Identification of Antibacterial Sterols from Korean Wild Mushroom Daedaleopsis confragosa via Bioactivity- and LC-MS/MS Profile-Guided Fractionation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction of Metabolites from D. confragosa and Bioactivity-Guided Fractionation
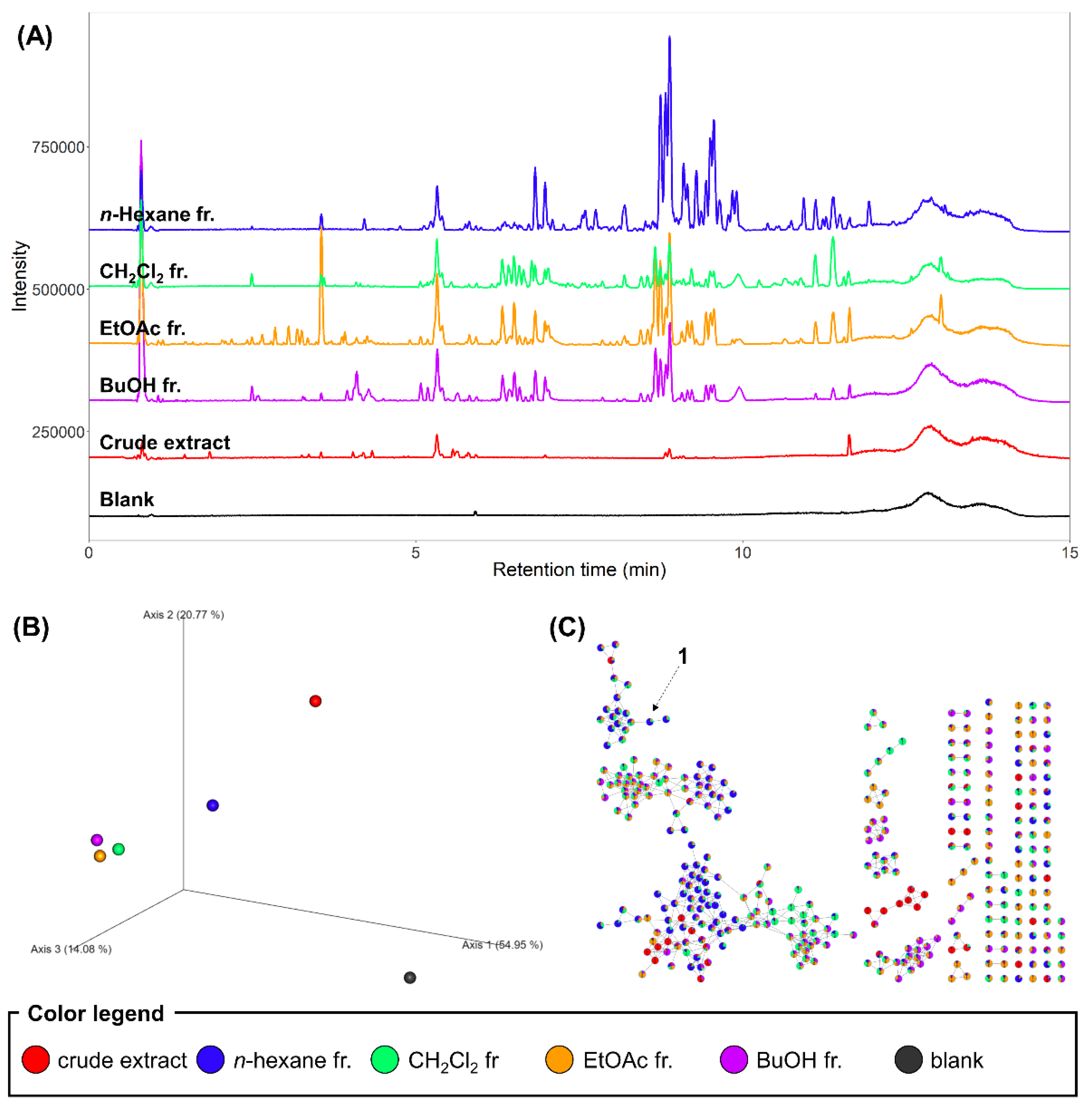
2.2. LC–MS/MS Analysis of the Extract and Fractions
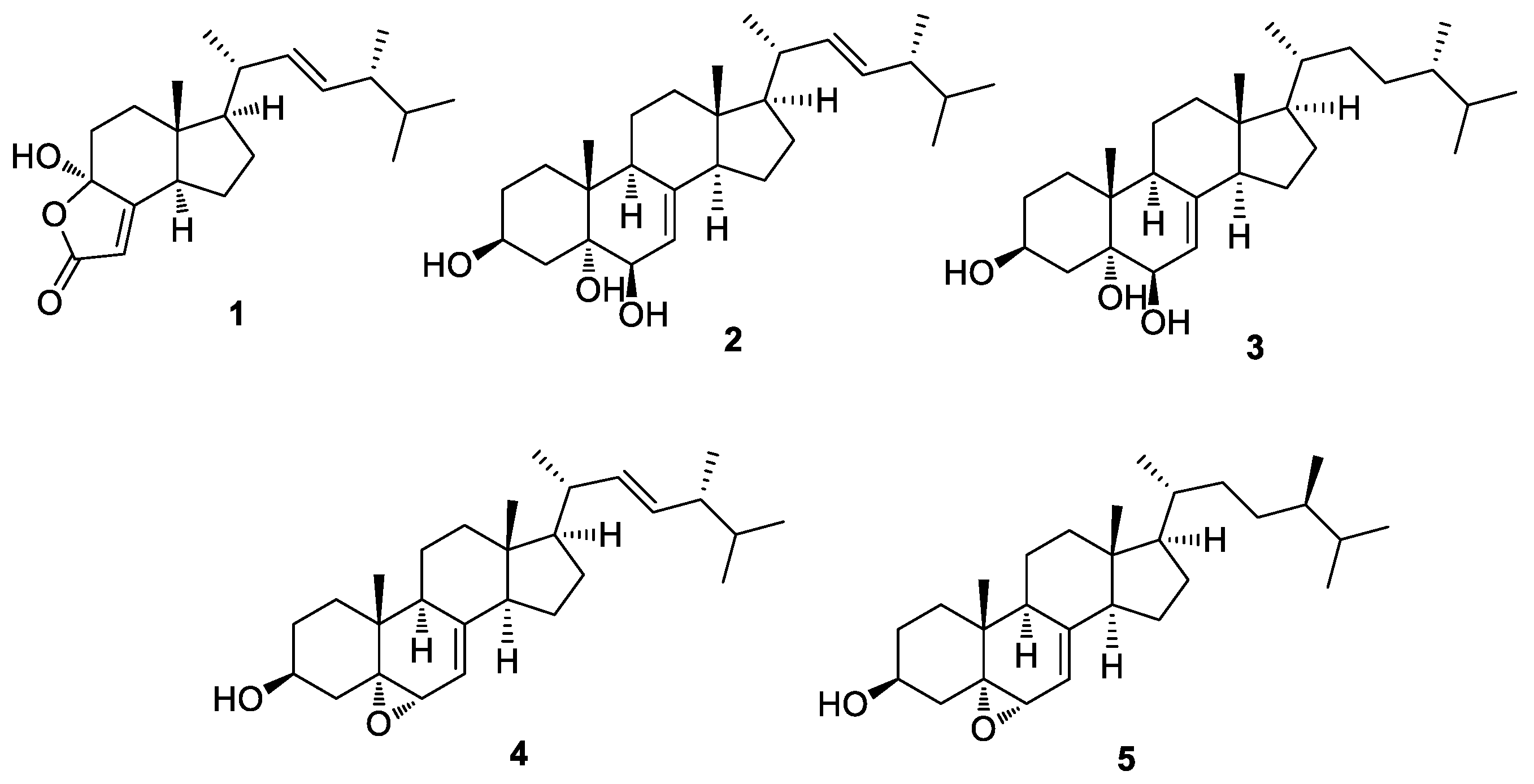
2.3. Isolation and Chemical Characterization of the Compounds
2.4. Evaluation of Antibacterial Activity of the Isolated Compounds against H. pylori
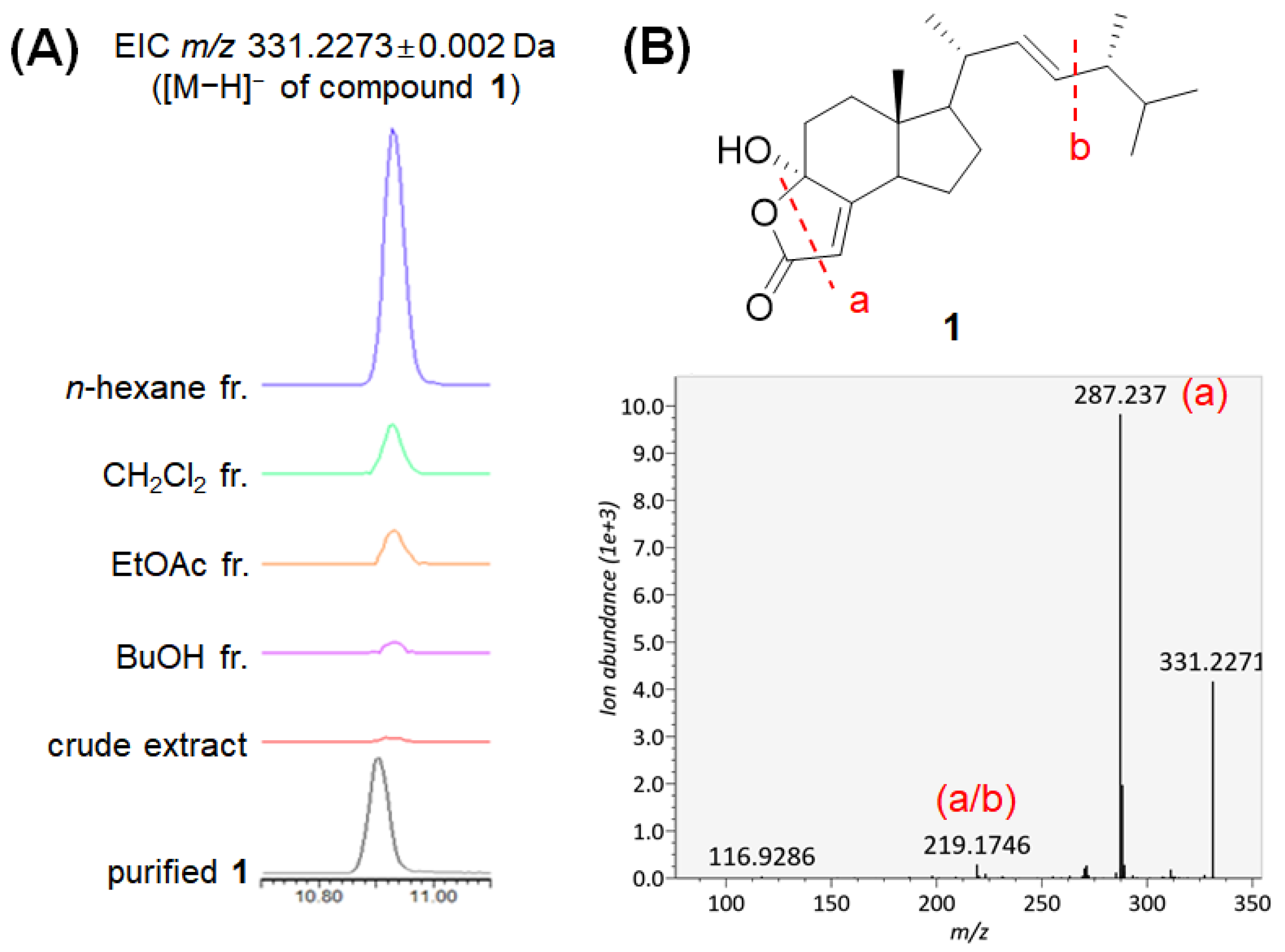
2.5. Distribution Analysis of Compound 1 in D. confragosa and Other Fungi
3. Materials and Methods
3.1. General Experimental Procedure
3.2. Mushroom Material
3.3. Extraction of D. Confragosa and Solvent Partition
3.4. LC–MS/MS Analysis
3.5. Isolation of Compounds from the Hexane and CH2Cl2 Fractions
3.6. Helicobacter Pylori Culture
3.7. Anti-Helicobacter Pylori Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Patel, S.; Goyal, A. Recent developments in mushrooms as anti-cancer therapeutics: A review. 3 Biotech 2011, 2, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Paterson, R.; Lima, N. Biomedical effects of mushrooms with emphasis on pure compounds. Biomed. J. 2014, 37, 357–368. [Google Scholar] [CrossRef]
- Ha, J.W.; Kim, J.; Kim, H.; Jang, W.; Kim, K.H. Mushrooms: An important source of natural bioactive compounds. Nat. Prod. Sci. 2020, 26, 118–131. [Google Scholar]
- Lee, S.; Ryoo, R.; Choi, J.H.; Kim, J.H.; Kim, S.H.; Kim, K.H. Trichothecene and tremulane sesquiterpenes from a hallucinogenic mushroom Gymnopilus junonius and their cytotoxicity. Arch. Pharmacal Res. 2020, 43, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, D.; Ryoo, R.; Kim, J.