Molecular and Pharmacological Evidence for the Expression of Multiple Functional P2 Purinergic Receptors in Human Adipocytes
Abstract
:1. Introduction
2. Results
2.1. Human Adipocytes/Adipose Tissue Express mRNA for Multiple P2X and P2Y Receptor Subtypes
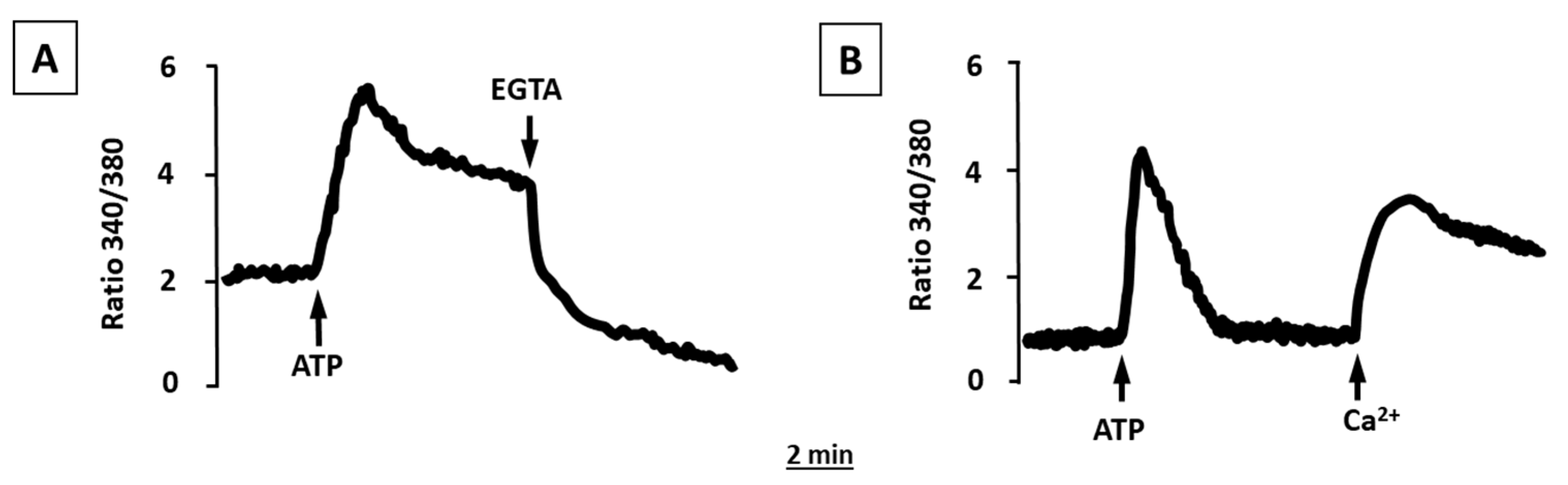
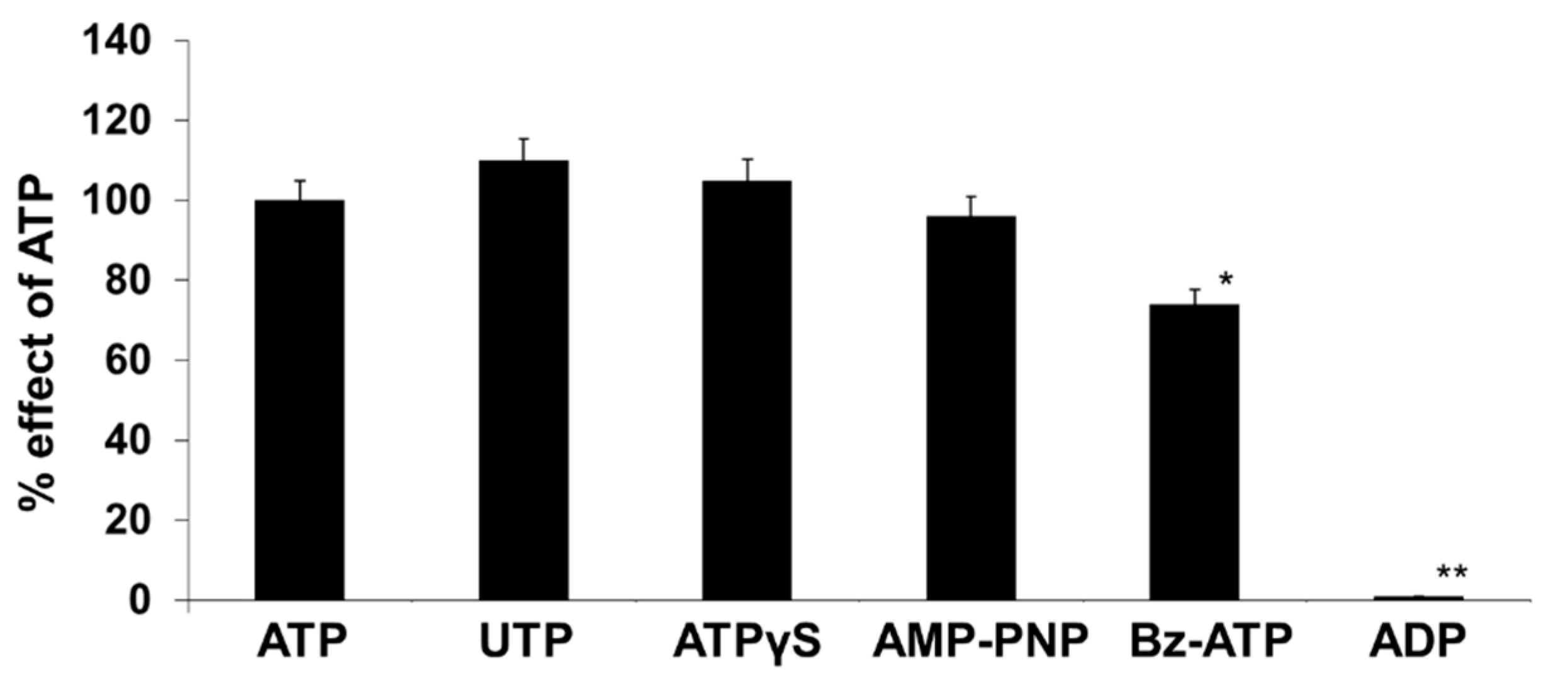
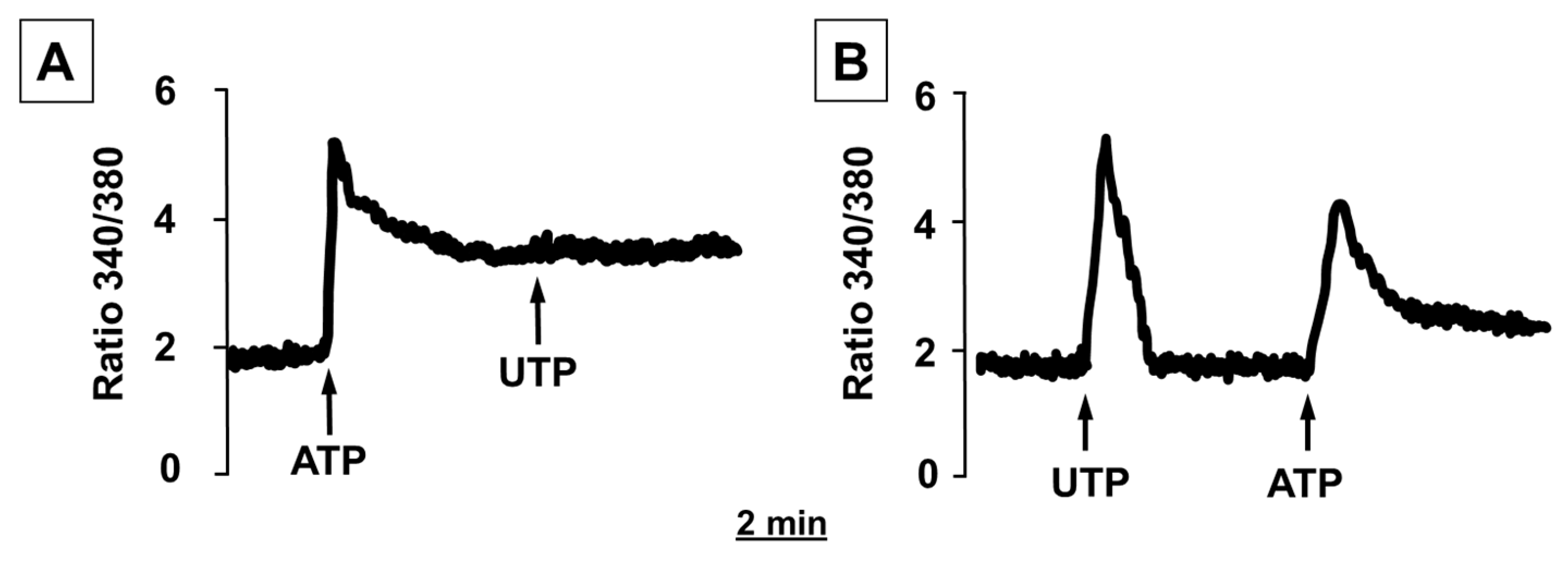
2.2. ATP and UTP Induce [Ca2+]i Changes in Human Adipocytes
2.3. Extracellular ATP Induces IL-6 Release from Human Adipocytes
2.4. Hydrolysis of Extracellular ATP Inhibits IL-6 Secretion by Human Adipocytes
3. Discussion
4. Materials and Methods
4.1. Ex Vivo Adipose Tissue Sampling and In Vitro Differentiation of Human Pre-Adipocytes to Mature Adipocytes
4.2. RNA Extraction
4.3. Detection of P2 Purinergic Receptor RNAs by Reverse-Transcriptase Polymerase Chain Reaction (RT-PCR)
4.4. Sequencing
4.5. Measurement of [Ca2+]i in Human Adipocytes
4.6. Measurement of IL-6 Concentration after Adipocyte Stimulation with ATP
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Linden, J. Purinergic chemotaxis. Science 2006, 314, 1689–1690. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G. Purinergic nerves. Pharm. Rev. 1972, 24, 509–581. [Google Scholar] [PubMed]
- Giuliani, A.L.; Sarti, A.C.; Di Virgilio, F. Extracellular nucleotides and nucleosides as signalling molecules. Immunol. Lett. 2019, 205, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, C. That was then, this is now: The development of our knowledge and understanding of P2 receptor subtypes. Purinergic Signal. 2021, 17, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; La Sala, A.; Chiozzi, P.; Morelli, A.; Falzoni, S.; Girolomoni, G.; Idzko, M.; Dichmann, S.; Norgauer, J.; Di Virgilio, F. The P2 purinergic receptors of human dendritic cells: Identification and coupling to cytokine release. FASEB J. 2000, 14, 2466–2476. