −24.0 | −14.9 | 19.3 | 27.8 |
| (Maleimide_cocgly) | Endo b | −9.1 | −14.7 | 3.7 | −22.3 | −15.3 | 18.4 | 26.0 |
| Exo a | −8.3 | −16.7 | 3.1 | −26.5 | −16.9 | 19.7 | 29.5 | |
| Exo b | −8.3 | −16.8 | 2.4 | −25.2 | −16.4 | 19.2 | 27.6 |
| System | RC | TS | P |
|---|---|---|---|
| Endo a | 1.923 | 1.873 | 1.904 |
| Endo b | 1.913 | 1.877 | 1.901 |
| Exo a | 1.874 | 1.855 | 1.861 |
| Exo b | 1.859 | 1.815 | 1.826 |
| HB? | Endo/Exo | RC | TS | P | TS − RC | TS − P |
|---|---|---|---|---|---|---|
| no | Endo a | −7.0 | 12.7 | −11.7 | 19.6 | 24.3 |
| Endo b | −6.8 | 11.4 | −12.2 | 18.2 | 23.6 | |
| Exo a | −7.3 | 13.1 | −13.3 | 20.3 | 26.4 | |
| Exo b | −8.1 | 12.0 | −13.4 | 20.1 | 25.4 |
| Exp. System | Rel. kDA | Comp. System | Rel. kDA | Diff. in Comp. ΔG‡ |
|---|---|---|---|---|
| No HB | 1.0 | |||
| <Model Series> | 1.0 | Poss. HB from Furan | 1.0 | 0.0 |
| <4F400-2M400 + PPG425> | 1.0 | Glycol additive | 3.5 | −0.7 |
| 4F400-ester-2M400 | 1.1 | Ester | 0.7 | +0.3 |
| <FGE> (OH free) | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mangialetto, J.; Gorissen, K.; Vermeersch, L.; Van Mele, B.; Van den Brande, N.; De Vleeschouwer, F. Hydrogen-Bond-Assisted Diels–Alder Kinetics or Self-Healing in Reversible Polymer Networks? A Combined Experimental and Theoretical Study. Molecules 2022, 27, 1961. https://doi.org/10.3390/molecules27061961
Mangialetto J, Gorissen K, Vermeersch L, Van Mele B, Van den Brande N, De Vleeschouwer F. Hydrogen-Bond-Assisted Diels–Alder Kinetics or Self-Healing in Reversible Polymer Networks? A Combined Experimental and Theoretical Study. Molecules. 2022; 27(6):1961. https://doi.org/10.3390/molecules27061961
Chicago/Turabian StyleMangialetto, Jessica, Kiano Gorissen, Lise Vermeersch, Bruno Van Mele, Niko Van den Brande, and Freija De Vleeschouwer. 2022. "Hydrogen-Bond-Assisted Diels–Alder Kinetics or Self-Healing in Reversible Polymer Networks? A Combined Experimental and Theoretical Study" Molecules 27, no. 6: 1961. https://doi.org/10.3390/molecules27061961
APA StyleMangialetto, J., Gorissen, K., Vermeersch, L., Van Mele, B., Van den Brande, N., & De Vleeschouwer, F. (2022). Hydrogen-Bond-Assisted Diels–Alder Kinetics or Self-Healing in Reversible Polymer Networks? A Combined Experimental and Theoretical Study. Molecules, 27(6), 1961. https://doi.org/10.3390/molecules27061961