-C.; Park, H.B.; Kang, K.S.; Kim, K.H. Calvatianone, a Sterol Possessing a 6/5/6/5-Fused Ring System with a Contracted Tetrahydrofuran B-ring, from the fruiting bodies of Calvatia nipponica. J. Nat. Prod. 2020, 83, 2737–2742. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, C.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Youn, U.J.; Ryoo, R.; Bae, H.Y.; Kim, K.H. Ergopyrone, a Styrylpyrone-Fused Steroid with a Hexacyclic 6/5/6/6/6/5 Skeleton from a Mushroom Gymnopilus orientispectabilis. Org. Lett. 2021, 23, 3315–3319. [Google Scholar] [CrossRef] [PubMed]
- Rho, T.; Jeong, H.W.; Hong, Y.D.; Yoon, K.; Cho, J.Y.; Yoon, K.D. Identification of a novel triterpene saponin from Panax ginseng seeds, pseudoginsenoside RT8, and its antiinflammatory activity. J. Ginseng Res. 2020, 44, 145–153. [Google Scholar] [CrossRef]
- Lee, S.R.; Kang, H.; Yoo, M.J.; Yu, J.S.; Lee, S.; Yi, S.A.; Kim, K.H. Anti-adipogenic pregnane steroid from a Hydractinia-associated fungus, Cladosporium sphaerospermum SW67. Nat. Prod. Sci. 2020, 26, 230–235. [Google Scholar]
- Yu, J.S.; Park, M.; Pang, C.; Rashan, L.; Jung, W.H.; Kim, K.H. Antifungal Phenols from Woodfordia uniflora Collected in Oman. J. Nat. Prod. 2020, 83, 2261–2268. [Google Scholar] [CrossRef]
- Lee, D.; Choi, S.; Yamabe, N.; Kim, K.H.; Kang, K.S. Recent Findings on the Mechanism of Cisplatin-Induced Renal Cytotoxicity. Nat. Prod. Sci. 2020, 26, 28–49. [Google Scholar]
- Baek, S.C.; Lee, B.S.; Yi, S.A.; Yu, J.S.; Lee, J.; Ko, Y.-J.; Pang, C.; Kim, K.H. Discovery of Dihydrophaseic Acid Glucosides from the Florets of Carthamus tinctorius. Plants 2020, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.S.; Lee, S.; Yu, J.S.; Baek, S.C.; Cho, Y.-C.; Kim, K.H. Megastigmane Derivatives from the Cladodes of Opuntia humifusa and Their Nitric Oxide Inhibitory Activities in Macrophages. J. Nat. Prod. 2020, 83, 684–692. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.-C.; Ko, Y.-J.; Kim, S.