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Jun, D.J.; Suh, B.C.; Choi, B.H.; Lee, J.H.; Do, M.S.; Suh, B.S.; Ha, H.; Kim, K.T. Dual roles of P2 purinergic receptors in insulin-stimulated leptin production and lipolysis in differentiated rat white adipocytes. J. Biol. Chem. 2005, 280, 28556–28563. [Google Scholar] [CrossRef] [Green Version]
- Schödel, J.; Weise, I.; Klinger, R.; Schmidt, M. Stimulation of lipogenesis in rat adipocytes by ATP, a ligand for P2-receptors. Biochem. Biophys. Res. Commun. 2004, 321, 767–773. [Google Scholar] [CrossRef]
- Leaver, E.V.; Pappone, P.A. Beta-adrenergic potentiation of endoplasmic reticulum Ca2+ release in brown fat cells. Am. J. Physiol.-Cell Physiol. 2002, 282, 1016–1024. [Google Scholar] [CrossRef]
- Wilson, S.M.; Lee, S.C.; Shook, S.; Pappone, P.A. ATP and beta-adrenergic stimulation enhance voltage-gated K current inactivation in brown adipocytes. Am. J. Physiol.-Cell Physiol. 2000, 279, 1847–1858. [Google Scholar] [CrossRef]
- Omatsu-Kanbe, M.; Matsuura, H. Inhibition of store-operated Ca2+ entry by extracellular ATP in rat brown adipocytes. J. Physiol. 1999, 521, 601–615. [Google Scholar] [CrossRef]
- Wilson, S.M.; Pappone, P.A. P2 receptor modulation of voltage-gated potassium currents in brown adipocytes. J. Gen. Physiol. 1999, 113, 125–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.C.; Pappone, P.A. Effects of P2 purinergic receptor stimulation in brown adipocytes. Am. J. Physiol.-Cell Physiol. 1997, 273, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Loffler, G. Induction of aromatase activity in human adipose tissue stromal cells by extracellular nucleotides: Evidence for P2-purinoceptors in adipose tissue. Eur. J. Biochem. 1998, 252, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, M.; Novak, I. Purinergic receptors in adipose tissue as potential targets in metabolic disorders. Front. Pharmacol. 2017, 8, 878. [Google Scholar] [CrossRef] [PubMed]
