Light Promotes the Immobilization of U(VI) by Ferrihydrite
Abstract
:1. Introduction
2. Results and Discussion
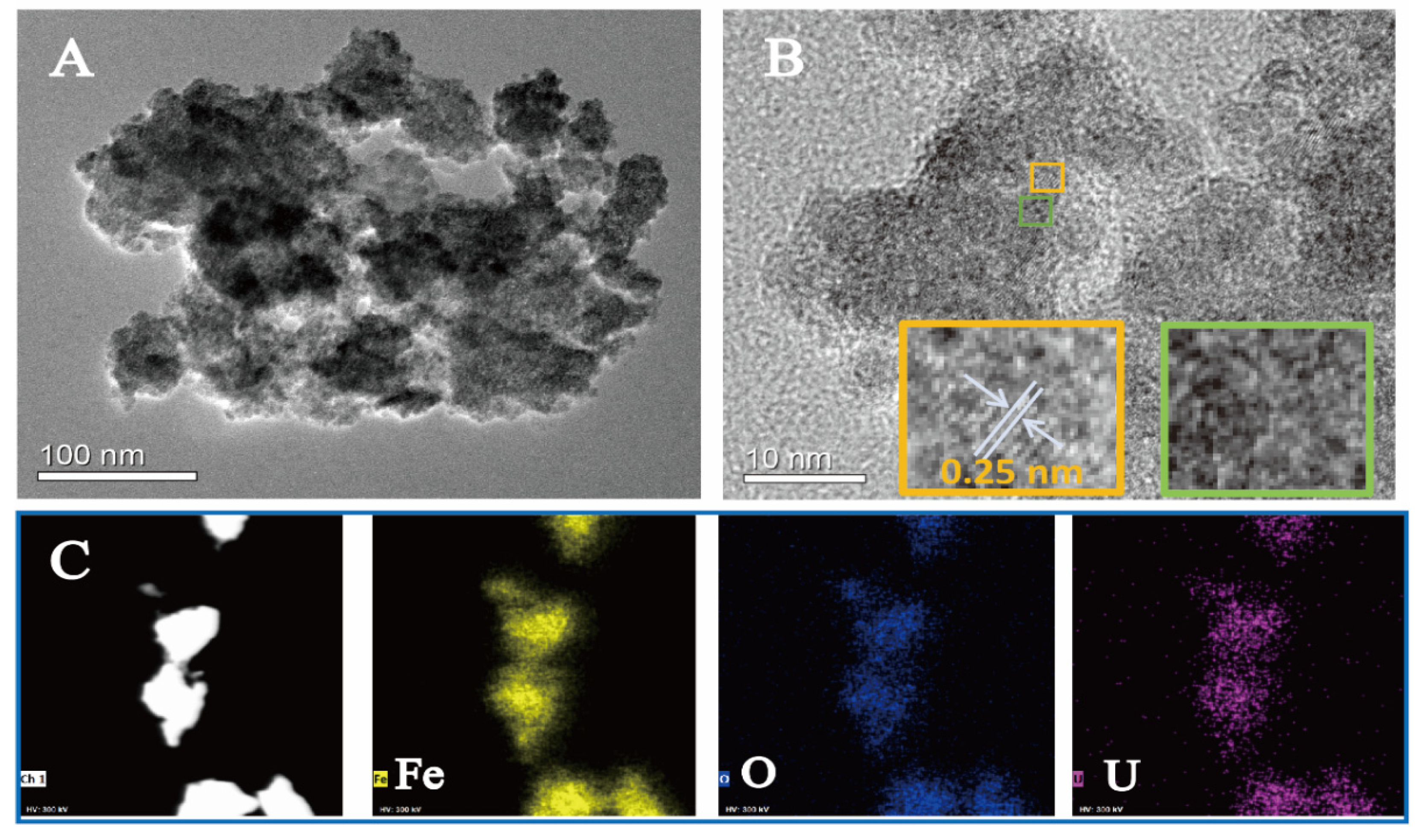
2.1. Physicochemical Properties of Ferrihydrite
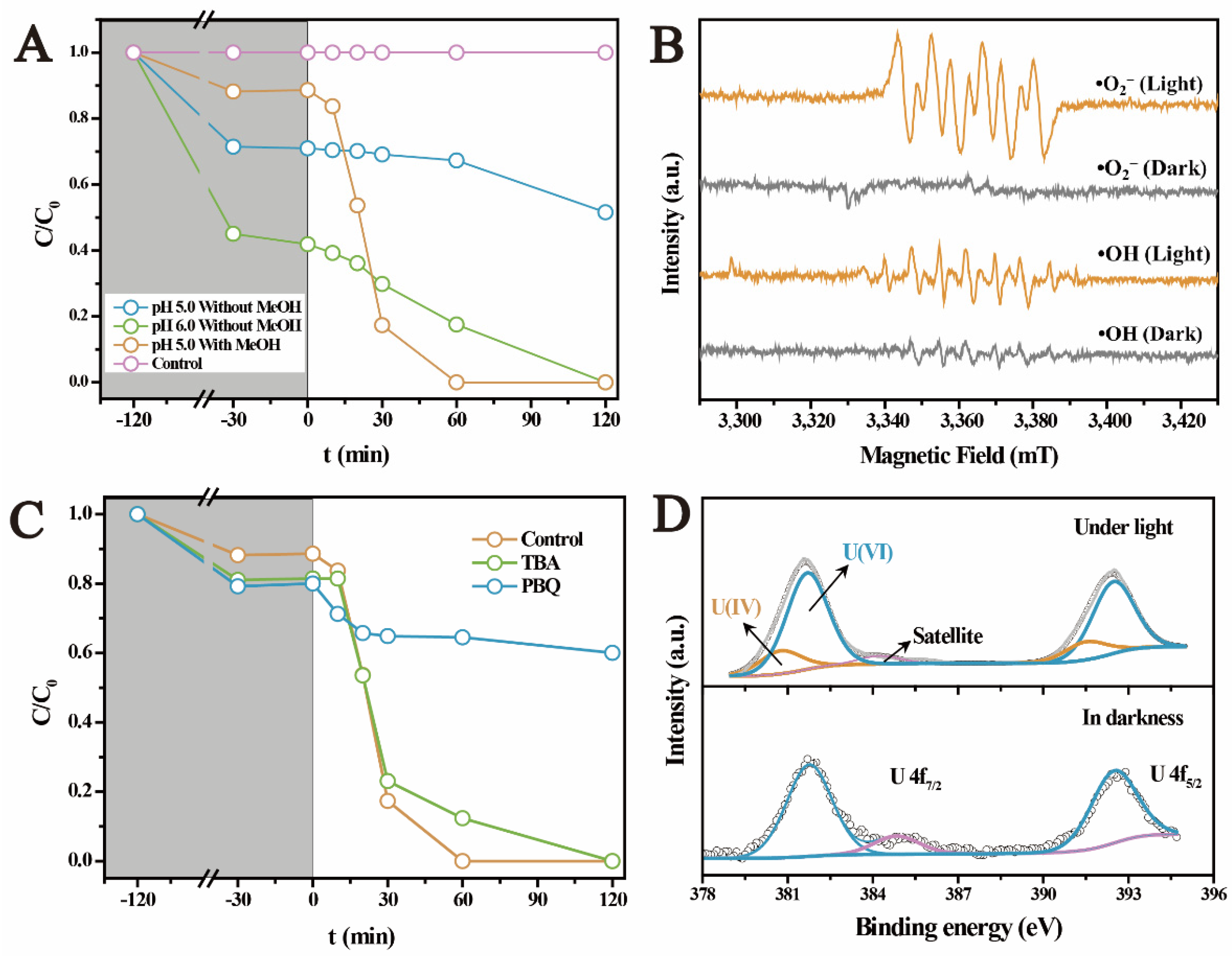
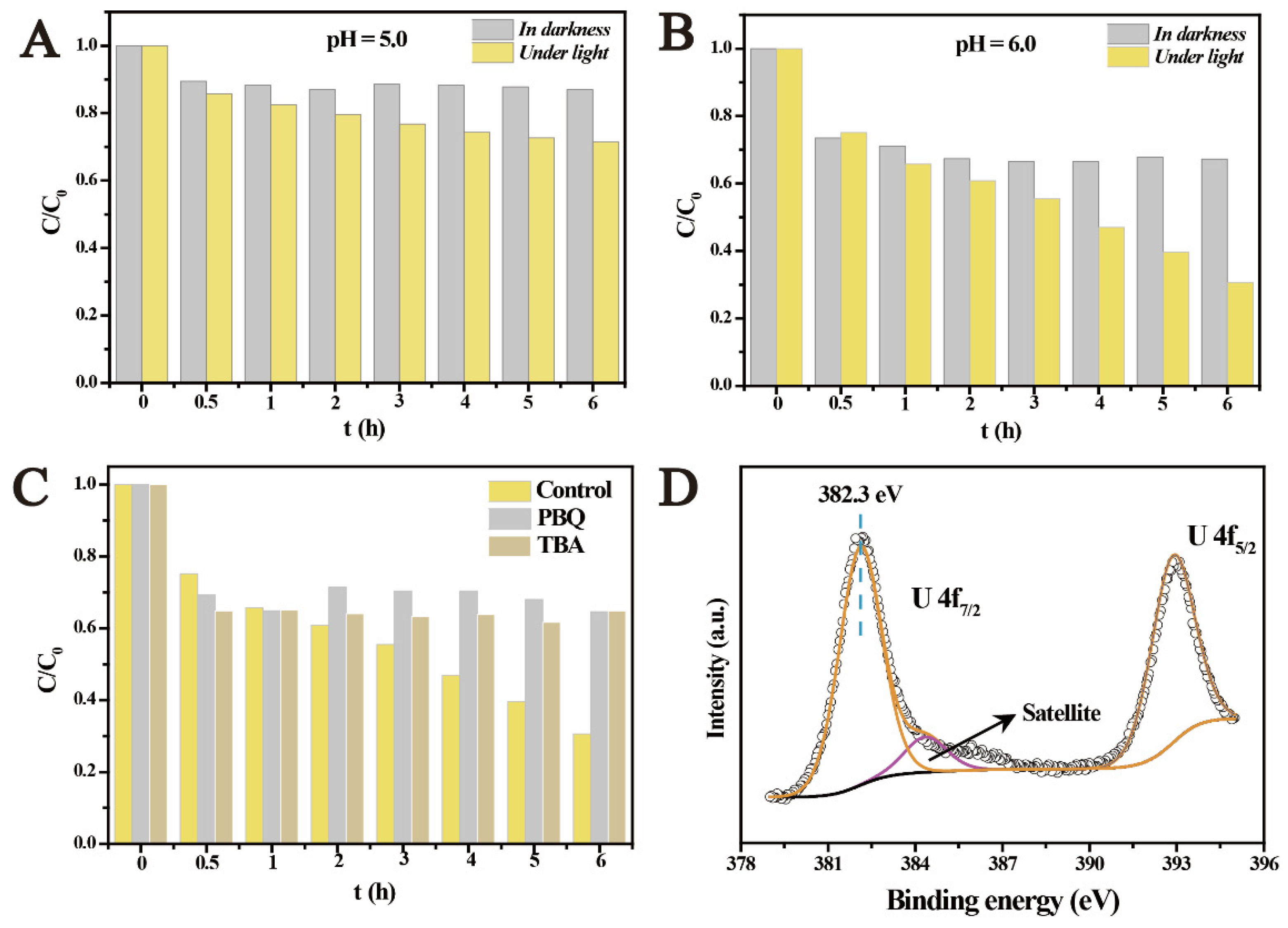