-H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharmacal Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Pham, H.T.; Lee, K.H.; Jeong, E.; Woo, S.; Yu, J.; Kim, W.-Y.; Lim, Y.W.; Kim, K.H.; Bin Kang, K. Species Prioritization Based on Spectral Dissimilarity: A Case Study of Polyporoid Fungal Species. J. Nat. Prod. 2021, 84, 298–309. [Google Scholar] [CrossRef]
- Mukhin, V.A.; Zhuykova, E.V.; Vladykina, V.D.; Badalyan, S.M. Notes on medicinal polypore species from the genus Daedaleopsis (Agaricomycetes), distributed in the Asian part of Russia. Int. J. Med. 2020, 22, 775–780. [Google Scholar] [CrossRef]
- Ko, K.S.; Jung, H. Molecular phylogeny of Trametes and related genera. Antonie Van Leeuwenhoek 1999, 75, 191–199. [Google Scholar] [CrossRef]
- Nothias, L.F.; Petras, D.; Schmid, R.; Dührkop, K.; Rainer, J.; Sarvepalli, A.; Dorrestein, P.C. Feature-based molecular networking in the GNPS analysis environment. Nat. Methods. 2020, 17, 905–908. [Google Scholar] [CrossRef]
- Zhao, J.-Y.; Feng, T.; Li, Z.-H.; Dong, Z.-J.; Zhang, H.-B.; Liu, J.-K. Sesquiterpenoids and an ergosterol from cultures of the fungus Daedaleopsis tricolor. Nat. Prod. Bioprospecting 2013, 3, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Shim, S.H.; Kim, J.S.; Kang, S.S. Constituents from the fruiting bodies of Ganoderma applanatum and their aldose reductase inhibitory activity. Arch. Pharmacal Res. 2006, 29, 479–483. [Google Scholar] [CrossRef]
- Iorizzi, M.; Minale, L.; Riccio, R.; Lee, J.-S.; Yasumoto, T. Polar Steroids from the Marine Scallop Patinopecten yessoensis. J. Nat. Prod. 1988, 51, 1098–1103. [Google Scholar] [CrossRef]
