A Comprehensive Computational Investigation into the Conserved Virulent Proteins of Shigella species Unveils Potential Small-Interfering RNA Candidates as a New Therapeutic Strategy against Shigellosis
Abstract
1. Introduction
2. Methods and Materials
2.1. Sequence Retrieval and Multiple Sequence Alignment for Determination of Conserved Regions
2.2. Recognition of Target Sequence and Designing of Potential siRNA Candidates
2.3. Determination of Off-Target Similarity
2.4. Prediction of Free Energy of Folding and Calculation of GC Content
2.5. Evaluation of the Thermodynamics Involved in the Formation of Secondary Structure of siRNA and Target and Visualization of siRNA-Target Binding
2.6. Determination of Heat Stability and Prediction of Functional Efficiency and Target Accessibility of the Potential siRNA Candidates
2.7. Validation of the Functional Efficiency of the siRNA Candidates
2.8. Prediction of Immunotoxicity of the Predicted siRNA Candidates
2.9. Designing of Tertiary (3D) Structure of the siRNA Candidates and Validation
3. Results
3.1. Retrieval of Nucleotide Sequences of Conserved Virulent Proteins of Shigella sp.
3.2. Designing siRNA Candidates by Using a Combination of First-Generation and Second-Generation Algorithms
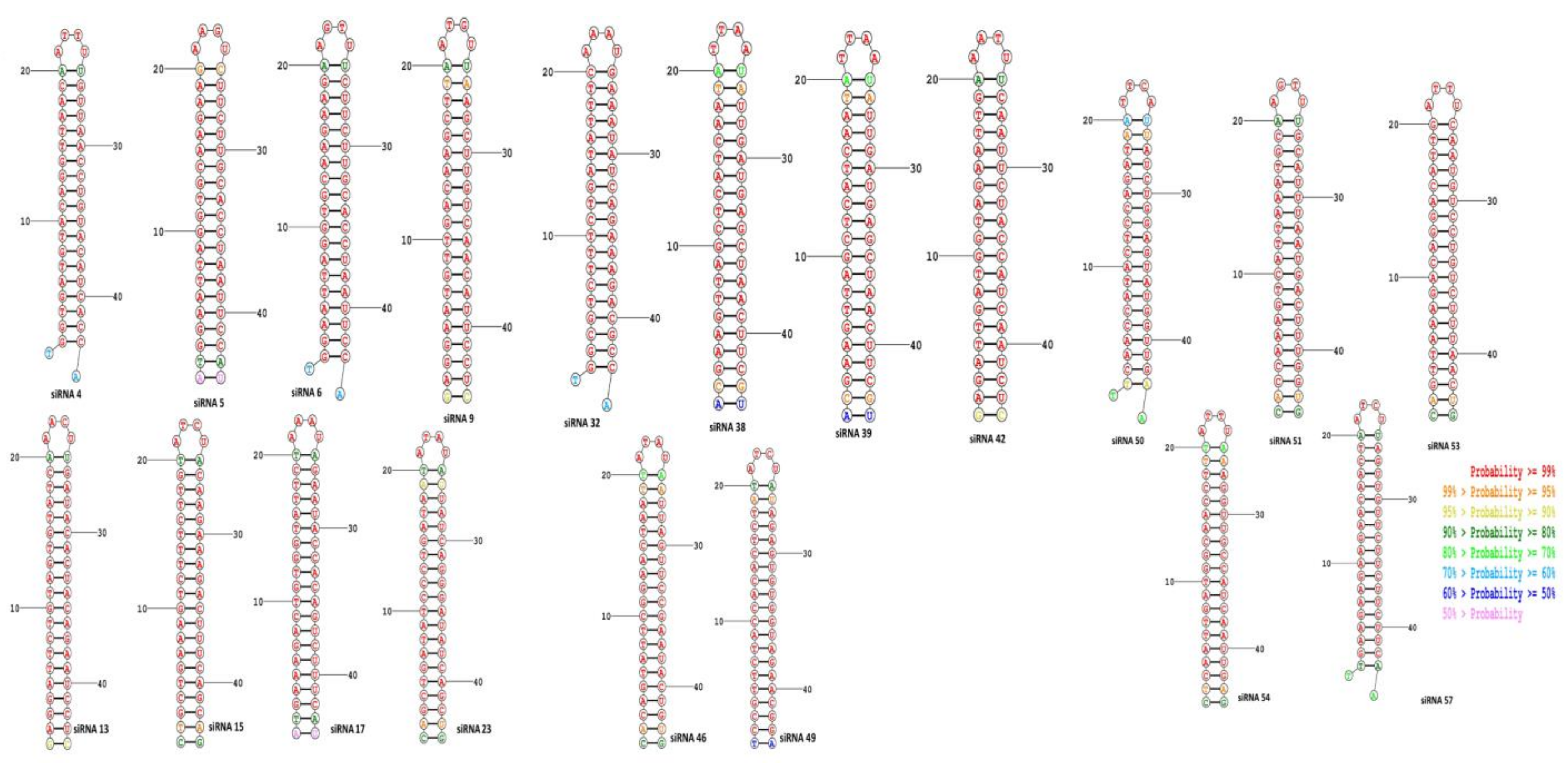
3.3. Evaluation of Binding Energy and Visualization of Secondary Structures of siRNA-Target Duplex
3.4. Determination of Heat Stability of siRNA–Target Duplex
3.5. Prediction of Functional Efficiency and Target Accessibility of the siRNA Candidates
3.6. Validation of the Functional Efficiency of the siRNA Candidates
3.7. Prediction of Immunotoxicity of the siRNA Candidates
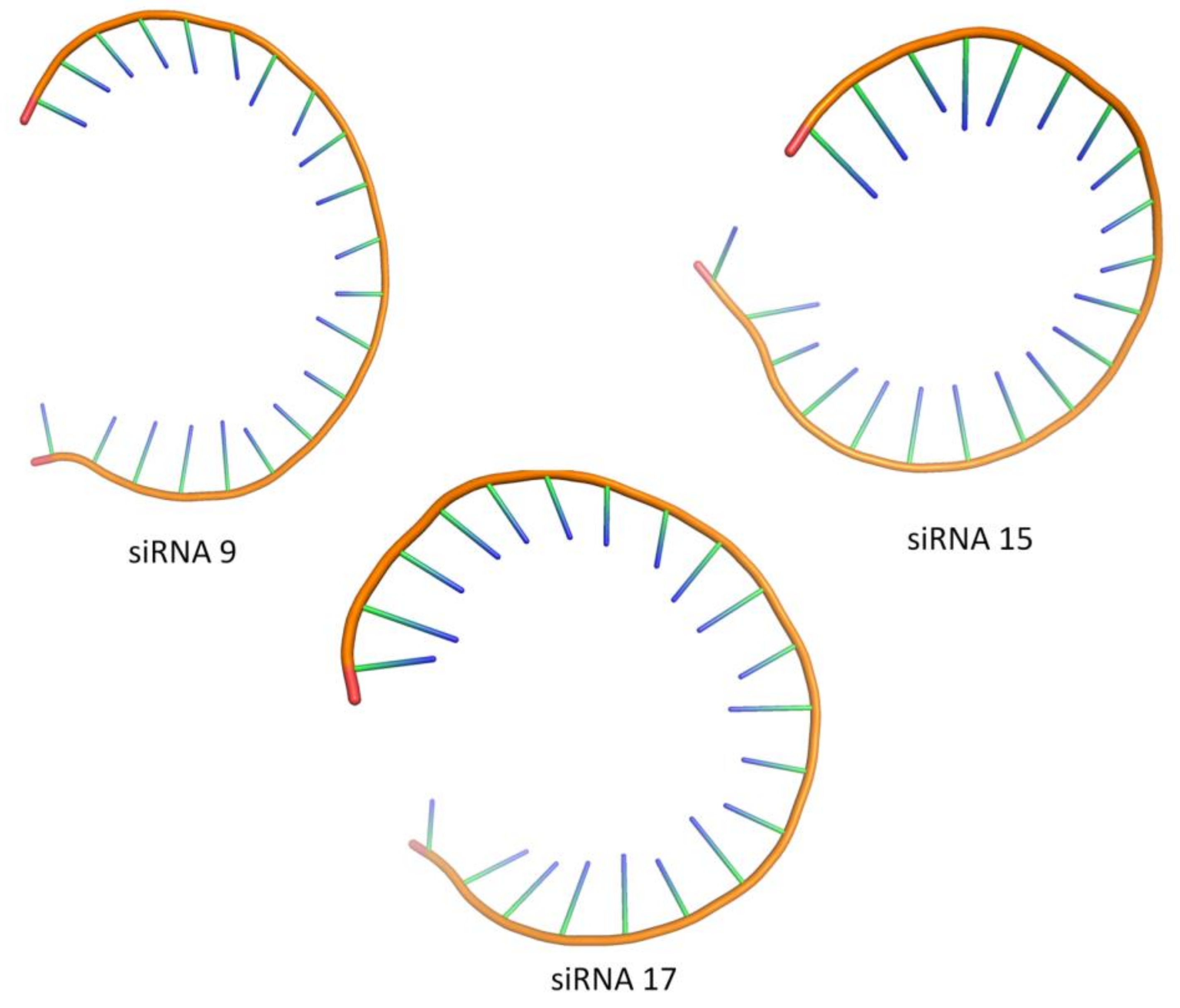
3.8. Designing of Tertiary Structure of the siRNA Candidates and Validation
4. Discussion
5. Limitations of the Study
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C., Jr.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Baker, S.; The, H.C. Recent insights into Shigella: A major contributor to the global diarrhoeal disease burden. Curr. Opin. Infect. Dis. 2018, 31, 449. [Google Scholar] [CrossRef] [PubMed]
- Thompson, C.N.; Duy, P.T.; Baker, S. The rising dominance of Shigella sonnei: An intercontinental shift in the etiology of bacillary dysentery. PLoS Negl. Trop. Dis. 2015, 9, e0003708. [Google Scholar] [CrossRef] [PubMed]
- Vinh, H.; Nhu, N.T.K.; Nga, T.V.T.; Duy, P.T.; Campbell, J.I.; Hoang, N.V.M.; Boni, M.F.; My, P.V.T.; Parry, C.; Van Minh, P.; et al. A changing picture of shigellosis in southern Vietnam: Shifting species dominance, antimicrobial susceptibility and clinical presentation. BMC Infect. Dis. 2009, 9, 204. [Google Scholar] [CrossRef] [PubMed]
- Bangtrakulnonth, A.; Vieira, A.R.; Lo Fo Wong, D.M.; Pornreongwong, S.; Pulsrikarn, C.; Sawanpanyalert, P.; Hendriksen, R.S.; Aarestrup, F.M. Shigella from humans in Thailand during 1993 to 2006: Spatial-time trends in species and serotype distribution. Foodborne Pathog. Dis. 2008, 5, 773–784. [Google Scholar] [CrossRef]
- Ud-Din, A.I.; Wahid, S.U.; Latif, H.A.; Shahnaij, M.; Akter, M.; Azmi, I.J.; Hasan, T.N.; Ahmed, D.; Hossain, M.A.; Faruque, A.S.G.; et al. Changing trends in the prevalence of Shigella species: Emergence of multi-drug resistant Shigella sonnei biotype g in Bangladesh. PLoS ONE 2013, 8, e82601. [Google Scholar] [CrossRef] [PubMed]
- Riddle, M.S. Is a Shigella vaccine needed for travellers and the military? J. Travel Med. 2018, 25, tay049. [Google Scholar] [CrossRef]
- Klontz, K.C.; Singh, N. Treatment of drug-resistant Shigella infections. Expert Rev. Anti-Infect. Ther. 2015, 13, 69–80. [Google Scholar] [CrossRef]
- Murphy, G.S.; Bodhidatta, L.; Echeverria, P.; Tansuphaswadikul, S.; Hoge, C.W.; Imlarp, S.; Tamura, K. Ciprofloxacin and loperamide in the treatment of bacillary dysentery. Ann. Intern. Med. 1993, 118, 582–586. [Google Scholar] [CrossRef]
- Darton, T.C.; Tuyen, H.T.; Newton, P.N.; Dance, D.A.; Phetsouvanh, R.; Davong, V.; Campbell, J.I.; Hoang, N.V.M.; Thwaites, G.E.; Parry, C.M.; et al. Azithromycin resistance in Shigella spp. in Southeast Asia. Antimicrob. Agents Chemother. 2018, 62, e01748–e01817. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.C.; Berkley, J.A. Guidelines for the treatment of dysentery (shigellosis): A systematic review of the evidence. Paediatr. Int. Child Health 2018, 38 (Suppl. 1), S50–S65. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Brown, J.D.; Willcox, S.J.; Franklin, N.; Hazelton, B.; Howard, P.; Reinten, T.; Sheppeard, V.; O’Sullivan, M. Shigella species epidemiology and antimicrobial susceptibility: The implications of emerging azithromycin resistance for guiding treatment, guidelines and breakpoints. J. Antimicrob. Chemother. 2017, 72, 3181–3186. [Google Scholar] [CrossRef]