2.2. The Photocatalytic U(VI) Reduction under Anaerobic Conditions
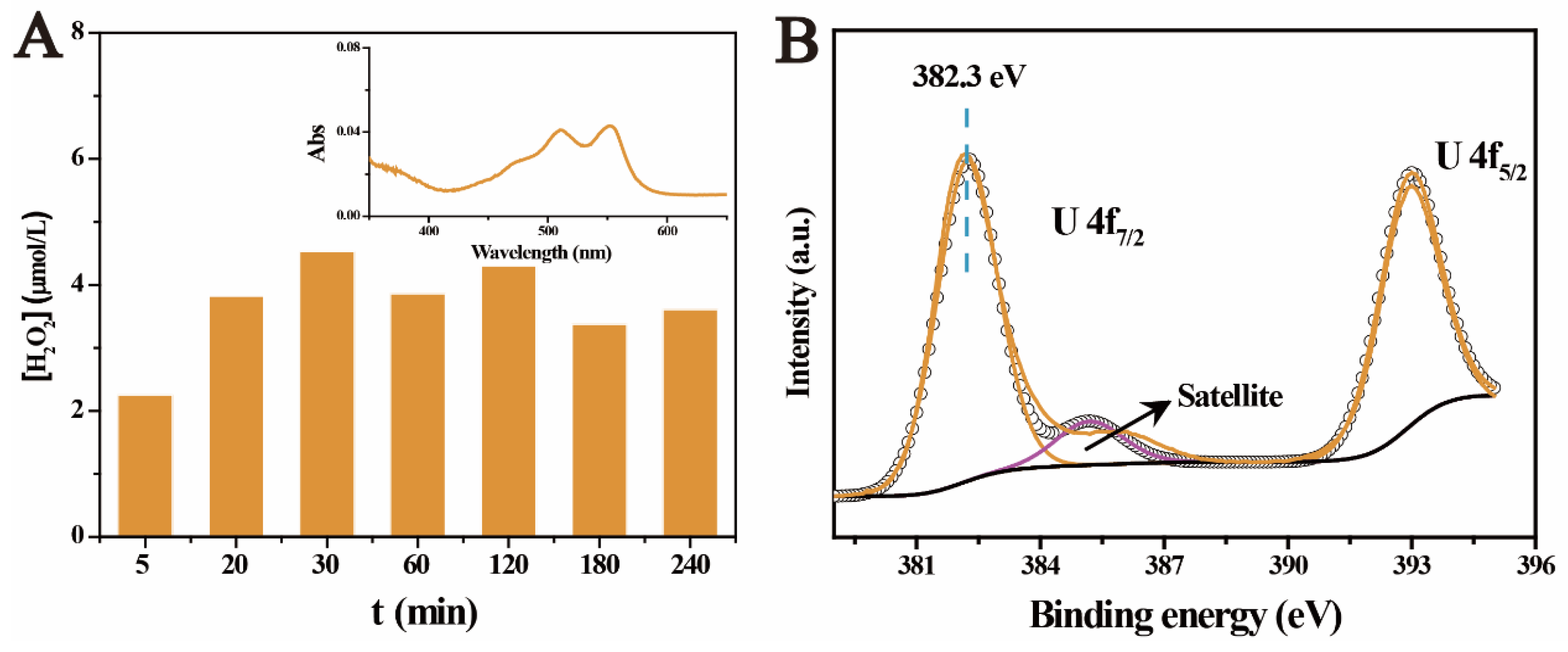
2.3. The Photocatalytic Immobilization of U(VI) under Aerobic Conditions
3. Materials and Methods
3.1. Materials
3.2. Photocatalysis Test
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Liu, C.; Hsu, P.-C.; Xie, J.; Zhao, J.; Wu, T.; Wang, H.; Liu, W.; Zhang, J.; Chu, S.; Cui, Y. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat. Energy 2017, 2, 17007. [Google Scholar] [CrossRef]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- NEA; IAEA. Uranium 2020: Resources, Production and Demand. A Joint Report by NEA and IAEA; NEA No. 7551. 2020. Available online: https://www.oecd-nea.org/jcms/pl_52718/uranium-2020-resources-production-and-demand?details=true (accessed on 10 January 2022).