- Abbracchio, M.P.; Burnstock, G.; Boeynaems, J.M.; Barnard, E.A.; Boyer, J.L.; Kennedy, C.; Knight, G.E.; Fumagalli, M.; Gachet, C.; Jacobson, K.A.; et al. International Union of Pharmacology LVIII: Update on the P2Y G protein-coupled nucleotide receptors: From molecular mechanisms and pathophysiology to therapy. Pharmacol. Rev. 2006, 58, 281–341. [Google Scholar] [CrossRef]
- Illes, P.; Müller, C.E.; Jacobson, K.A.; Grutter, T.; Nicke, A.; Fountain, S.J.; Kennedy, C.; Schmalzing, G.; Jarvis, M.F.; Stojilkovic, S.S.; et al. Update of P2X receptor properties and their pharmacology: IUPHAR Review 30. Br. J. Pharmacol. 2021, 178, 489–514. [Google Scholar] [CrossRef]
- Samways, D.S.; Li, Z.; Egan, T.M. Principles and properties of ion flow in P2X receptors. Front. Cell. Neurosci. 2014, 8, 6. [Google Scholar] [CrossRef] [Green Version]
- Alexander, S.P.; Christopoulos, A.; Davenport, A.P.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; et al. The concise guide to pharmacology 2021/22: G protein-coupled receptors. Br. J. Pharmacol. 2021, 178 (Suppl. S1), S27–S156. [Google Scholar] [CrossRef]
- Vettor, R.; Milan, G.; Rossato, M.; Federspil, G. Review article: Adipocytokines and insulin resistance. Aliment. Pharm. Ther. 2005, 22, 3–10. [Google Scholar] [CrossRef]
- Qi, L.; Zhang, C.; van Dam, R.M.; Hu, F.B. Interleukin-6 genetic variability and adiposity: Associations in two prospective cohorts and systematic review in 26,944 individuals. J. Clin. Endocrinol. Metab. 2007, 92, 3618–3625. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Jansson, P.A.; Pellmé, F.; Laakso, M.; Smith, U. Effect of the interleukin-6 (-174) g/c promoter polymorphism on adiponectin and insulin sensitivity. Obes. Res. 2005, 13, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Autieri, M.V.; Scalia, R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol.-Cell Physiol. 2021, 320, C375–C391. [Google Scholar] [CrossRef] [PubMed]