- Camacho, A.I.; Irache, J.M.; Gamazo, C. Recent progress towards development of a Shigella vaccine. Expert Rev. Vaccines 2013, 12, 43–55. [Google Scholar] [CrossRef]
- Giersing, B.K.; Porter, C.K.; Kotloff, K.; Neels, P.; Cravioto, A.; MacLennan, C.A. How can controlled human infection models accelerate clinical development and policy pathways for vaccines against Shigella? Vaccine 2019, 37, 4778–4783. [Google Scholar] [CrossRef] [PubMed]
- Livio, S.; Strockbine, N.A.; Panchalingam, S.; Tennant, S.M.; Barry, E.M.; Marohn, M.E.; Antonio, M.; Hossain, A.; Mandomando, I.; Ochieng, J.B.; et al. Shigella isolates from the global enteric multicenter study inform vaccine development. Clin. Infect. Dis. 2014, 59, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.N.; Trofa, A.; Sadoff, J.; Chu, C.; Bryla, D.; Shiloach, J.; Cohen, D.; Ashkenazi, S.; Lerman, Y.; Egan, W. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect. Immun. 1993, 61, 3678–3687. [Google Scholar] [CrossRef] [PubMed]
- Ravenscroft, N.; Braun, M.; Schneider, J.; Dreyer, A.M.; Wetter, M.; Haeuptle, M.A.; Kemmler, S.; Steffen, M.; Sirena, D.; Herwig, S.; et al. Characterization and immunogenicity of a Shigella flexneri 2a O-antigen bioconjugate vaccine candidate. Glycobiology 2019, 29, 669–680. [Google Scholar] [CrossRef]
- Cohen, D.; Atsmon, J.; Artaud, C.; Meron-Sudai, S.; Gougeon, M.-L.; Bialik, A.; Goren, S.; Asato, V.; Ariel-Cohen, O.; Reizis, A.; et al. Safety and immunogenicity of a synthetic carbohydrate conjugate vaccine against Shigella flexneri 2a in healthy adult volunteers: A phase 1, dose-escalating, single-blind, randomised, placebo-controlled study. Lancet Infect. Dis. 2020, 21, 546–558. [Google Scholar] [CrossRef]
- Kotloff, K.L.; Taylor, D.N.; Sztein, M.B.; Wasserman, S.S.; Losonsky, G.A.; Nataro, J.P.; Venkatesan, M.; Hartman, A.; Picking, W.D.; Katz, D.E.; et al. Phase I evaluation of ΔvirG Shigella sonnei live, attenuated, oral vaccine strain WRSS1 in healthy adults. Infect. Immun. 2002, 70, 2016–2021. [Google Scholar] [CrossRef][Green Version]
- Barry, E.M.; Pasetti, M.F.; Sztein, M.B.; Fasano, A.; Kotloff, K.L.; Levine, M.M. Progress and pitfalls in Shigella vaccine research. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 245. [Google Scholar] [CrossRef]
- Germane, K.L.; Ohi, R.; Goldberg, M.B.; Spiller, B.W. Structural and functional studies indicate that Shigella VirA is not a protease and does not directly destabilize microtubules. Biochemistry. 2008, 47, 10241–10243. [Google Scholar] [CrossRef][Green Version]
- Campbell-Valois, F.-X.; Sachse, M.; Sansonetti, P.J.; Parsot, C. Escape of actively secreting Shigella flexneri from ATG8/LC3-positive vacuoles formed during cell-to-cell spread is facilitated by IcsB and VirA. MBio 2015, 6, e02567–e02614. [Google Scholar] [CrossRef] [PubMed]
- Tobe, T.; Nagai, S.; Okada, N.; Adter, B.; Yoshikawa, M.; Sasakawa, C. Temperature-regulated expression of invasion genes in Shigella flexneri is controlled through the transcriptional activation of the virB gene on the large plasmid. Mol. Microbiol. 1991, 5, 887–893. [Google Scholar] [CrossRef]
- Emanuele, A.A.; Adams, N.E.; Chen, Y.-C.; Maurelli, A.T.; Garcia, G.A. Potential novel antibiotics from HTS targeting the virulence-regulating transcription factor, VirF, from Shigella flexneri. J. Antibiot. 2014, 67, 379–386. [Google Scholar] [CrossRef]
- Phalipon, A.; Sansonetti, P.J. Shigella’s ways of manipulating the host intestinal innate and adaptive immune system: A tool box for survival? Immunology and cell biology. Immunol. Cell Biol. 2007, 85, 119–129. [Google Scholar] [CrossRef]
- Martinez-Becerra, F.J.; Kissmann, J.M.; Diaz-McNair, J.; Choudhari, S.P.; Quick, A.M.; Mellado-Sanchez, G.; Clements, J.D.; Pasetti, M.; Picking, W.L. Broadly protective Shigella vaccine based on type III secretion apparatus proteins. Infect. Immun. 2012, 80, 1222–1231. [Google Scholar] [CrossRef]
- Martinez-Becerra, F.J.; Chen, X.; Dickenson, N.E.; Choudhari, S.P.; Harrison, K.; Clements, J.D.; Picking, W.D.; Van De Verg, L.L.; Walker, R.I.; Picking, W.L. Characterization of a novel fusion protein from IpaB and IpaD of Shigella spp. and its potential as a pan-Shigella vaccine. Infect. Immun. 2013, 81, 4470–4477. [Google Scholar] [CrossRef] [PubMed]
- Chitradevi, S.T.S.; Kaur, G.; Uppalapati, S.; Yadav, A.; Singh, D.; Bansal, A. Co-administration of rIpaB domain of Shigella with rGroEL of S. Typhi enhances the immune responses and protective efficacy against Shigella infection. Cell. Mol. Immunol. 2015, 12, 757–767. [Google Scholar] [CrossRef]