- Wang, X.; Dai, X.; Shi, C.; Wan, J.; Silver, M.A.; Zhang, L.; Chen, L.; Yi, X.; Chen, B.; Zhang, D.; et al. A 3,2-Hydroxypyridinone-based Decorporation Agent that Removes Uranium from Bones In Vivo. Nat. Commun. 2019, 10, 2570. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Rodriguez, A.; Luna-Velasco, A.; Field, J.A.; Sierra-Alvarez, R. Anaerobic bioremediation of hexavalent uranium in groundwater by reductive precipitation with methanogenic granular sludge. Water Res. 2010, 44, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Ilton, E.S.; Haiduc, A.; Cahill, A.C.L.; Felmy, A.R. Mica Surfaces Stabilize Pentavalent Uranium. Inorg. Chem. 2005, 44, 2986–2988. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qiang, S.; Wang, Y.; Wu, W.; Li, P.; Qin, H.; Fan, Q. Adsorption of U(VI) on the natural soil around a very low-level waste repository. J. Environ. Radioact. 2021, 233, 106619. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, J.; Li, P.; Qin, H.; Liang, J.; Fan, Q. The adsorption of U(VI) on magnetite, ferrihydrite and goethite. Environ. Technol. Innov. 2021, 23, 101615. [Google Scholar] [CrossRef]
- Bone, S.E.; Dynes, J.J.; Cliff, J.; Bargar, J.R. Uranium(IV) adsorption by natural organic matter in anoxic sediments. Proc. Natl. Acad. Sci. USA 2017, 114, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, J.W.; Anders, E. Chemical composition of Earth, Venus, and Mercury. Proc. Natl. Acad. Sci. USA 1980, 77, 6973–6977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, R.C.; Anizelli, P.R.; Di Mauro, E.; Valezi, D.F.; Da Costa, A.C.S.; Zaia, C.T.B.V.; Zaia, D.A.M. The effect of pH and ionic strength on the adsorption of glyphosate onto ferrihydrite. Geochem. Trans. 2019, 20, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, E.; McLennan, S.M.; Sklute, E.C.; Dyar, M.D. Stability and fate of ferrihydrite during episodes of water/rock inter-actions on early Mars: An experimental approach. J. Geophys. Res. Planets 2017, 122, 358–382. [Google Scholar] [CrossRef]
- Boily, J.-F.; Song, X. Direct identification of reaction sites on ferrihydrite. Commun. Chem. 2020, 3, 79. [Google Scholar] [CrossRef]
- Yang, Y.; Takizawa, S.; Sakai, H.; Murakami, M.; Watanabe, N. Removal of organic matter and phosphate using ferrihydrite for reduction of microbial regrowth potential. Water Sci. Technol. 2012, 66, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.; Fu, Q.L.; Xiong, J.W.; Hong, C.; Hu, H.Q.; Violante, A. Adsorption of phosphate onto ferrihydrite and ferrihydrite-humic acid complexes. Pedosphere 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Tokoro, C.; Kadokura, M.; Kato, T. Mechanism of arsenate coprecipitation at the solid/liquid interface of ferrihydrite: A perspective review. Adv. Powder Technol. 2020, 31, 859–866. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Lu, A.H.; Li, Y.; Ding, H.R.; Xu, X.M.; Li, Y.; Ren, G.; Liang, J.; Liu, Y.; Hong, H.; Chen, N.; et al. Photoelectric conversion on Earth’s surface via widespread Fe- and Mn-mineral coatings. Proc. Natl. Acad. Sci. USA 2019, 116, 9741–9746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, D.H.; Ng, T.W.; An, T.C.; Li, G.; Li, Y.; Yip, H.Y.; Zhao, H.J.; Lu, A.H.; Wong, P.K. A recyclable mineral catalyst for visible-light-driven photocatalytic inactivation of bacteria: Natural magnetic sphalerite. Environ. Sci. Technol. 2013, 47, 11166–11173. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Cheng, Q.; Wang, J.; Wu, Q.; Zhang, W. Combined first-principles calculations and experimental study on the photocatalytic mechanism of natural dolomite. RSC Adv. 2021, 11, 24416–24423. [Google Scholar] [CrossRef]
- Tang, X.; Tang, R.; Xiong, S.; Zheng, J.; Li, L.; Zhou, Z.; Gong, D.; Deng, Y.; Su, L.; Liao, C. Application of natural minerals in photocatalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 812, 152434. [Google Scholar] [CrossRef] [PubMed]