- Saltiel, A.R.; Olefsky, J.M. Inflammatory mechanisms linking obesity and metabolic disease. J. Clin. Investig. 2017, 127, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.Z.; Libby, P. Obesity, inflammation, and atherosclerosis. Nat. Rev. Cardiol. 2009, 6, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Chiozzi, P.; Morelli, A.; Fellin, R.; Di Virgilio, F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J. Cell. Sci. 1999, 112, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, C.M.; Beilby, J.P.; Humphries, S.E.; Palmer, L.J.; Thompson, P.L.; Hung, J. Association of an allelic variant of interleukin-6 with subclinical carotid atherosclerosis in an Australian community population. Eur. Heart J. 2003, 24, 1494–1499. [Google Scholar] [CrossRef] [Green Version]
- Battineni, G.; Sagaro, G.G.; Chintalapudi, N.; Amenta, F.; Tomassoni, D.; Tayebati, S.K. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int. J. Mol. Sci. 2021, 22, 4798. [Google Scholar] [CrossRef]
- Hildreth, A.D.; Ma, F.; Wong, Y.Y.; Sun, R.; Pellegrini, M.; O’Sullivan, T.E. Single-cell sequencing of human white adipose tissue identifies new cell states in health and obesity. Nat. Immunol. 2021, 22, 639–653. [Google Scholar] [CrossRef]
- Huh, J.Y.; Park, Y.J.; Kim, J.B. Adipocyte CD1d determines adipose inflammation and insulin resistance in obesity. Adipocyte 2018, 7, 129–136. [Google Scholar] [CrossRef] [Green Version]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014, 40, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Piché, M.E.; Weisnagel, S.J.; Corneau, L.; Nadeau, A.; Bergeron, J.; Lemieux, S. Contribution of abdominal visceral obesity and insulin resistance to the cardiovascular risk profile of postmenopausal women. Diabetes 2005, 54, 770–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Virgilio, F.; Sarti, A.C.; Coutinho-Silva, R. Purinergic signaling, DAMPs, and inflammation. Am. J. Physiol.