- Heine, S.J.; Diaz-McNair, J.; Martinez-Becerra, F.J.; Choudhari, S.P.; Clements, J.D.; Picking, W.L.; Pasetti, M.F. Evaluation of immunogenicity and protective efficacy of orally delivered Shigella type III secretion system proteins IpaB and IpaD. Vaccine 2013, 31, 2919–2929. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.; Suzuki, M.; Ohya, K.; Iwai, H.; Ishijima, N.; Koleske, A.J.; Fukui, Y.; Sasakawa, C. Shigella IpgB1 promotes bacterial entry through the ELMO–Dock180 machinery. Nat. Cell Biol. 2007, 9, 121–128. [Google Scholar] [CrossRef]
- Sun, C.H.; Wacquier, B.; Aguilar, D.I.; Carayol, N.; Denis, K.; Boucherie, S.; Valencia-Gallardo, C.; Simsek, C.; Erneux, C.; Lehman, A.; et al. The Shigella type III effector IpgD recodes Ca2+ signals during invasion of epithelial cells. EMBO J. 2017, 36, 2567–2580. [Google Scholar] [CrossRef]
- Niebuhr, K.; Jouihri, N.; Allaoui, A.; Gounon, P.; Sansonetti, P.J.; Parsot, C. IpgD, a protein secreted by the type III secretion machinery of Shigella flexneri, is chaperoned by IpgE and implicated in entry focus formation. Mol. Microbiol. 2000, 38, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Mattock, E.; Blocker, A.J. How do the virulence factors of Shigella work together to cause disease? Front. Cell. Infect. Microbiol. 2017, 7, 64. [Google Scholar] [CrossRef] [PubMed]
- Schuch, R.; Maurelli, A.T. Spa33, a cell surface-associated subunit of the Mxi-Spa type III secretory pathway of Shigella flexneri, regulates Ipa protein traffic. Infect. Immun. 2001, 69, 2180–2189. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Botteaux, A.; Sory, M.P.; Biskri, L.; Parsot, C.; Allaoui, A. MxiC is secreted by and controls the substrate specificity of the Shigella flexneri type III secretion apparatus. Mol. Microbiol. 2009, 71, 449–460. [Google Scholar] [CrossRef]
- Zurawski, D.V.; Mumy, K.L.; Faherty, C.S.; McCormick, B.A.; Maurelli, A.T. Shigella flexneri T3SS effectors OspB and OspF target the nucleus to down-regulate the host inflammatory response via interactions with retinoblastoma protein. Mol. Microbiol. 2009, 71, 350. [Google Scholar] [CrossRef]
- Kim, D.W.; Lenzen, G.; Page, A.-L.; Legrain, P.; Sansonetti, P.J.; Parsot, C. The Shigella flexneri effector OspG interferes with innate immune responses by targeting ubiquitin-conjugating enzymes. Proc. Natl. Acad. Sci. USA 2005, 102, 14046–14051. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Pei, Y.; Tuschl, T. On the art of identifying effective and specific siRNAs. Nat. Methods 2006, 3, 670–676. [Google Scholar] [CrossRef]
- Islam, M.O.; Palit, P.; Shawon, J.; Hasan, M.K.; Mahmud, A.; Mahfuz, M.; Ahmed, T.; Mondal, D. Exploring novel therapeutic strategies against vivax malaria through an integrated computational investigation to inhibit the merozoite surface protein− 1 of Plasmodium vivax. Inform. Med. Unlocked 2020, 21, 100471. [Google Scholar] [CrossRef]
- Chowdhury, F.T.; Shohan, M.U.; Islam, T.; Mimu, T.T.; Palit, P. A Therapeutic Approach Against Leishmania donovani by Predicting RNAi Molecules Against the Surface Protein, gp63. Curr. Bioinform. 2019, 14, 541–550. [Google Scholar] [CrossRef]
- Chowdhury, U.F.; Shohan, M.U.S.; Hoque, K.I.; Beg, M.A.; Siam, M.K.S.; Moni, M.A. A computational approach to design potential siRNA molecules as a prospective tool for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2. Genomics 2020, 113, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, P.S.; Sharma, S.; Naaz, F.; Kumar, D.; Raikwar, A.; Patil, S.A. Inhibition of Mycobacterium tuberculosis tRNA-Ligases Using siRNA-Based Gene Silencing Method: A Computational Approach. J. Comput. Biol. 2020, 27, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Thornbrough, J.M.; Hundley, T.; Valdivia, R.; Worley, M.J. Human genome-wide RNAi screen for host factors that modulate intracellular Salmonella growth. PLoS ONE 2012, 7, e38097. [Google Scholar] [CrossRef]
- Kühbacher, A.; Emmenlauer, M.; Rämo, P.; Kafai, N.; Dehio, C.; Cossart, P.; Pizarro-Cerdá, J. Genome-wide siRNA screen identifies complementary signaling pathways involved in Listeria infection and reveals different actin nucleation mechanisms during Listeria cell invasion and actin comet tail formation. MBio 2015, 6, e00598–e00615. [Google Scholar] [CrossRef]
- Jenuth, J.P. Bioinformatics Methods and Protocols; Springer: Berlin/Heidelberg, Germany, 2000; pp. 301–312. [Google Scholar]
- Sievers, F.; Higgins, D.G. Clustal Omega, accurate alignment of very large numbers of sequences. In Multiple Sequence Alignment Methods; Humana Press: Totowa, NJ, USA, 2014; pp. 105–116. [Google Scholar]
- Naito, Y.; Yoshimura, J.; Morishita, S.; Ui-Tei, K. siDirect 2.0: Updated software for designing functional siRNA with reduced seed-dependent off-target effect. BMC Bioinform. 2009, 10, 392. [Google Scholar] [CrossRef]