- Bok, J.W.; Lermer, L.; Chilton, J.; Klingeman, H.G.; Towers, G.N. Antitumor sterols from the mycelia of Cordyceps sinensis. Phytochemistry 1999, 51, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Liao, X.; Du, B.; Zhou, X.; Huang, Q.; Wu, C. A series of new 5, 6-epoxysterols from a Chinese sponge Ircinia aruensis. Steroids 2008, 73, 568–573. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.J.; George, A.E.; Trainor, E.A.; Horton, K.E.; Hildebrandt, E.; Testerman, T.L. Cholesterol Enhances Helicobacter pylori Resistance to Antibiotics and LL-37. Antimicrob. Agents Chemother. 2011, 55, 2897–2904. [Google Scholar] [CrossRef] [Green Version]
- Chey, W.D.; Wong, B.C. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am. J. Gastroenterol. 2007, 102, 1808–1825. [Google Scholar] [CrossRef]
- Kobayashi, J.; Kawakubo, M.; Fujii, C.; Arisaka, N.; Miyashita, M.; Sato, Y.; Nakayama, J. Cholestenone functions as an antibiotic against Helicobacter pylori by inhibiting biosynthesis of the cell wall component CGL. Proc. Natl. Acad. Sci. USA 2021, 118, e2016469118. [Google Scholar] [CrossRef]
- Wang, M.; Jarmusch, A.K.; Vargas, F.; Aksenov, A.A.; Gauglitz, J.M.; Weldon, K.; Petras, D.; Da Silva, R.; Quinn, R.; Melnik, A.V.; et al. Mass spectrometry searches using MASST. Nat. Biotechnol. 2020, 38, 23–26. [Google Scholar] [CrossRef]
- Kim, D.; Shin, Y.; Lee, S.; Wimonmuang, K.; Bin Kang, K.; Lee, Y.; Yun, S. FgPKS7 is an essential player in mating-type-mediated regulatory pathway required for completing sexual cycle in Fusarium graminearum. Environ. Microbiol. 2021, 23, 1972–1990. [Google Scholar] [CrossRef]
- Lee, S.B.; Taylor, J.W. Isolation of DNA from fungal mycelia and single spores. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Sninsky, D.H.G.J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 282–287. [Google Scholar]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with Global Natural Products Social Molecular Networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Concentrations | Inhibition (%) |
|---|---|---|
| Methanolic extract | 100 μg/mL | 22.4 |
| n-hexane fraction | 38.2 | |