-Cell Physiol. 2020, 318, 832–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossato, M.; Di Vincenzo, A.; Pagano, C.; El Hadi, H.; Vettor, R. The P2X7 Receptor and NLRP3 Axis in Non-Alcoholic Fatty Liver Disease: A Brief Review. Cells 2020, 9, 1047. [Google Scholar] [CrossRef]
- Rossato, M.; Granzotto, M.; Macchi, V.; Porzionato, A.; Petrelli, L.; Calcagno, A.; Vencato, J.; De Stefani, D.; Silvestrin, V.; Rizzuto, R.; et al. Human white adipocytes express the cold receptor TRPM8 which activation induces UCP1 expression, mitochondrial activation and heat production. Mol. Cell. Endocrinol. 2014, 383, 137–146. [Google Scholar] [CrossRef]
- Foresta, C.; Rossato, M.; Nogara, A.; Gottardello, F.; Bordon, P.; Di Virgilio, F. Role of P2-purinergic receptors in rat Leydig cell steroidogenesis. Biochem. J. 1996, 320, 499–504. [Google Scholar] [CrossRef] [Green Version]




| Gene | Forward (5′-3′) | Reverse | Annealing Temperature (°C) | N. of Cycle | Expected Product (bp) | Positive Control |
|---|---|---|---|---|---|---|
| P2X1 | TTCAGGTTTGCCAGGCACTTT | CCCCAAAGATGCCAATTCCA | 59 | 40 | 160 | PBMC |
| P2X2 | GGTGTCATCGGGGTCATTAT | ACCTGAAGTTGTAGCCGTACGA | 56 | 40 | 130 | A-172 |
| P2X3 | CACCTATGAGACCACCAAGTCG | CGTCACGTAATCAGACACATCC | 59 | 40 | 222 | A-172 |
| P2X4 | TGTGATACCAGCTCAGGAGGAAAAC | GCATCATAAATGCACGACTTGAGGT | 66 | 35 | 396 | PBMC |
| P2X5 | CTTCTCCAAAAGCAATGTGATGGAC | GATGAGTACCAGGTCGCAGAAGAAA | 65 | 39 | 406 | PBMC |
| P2X6 | ATGGAATCCGCTTCGACATC | TCCTCCAGTAGAAATGGGCTTC | 60 | 38 | 165 | HEK-293 |
| P2X7 | TGATAAAAGTCTTCGGGATCCGTTT | CCTGGACAAATCTGTGAAGTCCATC | 65 | 39 | 398 | PBMC |
| P2Y1 | ATGTGTGCTTTCAATGACAGGGTTT | TGTGGATGTGGGCATTTCTACTTCT | 66 | 38 | 399 | PBMC |
| P2Y2 | GTGTGCATTCATGAGTGAGGAACC | ATCAGACACAGCCAGGTGGAACATA | 68 | 40 | 313 | PBMC |
| P2Y4 | CCACCTGGCATTGTCAGACACC | GAGTGACCAGGCAGGCACGC | 61 | 38 | 425 | PBMC |