- Naito, Y.; Ui-Tei, K. Designing functional siRNA with reduced off-target effects. In siRNA Design; Humana Press: Totowa, NJ, USA, 2013; pp. 57–68. [Google Scholar]
- Ichihara, M.; Murakumo, Y.; Masuda, A.; Matsuura, T.; Asai, N.; Jijiwa, M.; Takagishi, M.; Shinmi, J.; Yatsuya, H.; Qiao, S.L.; et al. Thermodynamic instability of siRNA duplex is a prerequisite for dependable prediction of siRNA activities. Nucleic Acids Res. 2007, 35, e123. [Google Scholar] [CrossRef]
- Takasaki, S.; Kotani, S.; Konagaya, A. An effective method for selecting siRNA target sequences in mammalian cells. Cell Cycle 2004, 3, 788–793. [Google Scholar] [CrossRef]
- Huesken, D.; Lange, J.; Mickanin, C.; Weiler, J.; Asselbergs, F.; Warner, J.; Meloon, B.; Engel, S.; Rosenberg, A.; Cohen, D.; et al. Design of a genome-wide siRNA library using an artificial neural network. Nat. Biotechnol. 2005, 23, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Katoh, T.; Suzuki, T. Specific residues at every third position of siRNA shape its efficient RNAi activity. Nucleic Acids Res. 2007, 35, e27. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36 (Suppl. 2), W5–W9. [Google Scholar] [CrossRef]
- Reuter, J.S.; Mathews, D.H. RNAstructure: Software for RNA secondary structure prediction and analysis. BMC Bioinform. 2010, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Kibbe, W.A. OligoCalc: An online oligonucleotide properties calculator. Nucleic Acids Res. 2007, 35 (Suppl. 2), W43–W46. [Google Scholar] [CrossRef] [PubMed]
- Seetin, M.G.; Mathews, D.H. RNA structure prediction: An overview of methods. In Bacterial Regulatory RNA; Springer: Berlin/Heidelberg, Germany, 2012; pp. 99–122. [Google Scholar]
- Lu, Z.J.; Mathews, D.H. OligoWalk: An online siRNA design tool utilizing hybridization thermodynamics. Nucleic Acids Res. 2008, 36 (Suppl. 2), W104–W108. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.H. Using OligoWalk to identify efficient siRNA sequences. In RNA Therapeutics; Humana Press: Totowa, NJ, USA, 2010; pp. 107–119. [Google Scholar]
- Kumar, M.; Lata, S.; Raghava, G. (Eds.) siRNApred: SVM based method for predicting efficacy value of siRNA. In Proceedings of the 1st International Conference on Open Source for Computer Aided Drug Discovery (OSCADD), Chandigarh, India, 22–26 March 2009. CSIR-IMTECH. [Google Scholar]
- Chaudhary, K.; Nagpal, G.; Dhanda, S.K.; Raghava, G.P. Prediction of Immunomodulatory potential of an RNA sequence for designing non-toxic siRNAs and RNA-based vaccine adjuvants. Sci. Rep. 2016, 6, 20678. [Google Scholar] [CrossRef]
- Antczak, M.; Popenda, M.; Zok, T.; Sarzynska, J.; Ratajczak, T.; Tomczyk, K.; Adamiak, R.W.; Szachniuk, M. New functionality of RNAComposer: Application to shape the axis of miR160 precursor structure. Acta Biochim. Pol. 2016, 63, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Popenda, M.; Szachniuk, M.; Antczak, M.; Purzycka, K.J.; Lukasiak, P.; Bartol, N.; Blazewicz, J.; Adamiak, R.W. Automated 3D structure composition for large RNAs. Nucleic Acids Res. 2012, 40, e112. [Google Scholar] [CrossRef]
- Davis, I.W.; Leaver-Fay, A.; Chen, V.B.; Block, J.N.; Kapral, G.J.; Wang, X.Y.; Murray, L.W.; Arendall, W.B.; Snoeyink, J.; Richardson, J.S.; et al. MolProbity: All-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007, 35 (Suppl. 2), W375–W383. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Hajiaghayi, M.; Condon, A.; Hoos, H.H. Analysis of energy-based algorithms for RNA secondary structure prediction. BMC Bioinform. 2012, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.H. Predicting a set of minimal free energy RNA secondary structures common to two sequences. Bioinformatics 2005, 21, 2246–2253. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Chan, C.Y.; Lawrence, C.E. RNA secondary structure prediction by centroids in a Boltzmann weighted ensemble. RNA 2005, 11, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, Y.; Zhang, C.; Wei, Q.; Chen, J.; Chen, H.; Xu, D. Influence of mRNA features on siRNA interference efficacy. J. Bioinform. Comput. Biol. 2013, 11, 1341004. [Google Scholar] [CrossRef]
- Shao, Y.; Chan, C.Y.; Maliyekkel, A.; Lawrence, C.E.; Roninson, I.B.; Ding, Y. Effect of target secondary structure on RNAi efficiency. RNA 2007, 13, 1631–1640. [Google Scholar] [CrossRef]
- Umu, S.U.; Gardner, P.P. A comprehensive benchmark of RNA–RNA interaction prediction tools for all domains of life. Bioinformatics 2017, 33, 988–996. [Google Scholar] [CrossRef]
- Nazipova, N.N.; Shabalina, S.A. Understanding off-target effects through hybridization kinetics and thermodynamics. Cell Biol. Toxicol. 2020, 36, 11–15. [Google Scholar] [CrossRef]