- Shelton, T.L.; Bensema, B.L.; Brune, N.K.; Wong, C.; Yeh, M.; Osterloh, F.E. Photocatalytic water oxidation with iron oxide hydroxide (rust) nanoparticles. J. Photon. Energy 2016, 7, 012003. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Stengl, V.; Subrt, J.; Houskova, V.; Kalenda, P. Photocatalytic efficiency of iron oxides: Degradation of 4-chlorophenol. J. Phys. Chem. Solids 2007, 68, 721–724. [Google Scholar] [CrossRef]
- Mohan, H.; Ramasamy, M.; Ramalingam, V.; Natesan, K.; Duraisamy, M.; Venkatachalam, J.; Shin, T.; Seralathan, K.K. Enhanced visible light-driven photocatalysis of iron-oxide/titania composite: Norfloxacin degradation mechanism and toxicity study. J. Hazard. Mater. 2021, 412, 125330. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, S.; Zhu, M.; Ginder-vogel, M.; Yin, H.; Liu, F.; Tan, W.; Feng, X. The presence of ferrihydrite promotes abiotic Mn(II) oxidation and formation of birnessite. Soil Sci. Soc. Am. J. 2015, 79, 1297–1305. [Google Scholar] [CrossRef]
- Lan, S.; Wang, X.; Yang, P.; Qin, Z.; Zhu, M.; Zhang, J.; Liu, F.; Tan, W.; Huang, Q.; Feng, X. The catalytic effect of AQDS as an electron shuttle on Mn(II) oxidation to birnessite on ferrihydrite at circumneutral pH. Geochim. Cosmochim. Acta 2019, 247, 175–190. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, Y.; Liang, J.; Pan, D.; Qiang, S.; Fan, Q. An overview and recent progress in the heterogeneous photo-catalytic reduction of U(VI). J. Photochem. Photobiol. C Photochem. Rev. 2019, 41, 100320. [Google Scholar] [CrossRef]
- Li, Z.J.; Huang, Z.W.; Guo, W.L.; Wang, L.; Zheng, L.R.; Chai, Z.F.; Shi, W.Q. Enhanced photocatalytic removal of uranium(VI) from aqueous solution by magnetic TiO2/Fe3O4 and its graphene composite. Environ. Sci. Technol. 2017, 51, 5666–5674. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Peng, T.; Wang, Y.; Liang, J.; Pan, D.; Fan, Q. Heterostructure of anatase-rutile aggregates boosting the photoreduction of U(VI). Appl. Surf. Sci. 2019, 483, 670–676. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, J.; Liang, J.; Pan, D.; Li, P.; Fan, Q. Efficient recovery of uranium from saline lake brine through photocatalytic reduction. J. Mol. Liq. 2020, 308, 113007. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Wang, W.; Peng, T.; Liang, J.; Li, P.; Pan, D.; Fan, Q.; Wu, W. Visible light driven Ti3+ self-doped TiO2 for adsorption-photocatalysis of aqueous U(VI). Environ. Pollut. 2020, 262, 114373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, W.; Li, A.; Zhang, D.; Li, X.; Zhai, F.; Chen, L.; Chen, L.; Wang, Y.; Wang, S. Three mechanisms in one material: Uranium capture by a polyoxometalate–organic framework through combined complexation, chemical reduction, and pho-tocatalytic reduction. Angew. Chem. Int. Ed. 2019, 58, 16110–16114. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Wang, J.; Wang, Y.; Dong, L.; Wang, W.; Geng, R.; Ding, Z.; Luo, D.; Pan, D.; Liang, J.; et al. Ultrafast recovery of aqueous uranium: Photocatalytic U(VI) reduction over CdS/g-C3N4. Chem. Eng. J. 2021, 425, 131552. [Google Scholar] [CrossRef]
- Li, P.; Wang, J.; Wang, Y.; Liang, J.; He, B.; Pan, D.; Fan, Q.; Wang, X. Photoconversion of U(VI) by TiO2: An efficient strategy for seawater uranium extraction. Chem. Eng. J. 2019, 365, 231–241. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, T.; Li, K. One-step synthesis of mesoporous two-line ferrihydrite for effective elimination of arsenic contaminants from natural water. Dalton Trans. 2011, 40, 2062–2066. [Google Scholar] [CrossRef] [PubMed]