| CH2Cl2 fraction | 23.3 | |
| EtOAc fraction | 16.0 | |
| BuOH fraction | 15.6 | |
| Quercetin a | 100 μM | 22.2 |
| Metronidazole a | 73.5 |
| Compound | Concentrations | Inhibition (%) |
|---|---|---|
| 1 | 100 μM | 33.9 |
| 2 | 8.6 | |
| 3 | 18.5 | |
| 4 | 6.8 | |
| 5 | 3.4 | |
| Quercetin a | 100 μM | 22.2 |
| Metronidazole a | 73.5 |
| Dataset | Species | Family |
|---|---|---|
| MSV000084977 | Fusarium graminearum | Nectraiaceae |
| MSV000085071 | Leucoagaricus atrodisca | Agaricaceae |
| Lepiota spheniscuspora | ||
| Amanita pantherinoides | Amanitaceae | |
| Amanita protecta | ||
| Phylloporus arenicola | Boletaceae | |
| Suillelus amygdalinus | ||
| Bondarzewia occidentalis | Bondarzewiaceae | |
| Cantharellus californicus | Cantharellaceae | |
| Cantharellus subalbidus | ||
| Craterellus tubaeformis | ||
| Clavariadelphus mucronatus | Clavariadelphaceae | |
| Clavariadelphus occidentalis | ||
| Clavulina cinerea | Clavulinaceae | |
| Cortinarius alboglobosus | Cortinariaceae | |
| Cortinarius albogfragrans | ||
| Cortinarius alboviolaceus | ||
| Cortinarius anomalus | ||
| Cortinarius cinnamomeus | ||
| Cortinarius collinitus | ||
| Cortinarius caperatus | ||
| Cortinarius causticus | ||
| Cortinarius croceus | ||
| Clitopilus hobsonii | Entolomataceae | |
| Gomphus vlavatus | Gomphaceae | |
| Ramaria araispora var. araiospora | ||
| Ramaria formosa | ||
| Ramaria gelatonosa var. oregonensis | ||
| Ramaria rubripermanens | ||
| Ramaria sandaracina var. sandaracina | ||
| Hydnum umbilicatum | Hydnaceae | |
| Armillaria solidipes | Physalacriaceae | |
| Polyporus leptocephalus | Polyporaceae | |
| Trametes hirsuta | ||
| Trametes versicolor | ||
| Coprinopsis atramentaria | Psathyrellaceae | |
| Lactarius aestivus | Russulaceae | |
| Lactarius rubrilacteus | ||
| Lactarius scrobiculatus | ||
| Russula americana | ||
| Russula xerampelina | ||