- Lu, Z.J.; Mathews, D.H. Efficient siRNA selection using hybridization thermodynamics. Nucleic Acids Res. 2008, 36, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Mückstein, U.; Tafer, H.; Hackermüller, J.; Bernhart, S.H.; Stadler, P.F.; Hofacker, I.L. Thermodynamics of RNA–RNA binding. Bioinformatics 2006, 22, 1177–1182. [Google Scholar] [CrossRef]
- Bernhart, S.H.; Tafer, H.; Mückstein, U.; Flamm, C.; Stadler, P.F.; Hofacker, I.L. Partition function and base pairing probabilities of RNA heterodimers. Algorithms Mol. Biol. 2006, 1, 3. [Google Scholar] [CrossRef]
- Reynolds, A.; Leake, D.; Boese, Q.; Scaringe, S.; Marshall, W.S.; Khvorova, A. Rational siRNA design for RNA interference. Nat. Biotechnol. 2004, 22, 326–330. [Google Scholar] [CrossRef]
- Biesiada, M.; Purzycka, K.J.; Szachniuk, M.; Blazewicz, J.; Adamiak, R.W. Automated RNA 3D structure prediction with RNAComposer. In RNA Structure Determination; Humana Press: New York, NY, USA, 2016; pp. 199–215. [Google Scholar]
- Huo, J.; Le Bas, A.; Ruza, R.R.; Duyvesteyn, H.M.; Mikolajek, H.; Malinauskas, T.; Tan, T.K.; Rijal, P.; Dumoux, M.; Ward, P.N.; et al. Neutralizing nanobodies bind SARS-CoV-2 spike RBD and block interaction with ACE2. Nat. Struct. Mol. Biol. 2020, 27, 846–854. [Google Scholar] [CrossRef]
- Dana, H.; Chalbatani, G.M.; Mahmoodzadeh, H.; Karimloo, R.; Rezaiean, O.; Moradzadeh, A.; Mehmandoost, N.; Moazzen, F.; Mazraeh, A.; Marmari, V. Molecular mechanisms and biological functions of siRNA. Int. J. Biomed. Sci. 2017, 13, 48. [Google Scholar]
- Ryther, R.; Flynt, A.; Phillips Patton, J., Jr. siRNA therapeutics: Big potential from small RNAs. Gene Ther. 2005, 12, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Taxman, D.J.; Livingstone, L.R.; Zhang, J.; Conti, B.J.; Iocca, H.A.; Williams, K.L.; Lich, J.D.; Ting, J.P.; Reed, W. Criteria for effective design, construction, and gene knockdown by shRNA vectors. BMC Biotechnol. 2006, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- ElHefnawi, M.; Kim, T.; Kamar, M.A.; Min, S.; Hassan, N.M.; El-Ahwany, E. In silico design and experimental validation of siRNAs targeting conserved regions of multiple hepatitis C virus genotypes. PLoS ONE 2016, 11, e0159211. [Google Scholar] [CrossRef]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef]
- Heale, B.S.; Soifer, H.S.; Bowers, C.; Rossi, J.J. siRNA target site secondary structure predictions using local stable substructures. Nucleic Acids Res. 2005, 33, e30. [Google Scholar] [CrossRef] [PubMed]
- Vickers, T.A.; Wyatt, J.R.; Freier, S.M. Effects of RNA secondary structure on cellular antisense activity. Nucleic Acids Res. 2000, 28, 1340–1347. [Google Scholar] [CrossRef]
- Nawrot, B.; Sipa, K. Chemical and structural diversity of siRNA molecules. Curr. Top. Med. Chem. 2006, 6, 913–925. [Google Scholar] [CrossRef]
- Tafer, H.; Ameres, S.L.; Obernosterer, G.; Gebeshuber, C.A.; Schroeder, R.; Martinez, J.; Hofacker, I.L. The impact of target site accessibility on the design of effective siRNAs. Nat. Biotechnol. 2008, 26, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Monack, D.M. Newly described pattern recognition receptors team up against intracellular pathogens. Nat. Rev. Immunol. 2013, 13, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, Y.; Anderson, E.M.; Birmingham, A.; Reynolds, A.; Karpilow, J.; Robinson, K.; Leake, D.; Marshall, W.S.; Khvorova, A. Off-target effects by siRNA can induce toxic phenotype. RNA 2006, 12, 1188–1196. [Google Scholar] [CrossRef]
- Hajdin, C.E.; Ding, F.; Dokholyan, N.V.; Weeks, K.M. On the significance of an RNA tertiary structure prediction. RNA 2010, 16, 1340–1349. [Google Scholar] [CrossRef] [PubMed]

| Name of Webserver | URL | Function |
|---|---|---|
| NCBI Nucleotide | https://www.ncbi.nlm.nih.gov/nucleotide | Databases consisting of nucleotide sequences from sources such as: GenBank, RefSeq, TPA, and PDB. |
| Clustal Omega | https://www.ebi.ac.uk/Tools/msa/clustalo/ | Multiple sequence-alignment tools that use seeded guide trees and Hidden Markov Models (HMM) to generate alignments between three or more sequences. |
| siDirect 2.0 | http://sidirect2.rnai.jp/ | Candidate siRNA designing on the basis of the first generation algorithms for siRNA designing (Ui-Tei, Amarzguioui, and Reynolds rules) and using target mRNA sequences as query. |
| i-SCORE Designer | https://www.med.nagoya-u.ac.jp/neurogenetics/i_Score/i_score.html | Validation of potential siRNA candidates on the basis of the second generation rules for siRNA designing (i-Score‚ s-Biopredsi‚ and DSIR) |
| BLAST | http://www.ncbi.nlm.nih.gov/blast | Locating regions of similarity between biological sequences for the determination of off-target sequence similarity between candidate siRNA and other nucleotide sequences in the host system. |