| P2Y6 | CGCTTCCTCTTCTTGCCAACC | CCATCCTGGCGGCACAGGCGGC | 62 | 36 | 365 | Placenta |
| P2Y11 | CTACAGAGCGTATAGCCTGGTGCTG | CCATGTAGAGTAGAGGGTGGACACA | 70 | 39 | 365 | PBMC |
| P2Y12 | CATTCAAACCCTCCAGAATCAACAG | CGATCGATAGTTATCAGTCCCAGGAA | 68 | 36 | 413 | PBMC |
| P2Y13 | AAAAACACTTTGGTGGCCGACTT | ACAGCACGATGCCCACATACAT | 58 | 37 | 154 | PBMC |
| P2Y14 | CACTTCAAGACGACAAACG | GAATATCCATCCTGACACTCC | 60 | 38 | 108 | Placenta |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossato, M.; Favaretto, F.; Granzotto, M.; Crescenzi, M.; Boscaro, A.; Di Vincenzo, A.; Capone, F.; Dalla Nora, E.; Zabeo, E.; Vettor, R. Molecular and Pharmacological Evidence for the Expression of Multiple Functional P2 Purinergic Receptors in Human Adipocytes. Molecules 2022, 27, 1913. https://doi.org/10.3390/molecules27061913
Rossato M, Favaretto F, Granzotto M, Crescenzi M, Boscaro A, Di Vincenzo A, Capone F, Dalla Nora E, Zabeo E, Vettor R. Molecular and Pharmacological Evidence for the Expression of Multiple Functional P2 Purinergic Receptors in Human Adipocytes. Molecules. 2022; 27(6):1913. https://doi.org/10.3390/molecules27061913
Chicago/Turabian StyleRossato, Marco, Francesca Favaretto, Marnie Granzotto, Marika Crescenzi, Alessandra Boscaro, Angelo Di Vincenzo, Federico Capone, Edoardo Dalla Nora, Eva Zabeo, and Roberto Vettor. 2022. "Molecular and Pharmacological Evidence for the Expression of Multiple Functional P2 Purinergic Receptors in Human Adipocytes" Molecules 27, no. 6: 1913. https://doi.org/10.3390/molecules27061913
APA StyleRossato, M., Favaretto, F., Granzotto, M., Crescenzi, M., Boscaro, A., Di Vincenzo, A., Capone, F., Dalla Nora, E., Zabeo, E., & Vettor, R. (2022). Molecular and Pharmacological Evidence for the Expression of Multiple Functional P2 Purinergic Receptors in Human Adipocytes. Molecules, 27(6), 1913. https://doi.org/10.3390/molecules27061913