- Motonari, A.; Kei, N.; Ryo, T.; Jun, A.; Katsumi, T.; Yasushige, M.; Fumio, U. Comparison of electrochemical impedance spectroscopy between illumination and dark conditions. Chem. Lett. 2011, 40, 890–892. [Google Scholar]
- Iqbal, N.; Afzal, A.; Khan, I.; Khan, M.S.; Qurashi, A. Molybdenum impregnated g-C3N4 nanotubes as potentially active photocatalyst for renewable energy applications. Sci. Rep. 2021, 11, 16886. [Google Scholar] [CrossRef]
- Fang, J.; Fan, H.; Li, M.; Long, C. Nitrogen self-doped graphitic carbon nitride as efficient visible light photocatalyst for hydrogen evolution. J. Mater. Chem. A 2015, 3, 13819–13826. [Google Scholar] [CrossRef]
- Kamat, P.V.; Jin, S. Semiconductor photocatalysis: “tell us the complete story!”. ACS Energy Lett. 2018, 3, 622–623. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, Y.; Wang, W.; Ding, Z.; Geng, R.; Li, P.; Pan, D.; Liang, J.; Qin, H.; Fan, Q. Tunable mesoporous g-C3N4 nanosheets as a metal-free catalyst for enhanced visible-light-driven photocatalytic reduction of U(VI). Chem. Eng. J. 2020, 383, 123193. [Google Scholar] [CrossRef]
- Salomone, V.N.; Meichtry, J.M.; Schinelli, G.; Leyva, A.G.; Litter, M.I. Photochemical reduction of U(VI) in aqueous solution in the presence of 2-propanol. J. Photochem. Photobiol. A Chem. 2014, 277, 19–26. [Google Scholar] [CrossRef]
- Li, P.; Wang, Y.; Wang, J.; Dong, L.; Zhang, W.; Lu, Z.; Liang, J.; Pan, D.; Fan, Q. Carboxyl groups on g-C3N4 for boosting the photocatalytic U(VI) reduction in the presence of carbonates. Chem. Eng. J. 2021, 414, 128810. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Smith, S.C.; Dohnalkova, A.C.; Russell, C.K. Transformation of 2-line fer-rihydrite to 6-line ferrihydrite under oxic and anoxic conditions. Am. Mineral. 2003, 88, 1903–1914. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Jiang, S.; Song, S.; Sun, C. Constructing effective photocatalytic purification system with P-introduced g-C3N4 for elimination of UO22+. Appl. Surf. Sci. 2018, 430, 371–379. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Kim, Y.-H.; Lee, S.-Y.; Lee, J.-W.; Joe, K.-S.; Lee, E.-H.; Kim, J.-S.; Song, K.; Song, K.-C. Precipitation Characteristics of Uranyl Ions at Different pHs Depending on the Presence of Carbonate Ions and Hydrogen Peroxide. Environ. Sci. Technol. 2009, 43, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Debets, P. X-ray diffraction data on hydrated uranium peroxide. J. Inorg. Nucl. Chem. 1963, 25, 727–730. [Google Scholar] [CrossRef]
- Hsieh, H.-S.; Wu, R.; Jafvert, C. Light-Independent Reactive Oxygen Species (ROS) Formation through Electron Transfer from Carboxylated Single-Walled Carbon Nanotubes in Water. Environ. Sci. Technol. 2014, 48, 11330–11336. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, F.; Tan, W.; Li, W.; Feng, X.; Sparks, D.L. Characteristics of phosphate adsorption-desorption onto ferrihydrite: Comparison with well-crystalline Fe(hydr)oxides. Soil Sci. 2013, 178, 1–11. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, J.; Ding, Z.; Wang, W.; Song, J.; Li, P.; Liang, J.; Fan, Q. Light Promotes the Immobilization of U(VI) by Ferrihydrite. Molecules 2022, 27, 1859. https://doi.org/10.3390/molecules27061859
Wang Y, Wang J, Ding Z, Wang W, Song J, Li P, Liang J, Fan Q. Light Promotes the Immobilization of U(VI) by Ferrihydrite. Molecules. 2022; 27(6):1859. https://doi.org/10.3390/molecules27061859
Chicago/Turabian StyleWang, Yun, Jingjing Wang, Zhe Ding, Wei Wang, Jiayu Song, Ping Li, Jianjun Liang, and Qiaohui Fan. 2022. "Light Promotes the Immobilization of U(VI) by Ferrihydrite" Molecules 27, no. 6: 1859. https://doi.org/10.3390/molecules27061859
APA StyleWang, Y., Wang, J., Ding, Z., Wang, W., Song, J., Li, P., Liang, J., & Fan, Q. (2022). Light Promotes the Immobilization of U(VI) by Ferrihydrite. Molecules, 27(6), 1859. https://doi.org/10.3390/molecules27061859