| RNAstructure | https://rna.urmc.rochester.edu/RNAstructureWeb/ | Prediction and validation of the secondary structures of the siRNA candidates. |
| OligoCalC: Oligonucleotide Properties Calculator | http://biotools.nubic.northwestern.edu/OligoCalc.html | Calculating the GC content in the predicted siRNA candidates. |
| OligoWalk | http://rna.urmc.rochester.edu/cgi-bin/server_exe/oligowalk/oligowalk_form.cgi | Assessment of thermodynamic stability of the predicted candidate siRNA and determination of its strand, functional efficiency, and target accessibility. |
| siRNAPred | http://crdd.osdd.net/raghava/sirnapred/index.html | Validation of the functional efficiency of the predicted siRNA candidates against the Main21 dataset. |
| imRNA | https://webs.iiitd.edu.in/raghava/imrna/sirna.php | Prediction of the immunotoxicity of the designed siRNA candidates. |
| RNAComposer | http://rnacomposer.cs.put.poznan.pl/ | Prediction of tertiary structure siRNA candidates. |
| MOLprobity | http://molprobity.biochem.duke.edu/ | Validation of the tertiary structure of siRNA candidates. |
| siRNA | Gene | Target Sequence | Predicted siRNA Duplex siRNA Candidate at 37 °C (5′ to 3′) | Combined Second-Generation Algorithm Scoring a | Free Energy of Self-Folding of Guide Strand (kcal/mol) | GC Content (%) |
|---|---|---|---|---|---|---|
| siRNA 1 | IcsA | TGGAATGGATGCGTGGTATATAA | AUAUACCACGCAUCCAUUCCA | <90% threshold | ----- | ----- |
| GAAUGGAUGCGUGGUAUAUAA | ||||||
| siRNA 2 | IcsA | TACGGATTAACTCTGATATTATG | UUAUAUAUCCGCUAUUAGCAA | >90% threshold | 1.0 | 28.57 |
| GCUAAUAGCGGAUAUAUAAUU | ||||||
| siRNA 3 | IcsA | TTGCTAATAGCGGATATATAATT | UUAUAUAUCCGCUAUUAGCAA | >90% threshold | −1.30 | ----- |
| GCUAAUAGCGGAUAUAUAAUU | ||||||
| siRNA 4 | IcsA | TGGTGATGTACAGGTTAACAATT | UUGUUAACCUGUACAUCACCA | >90% threshold | 1.50 | 38.10 |
| GUGAUGUACAGGUUAACAAUU | ||||||
| siRNA 5 | IpaJ | ATGGAATTAGGTGCAAGAAGAAG | UCUUCUUGCACCUAAUUCCAU | >90% threshold | 1.80 | 38.10 |
| GGAAUUAGGUGCAAGAAGAAG | ||||||
| siRNA6 | IpaJ | TGGAATTAGGTGCAAGAAGAAGT | UUCUUCUUGCACCUAAUUCCA | >90% threshold | 1.80 | 38.10 |
| GAAUUAGGUGCAAGAAGAAGU | ||||||
| siRNA7 | IpgB | AAGGATCTTACAAATCTAGTATC | UACUAGAUUUGUAAGAUCCUU | >90% threshold | 1.70 | 28.57 |
| GGAUCUUACAAAUCUAGUAUC | ||||||
| siRNA8 | IpgD | AGCCATGATGGAACGATTAGATA | UCUAAUCGUUCCAUCAUGGCU | <90% threshold | ----- | ----- |
| CCAUGAUGGAACGAUUAGAUA | ||||||
| siRNA 9 | IpgD | GAGGAATGTTGACAAGCTTAATG | UUAAGCUUGUCAACAUUCCUC | >90% threshold | 1.80 | 38.10 |
| GGAAUGUUGACAAGCUUAAUG | ||||||
| siRNA 10 | IpgD | TGGAAATCCAAGAGATGAATACT | UAUUCAUCUCUUGGAUUUCCA | <90% threshold | ----- | ----- |
| GAAAUCCAAGAGAUGAAUACU | ||||||
| siRNA 11 | MxiC | TTCAGCTATACAGGCTAAATTAT | AAUUUAGCCUGUAUAGCUGAA | <90% threshold | ----- | ----- |
| CAGCUAUACAGGCUAAAUUAU | ||||||
| siRNA 12 | MxiC | GTGAAAGTGAGCAAATTCTTACT | UAAGAAUUUGCUCACUUUCAC | <90% threshold | ----- | ----- |
| GAAAGUGAGCAAAUUCUUACU | ||||||
| siRNA 13 | MxiC | GAGGATTCTGTAGTGTATCAAAC | UUGAUACACUACAGAAUCCUC | >90% threshold | 1.70 | 38.10 |
| GGAUUCUGUAGUGUAUCAAAC | ||||||
| siRNA 14 | OspB | ATGTACAAACAATCATTTCAAGA | UUGAAAUGAUUGUUUGUACAU | >90% threshold | 1.70 | 23.80 |
| GUACAAACAAUCAUUUCAAGA | ||||||
| siRNA 15 | OspB | CTGCTGAAAGTCTTTCTTGTATC | UACAAGAAAGACUUUCAGCAG | >90% threshold | 1.70 | 38.10 |
| GCUGAAAGUCUUUCUUGUAUC | ||||||
| siRNA 16 | OspB | ATCTTTGCTAGAGCAGATAAAAA | UUUAUCUGCUCUAGCAAAGAU | >90% threshold | −1.40 | ----- |
| CUUUGCUAGAGCAGAUAAAAA | ||||||
| siRNA 17 | OspB | ATGAAAGACTGTGGTATTCTAAA | UAGAAUACCACAGUCUUUCAU | >90% threshold | 1.60 | 33.33 |
| GAAAGACUGUGGUAUUCUAAA | ||||||
| siRNA 18 | OspF | ATGCTTTCTGCGAATGAAAGATT | UCUUUCAUUCGCAGAAAGCAU | <90% threshold | ----- | ----- |
| GCUUUCUGCGAAUGAAAGA | ||||||
| siRNA 19 | OspF | TGGAAGATAACTGATATGAATCG | AUUCAUAUCAGUUAUCUUCCA | >90% threshold | 1.80 | 28.57 |
| GAAGAUAACUGAUAUGAAUCG | ||||||
| siRNA 20 | OspF | ATGGAAGATAACTGATATGAATC | UUCAUAUCAGUUAUCUUCCAU | >90% threshold | 1.80 | 28.57 |
| GGAAGAUAACUGAUAUGAAUC | ||||||
| siRNA 21 | OspF | TCGCAATATAGTGCTTTATTACT | UAAUAAAGCACUAUAUUGCGA | >90% threshold | −2.0 | ----- |
| GCAAUAUAGUGCUUUAUUACU | ||||||
| siRNA 22 | OspG | GCCCATTCTCGGTAAGTTAATAG | AUUAACUUACCGAGAAUGGGC | <90% threshold | ----- | ----- |
| CCAUUCUCGGUAAGUUAAUAG | ||||||
| siRNA 23 | OspG | CAGCTGATATCCCTGATAATATA | UAUUAUCAGGGAUAUCAGCUG | >90% threshold | 1.50 | 38.10 |
| GCUGAUAUCCCUGAUAAUAUA | ||||||
| siRNA 24 | OspG | ATCTACAGTTGATATGTAAATTG | AUUUACAUAUCAACUGUAGAU | <90% threshold | ----- | ----- |
| CUACAGUUGAUAUGUAAAUUG | ||||||
| siRNA 25 | OspG | ATCCATTACGATCTTAATACAGG | UGUAUUAAGAUCGUAAUGGAU | >90% threshold | 1.60 | 28.57 |
| CCAUUACGAUCUUAAUACAGG | ||||||
| siRNA 26 | OspG | CGCAATATTTATGCTGAATATTA | AUAUUCAGCAUAAAUAUUGCG | >90% threshold | −2.40 | ----- |