| Pholiota flammans | Strophariaceae | |
| Clitocybe nuda | Tricholomataceae | |
| Collybia cirrhata | ||
| Leucopaxillus albissimus | ||
| Tricholoma inamoenum | ||
| Tricholoma mutabile | ||
| Tricholoma pardinum | ||
| Tricholoma portentosum | ||
| MSV000085974 | Cerrena unicolor | Polyporaceae |
| Coriolopsis strumose | ||
| Daedaleopsis tricolor | ||
| Microporus vernicipes | ||
| Polyporus brumalis | ||
| Trametes orientalis | ||
| Trametes suaveolens | ||
| Trametopsis cervina |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Na, M.W.; Lee, E.; Kang, D.-M.; Jeong, S.Y.; Ryoo, R.; Kim, C.-Y.; Ahn, M.-J.; Kang, K.B.; Kim, K.H. Identification of Antibacterial Sterols from Korean Wild Mushroom Daedaleopsis confragosa via Bioactivity- and LC-MS/MS Profile-Guided Fractionation. Molecules 2022, 27, 1865. https://doi.org/10.3390/molecules27061865
Na MW, Lee E, Kang D-M, Jeong SY, Ryoo R, Kim C-Y, Ahn M-J, Kang KB, Kim KH. Identification of Antibacterial Sterols from Korean Wild Mushroom Daedaleopsis confragosa via Bioactivity- and LC-MS/MS Profile-Guided Fractionation. Molecules. 2022; 27(6):1865. https://doi.org/10.3390/molecules27061865
Chicago/Turabian StyleNa, Myung Woo, Eunjin Lee, Dong-Min Kang, Se Yun Jeong, Rhim Ryoo, Chul-Young Kim, Mi-Jeong Ahn, Kyo Bin Kang, and Ki Hyun Kim. 2022. "Identification of Antibacterial Sterols from Korean Wild Mushroom Daedaleopsis confragosa via Bioactivity- and LC-MS/MS Profile-Guided Fractionation" Molecules 27, no. 6: 1865. https://doi.org/10.3390/molecules27061865
APA StyleNa, M. W., Lee, E., Kang, D.-M., Jeong, S. Y., Ryoo, R., Kim, C.-Y., Ahn, M.-J., Kang, K. B., & Kim, K. H. (2022). Identification of Antibacterial Sterols from Korean Wild Mushroom Daedaleopsis confragosa via Bioactivity- and LC-MS/MS Profile-Guided Fractionation. Molecules, 27(6), 1865. https://doi.org/10.3390/molecules27061865