| CAAUAUUUAUGCUGAAUAUUA | ||||||
| siRNA 27 | Spa33 | GACAATCAATGAACTAAAAATGT | AUUUUUAGUUCAUUGAUUGUC | <90% threshold | ----- | ----- |
| CAAUCAAUGAACUAAAAAUGU | ||||||
| siRNA 28 | Spa33 | AACTAAAAATGTATGTAGAAAAC | UUUCUACAUACAUUUUUAGUU | >90% threshold | 1.60 | 19.05 |
| CUAAAAAUGUAUGUAGAAAAC | ||||||
| siRNA 29 | Spa33 | ATGTATGTAGAAAACGAATTATT | UAAUUCGUUUUCUACAUACAU | >90% threshold | 1.60 | 28.10 |
| GUAUGUAGAAAACGAAUUAUU | ||||||
| siRNA 30 | Spa33 | TTCAAGTTTCCCGATGACATAGT | UAUGUCAUCGGGAAACUUGAA | >90% threshold | −1.30 | ----- |
| CAAGUUUCCCGAUGACAUAGU | ||||||
| siRNA 31 | VirF | TTCAACAAATCCTTCTTGATATT | UAUCAAGAAGGAUUUGUUGAA | <90% threshold | ----- | ----- |
| CAACAAAUCCUUCUUGAUAUU | ||||||
| siRNA 32 | VirF | TGGCGTCTTTCTGATATTTCAAA | UGAAAUAUCAGAAAGACGCCA | >90% threshold | 1.80 | 38.10 |
| GCGUCUUUCUGAUAUUUCAAA | ||||||
| siRNA 33 | VirF | GTCTTTCTGATATTTCAAATAAC | UAUUUGAAAUAUCAGAAAGAC | >90% threshold | −1.40 | ----- |
| CUUUCUGAUAUUUCAAAUAAC | ||||||
| siRNA 34 | VirF | AACTTGAATTTATCAGAAATAGC | UAUUUCUGAUAAAUUCAAGUU | <90% threshold | ----- | ----- |
| CUUGAAUUUAUCAGAAAUAGC | ||||||
| siRNA 35 | VirA | GCCTGAACAACGAGTTATTAACA | UUAAUAACUCGUUGUUCAGGC | <90% threshold | ----- | ----- |
| CUGAACAACGAGUUAUUAACA | ||||||
| siRNA 36 | VirA | CTGAACAACGAGTTATTAACAAT | UGUUAAUAACUCGUUGUUCAG | <90% threshold | ----- | ----- |
| GAACAACGAGUUAUUAACAAU | ||||||
| siRNA 37 | VirA | TACGAAGTTAGCTCATCAATATT | UAUUGAUGAGCUAACUUCGUA | >90% threshold | −0.90 | ----- |
| CGAAGUUAGCUCAUCAAUAUU | ||||||
| siRNA 38 | VirA | ACGAAGTTAGCTCATCAATATTA | AUAUUGAUGAGCUAACUUCGU | >90% threshold | 1.60 | 33.33 |
| GAAGUUAGCUCAUCAAUAUUA | ||||||
| siRNA 39 | VirA | CTCCAGAAAGTCGTCAAGTATCA | AUACUUGACGACUUUCUGGAG | >90% threshold | 1.60 | 42.86 |
| CCAGAAAGUCGUCAAGUAUCA | ||||||
| siRNA 40 | VirB | CTCCATTCTGGTAATAAAGTTTC | AACUUUAUUACCAGAAUGGAG | <90% threshold | ----- | ----- |
| CCAUUCUGGUAAUAAAGUUUC | ||||||
| siRNA 41 | VirB | AACGAATGTACGCGATCAAGAAT | UCUUGAUCGCGUACAUUCGUU | <90% threshold | ----- | ----- |
| CGAAUGUACGCGAUCAAGAAU | ||||||
| siRNA 42 | VirB | GAGATTGATGGTAGAATTGAAAT | UUCAAUUCUACCAUCAAUCUC | >90% threshold | 1.80 | 33.33 |
| GAUUGAUGGUAGAAUUGAAAU | ||||||
| siRNA 43 | VirB | AACTAGCAAACGATATACAAACA | UUUGUAUAUCGUUUGCUAGUU | >90% threshold | 1.80 | 28.57 |
| CUAGCAAACGAUAUACAAACA | ||||||
| siRNA 44 | VirB | TAGTTCTACACTACCAATATTAA | AAUAUUGGUAGUGUAGAACUA | >90% threshold | −1.10 | ----- |
| GUUCUACACUACCAAUAUUAA | ||||||
| siRNA 45 | IpaA | GGGAAAGAAGATGTGTTAAGAAG | UCUUAACACAUCUUCUUUCCC | <90% threshold | ----- | ----- |
| GAAAGAAGAUGUGUUAAGAAG | ||||||
| siRNA 46 | IpaA | CACAGTATTCGGAACTAATTATA | UAAUUAGUUCCGAAUACUGUG | >90% threshold | 1.90 | 30.96 |
| CAGUAUUCGGAACUAAUUAUA | ||||||
| siRNA 47 | IpaA | TTGCACTATAGCACAACAACACA | UGUUGUUGUGCUAUAGUGCAA | <90% threshold | ----- | ----- |
| GCACUAUAGCACAACAACACA | ||||||
| siRNA 48 | IpaA | CTCCTCAATACTGAAGTATCATC | UGAUACUUCAGUAUUGAGGAG | >90% threshold | −3.20 | ----- |
| CCUCAAUACUGAAGUAUCAUC | ||||||
| siRNA 49 | IpaA | TCCGTTCTACCACACTCTATATC | UAUAGAGUGUGGUAGAACGGA | >90% threshold | 1.70 | 42.86 |
| CGUUCUACCACACUCUAUAUC | ||||||
| siRNA 50 | IpaA | TTCAACCATACTCCAGATAATTC | AUUAUCUGGAGUAUGGUUGAA | >90% threshold | 1.50 | 35.71 |
| CAACCAUACUCCAGAUAAUUC | ||||||
| siRNA 51 | IpaB | CACCAAAGTCATTAAATGCAAGT | UUGCAUUUAAUGACUUUGGUG | >90% threshold | 1.50 | 33.33 |
| CCAAAGUCAUUAAAUGCAAGU | ||||||
| siRNA 52 | IpaB | AAGAAATACAACTCACTATCAAA | UGAUAGUGAGUUGUAUUUCUU | >90% threshold | 1.50 | 28.57 |
| GAAAUACAACUCACUAUCAAA | ||||||
| siRNA 53 | IpaB | CAGTTAAAGACAGGACATTGATT | UCAAUGUCCUGUCUUUAACUG | >90% threshold | 1.80 | 35.71 |
| GUUAAAGACAGGACAUUGAUU | ||||||
| siRNA 54 | IpaB | CTCAATTGATGGCAACCTTTATT | UAAAGGUUGCCAUCAAUUGAG | >90% threshold | 1.40 | 35.71 |
| CAAUUGAUGGCAACCUUUAUU | ||||||
| siRNA 55 | IpaB | CTCCTTTCAGATGCATTTACAAA | UGUAAAUGCAUCUGAAAGGAG | <90% threshold | ----- | ----- |
| CCUUUCAGAUGCAUUUACAAA | ||||||
| siRNA 56 | IpaB | GGCCAATTGCAGGAAGTAATTGC | AAUUACUUCCUGCAAUUGGCC | <90% threshold | ----- | ----- |
| CCAAUUGCAGGAAGUAAUUGC | ||||||
| siRNA 57 | IpaC | TTGAAGAAGAAGAACAACTAATC | UUAGUUGUUCUUCUUCUUCAA | >90% threshold | 1.80 | 30.96 |
| GAAGAAGAAGAACAACUAAUC | ||||||
| siRNA 58 | IpaC | AAGAAGAAGAACAACTAATCAGT | UGAUUAGUUGUUCUUCUUCUU | <90% threshold | 1.70 | 30.963 |
| GAAGAAGAACAACUAAUCAGU |
| siRNA | Target Sequence | Free Energy of Binding with Target (kcal/mol) | Melting Temperature of siRNA–Target Duplex(°C) | End-Diff a (kcal/mol) | Break-Targ.∆G b (kcal/mol) | Probability of Being Efficient siRNA | siRNA Validity Score (Binary) | Immunogenicity |
|---|---|---|---|---|---|---|---|---|
| siRNA 4 | TGGTGATGTACAGGTTAACAATT | −32.6 | 79.1 | 1.76 | −1.8 | 0.951 | 0.901 | Non-immunomodulatory (IMscore:4.2) |
| siRNA 5 | ATGGAATTAGGTGCAAGAAGAAG | −32.1 | 79.8 | 0.17 | −0.1 | 0.951 | 0.95 | Non-immunomodulatory (IMscore:3.5) |
| siRNA 6 | TGGAATTAGGTGCAAGAAGAAGT | −32.5 | 78.2 | 0.17 | −0.1 | 0.951 | 0.95 | Non-immunomodulatory (IMscore:4.2) |
| siRNA 9 | GAGGAATGTTGACAAGCTTAATG | −31.8 | 78.2 | 2.33 | −1.4 | 0.961 | 0.967 | Non-immunomodulatory (IMscore:3.4) |
| siRNA 13 | GAGGATTCTGTAGTGTATCAAAC | −32.7 | 78.7 | 2.33 | −0.7 | 0.974 | 0.975 | Non-immunomodulatory (IMscore:2.6) |
| siRNA 15 | CTGCTGAAAGTCTTTCTTGTATC | −31 | 78.3 | 2.09 | −1.8 | 0.967 | 1.026 | Non-immunomodulatory (IMscore:2.7) |
| siRNA 17 | ATGAAAGACTGTGGTATTCTAAA | −30.1 | 78.9 | 0.03 | −0.9 | 0.947 | 1.026 | Non-immunomodulatory (IMscore:3.1) |
| siRNA 23 | CAGCTGATATCCCTGATAATATA | −32.4 | 79.1 | 2.09 | −0.9 | 0.96 | 0.989 | Immunomodulatory (IMscore:4.7) |
| siRNA 32 | TGGCGTCTTTCTGATATTTCAAA | −31.9 | 76.6 | 1.76 | −1.8 | 0.957 | 1.009 | Immunomodulatory (IMscore:4.8) |
| siRNA 38 | ACGAAGTTAGCTCATCAATATTA | −29.4 | 74.9 | 1.7 | −0.3 | 0.931 | 1.012 | Non-immunomodulatory (IMscore:4.0) |
| siRNA 39 | CTCCAGAAAGTCGTCAAGTATCA | −33 | 80.4 | 2.16 | −0.3 | 0.952 | 0.949 | Non-immunomodulatory (IMscore:2.8) |
| siRNA 42 | GAGATTGATGGTAGAATTGAAAT | −30.2 | 74 | 1.87 | −1 | 0.968 | 0.965 | Immunomodulatory (IMscore:5.6) |
| siRNA 46 | CACAGTATTCGGAACTAATTATA | −28.6 | 74.6 | 1.23 | −0.3 | 0.936 | 0.971 | Non-immunomodulatory (IMscore:2.2) |
| siRNA 49 | TCCGTTCTACCACACTCTATATC | −34.3 | 82 | 1.03 | −0.1 | 0.935 | 1.006 | Non-immunomodulatory (IMscore:1.1) |
| siRNA 50 | TTCAACCATACTCCAGATAATTC | −30.3 | 78.5 | 1.46 | 0 | 0.886 | 0.886 | - |
| siRNA 51 | CACCAAAGTCATTAAATGCAAGT | −29 | 76.1 | 0.13 | −0.3 | 0.818 | 0.818 | - |
| siRNA 53 | CAGTTAAAGACAGGACATTGATT | −31.3 | 78.1 | 0.79 | −1.1 | 0.926 | 0.909 | Immunomodulatory (IMscore:5.6) |
| siRNA 54 | CTCAATTGATGGCAACCTTTATT | −31 | 78 | 1.23 | −1 | 0.891 | - | - |
| siRNA 57 | TTGAAGAAGAAGAACAACTAATC | −27.8 | 76.2 | 0.33 | −0.2 | 0.906 | 0.999 | Immunomodulatory (IMscore:7.3) |
| siRNA | Nucleic Acid Geometry | All Atom Contacts (clashsccore, Percentile) b | Validity of Predicted 3D Structure of siRNA | |||
|---|---|---|---|---|---|---|
| Probability of Wrong Sugar Puckers a (%) | Bad Backbone Confirmations a (%) | Bad Bonds a (%) | Bad Angles a (%) | |||
| siRNA 4 | 0.00 | 0.00 | 0.00 | 0.00 | 36.81, 9th | Low |
| siRNA 5 | 0.00 | 0.00 | 0.00 | 0.00 | 30.67, 15th | Low |
| siRNA 6 | 0.00 | 0.00 | 0.00 | 0.00 | 36.81, 9th | Low |
| siRNA 9 | 0.00 | 0.00 | 0.00 | 0.00 | 19.76, 33rd | Acceptable |
| siRNA 13 | 0.00 | 0.00 | 0.00 | 0.00 | 24.1, 23rd | Low |
| siRNA 15 | 0.00 | 4.76 | 0.00 | 0.00 | 19.23, 35th | Acceptable |
| siRNA 17 | 0.00 | 0.00 | 0.00 | 0.00 | 19.61, 34th | Acceptable |
| siRNA 38 | 4.76 | 57.14 | 0.00 | 0.00 | 22.52, 26th | Low |
| siRNA 39 | 0.00 | 0.00 | 0.00 | 0.00 | 22.46, 26th | Low |
| siRNA 46 | 0.00 | 0.00 | 0.00 | 0.00 | 29.76, 16th | Low |
| siRNA 49 | 0.00 | 28.57 | 0.00 | 0.00 | 19.03, 35th | Low |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palit, P.; Chowdhury, F.T.; Baruah, N.; Sarkar, B.; Mou, S.N.; Kamal, M.; Siddiqua, T.J.; Noor, Z.; Ahmed, T. A Comprehensive Computational Investigation into the Conserved Virulent Proteins of Shigella species Unveils Potential Small-Interfering RNA Candidates as a New Therapeutic Strategy against Shigellosis. Molecules 2022, 27, 1936. https://doi.org/10.3390/molecules27061936
Palit P, Chowdhury FT, Baruah N, Sarkar B, Mou SN, Kamal M, Siddiqua TJ, Noor Z, Ahmed T. A Comprehensive Computational Investigation into the Conserved Virulent Proteins of Shigella species Unveils Potential Small-Interfering RNA Candidates as a New Therapeutic Strategy against Shigellosis. Molecules. 2022; 27(6):1936. https://doi.org/10.3390/molecules27061936
Chicago/Turabian StylePalit, Parag, Farhana Tasnim Chowdhury, Namrata Baruah, Bonoshree Sarkar, Sadia Noor Mou, Mehnaz Kamal, Towfida Jahan Siddiqua, Zannatun Noor, and Tahmeed Ahmed. 2022. "A Comprehensive Computational Investigation into the Conserved Virulent Proteins of Shigella species Unveils Potential Small-Interfering RNA Candidates as a New Therapeutic Strategy against Shigellosis" Molecules 27, no. 6: 1936. https://doi.org/10.3390/molecules27061936
APA StylePalit, P., Chowdhury, F. T., Baruah, N., Sarkar, B., Mou, S. N., Kamal, M., Siddiqua, T. J., Noor, Z., & Ahmed, T. (2022). A Comprehensive Computational Investigation into the Conserved Virulent Proteins of Shigella species Unveils Potential Small-Interfering RNA Candidates as a New Therapeutic Strategy against Shigellosis. Molecules, 27(6), 1936. https://doi.org/10.3390/molecules27061936






