High Sorption and Selective Extraction of Actinides from Aqueous Solutions
Abstract
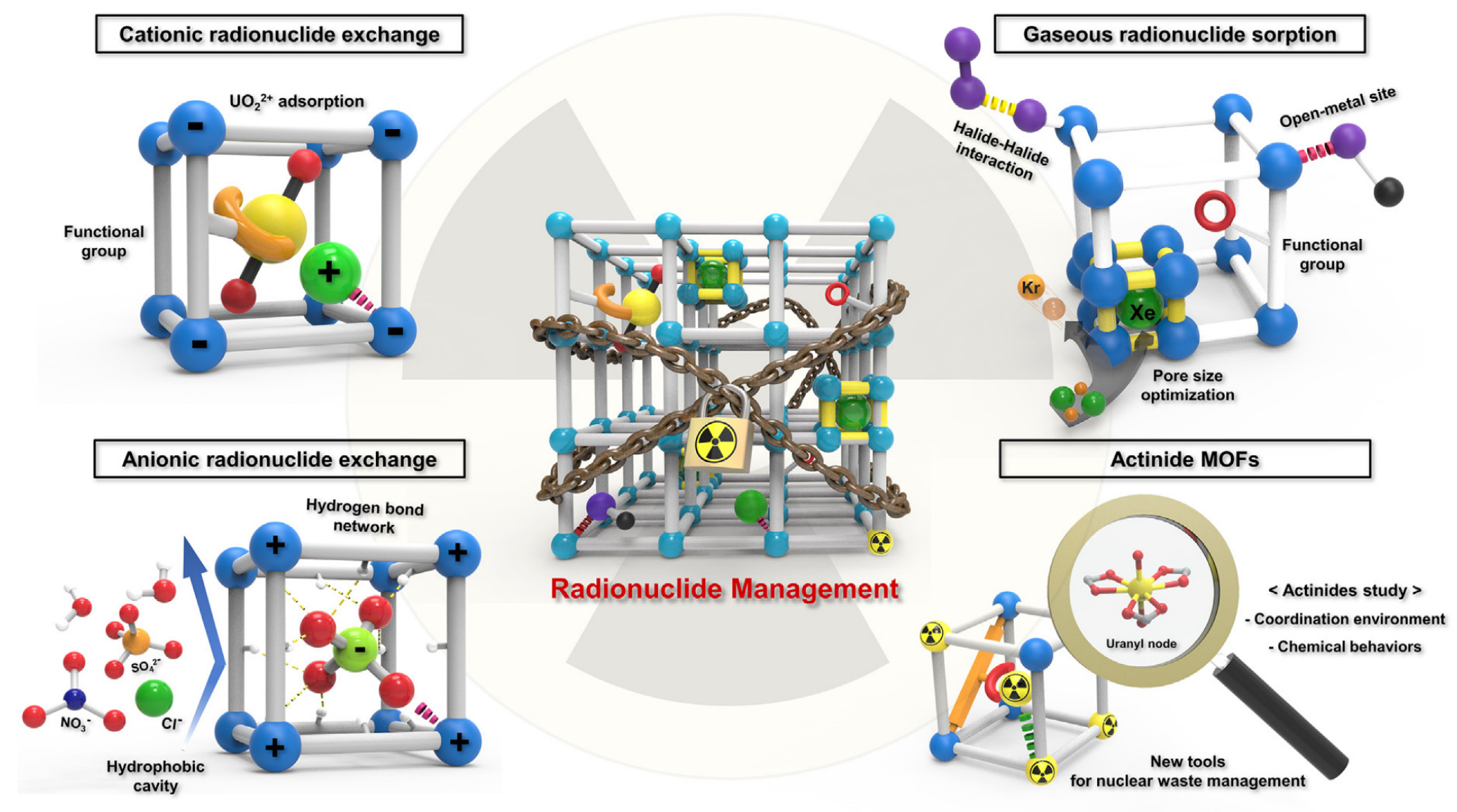
:1. Introduction
2. Interaction Mechanism of Actinides at Solid-Water Interfaces
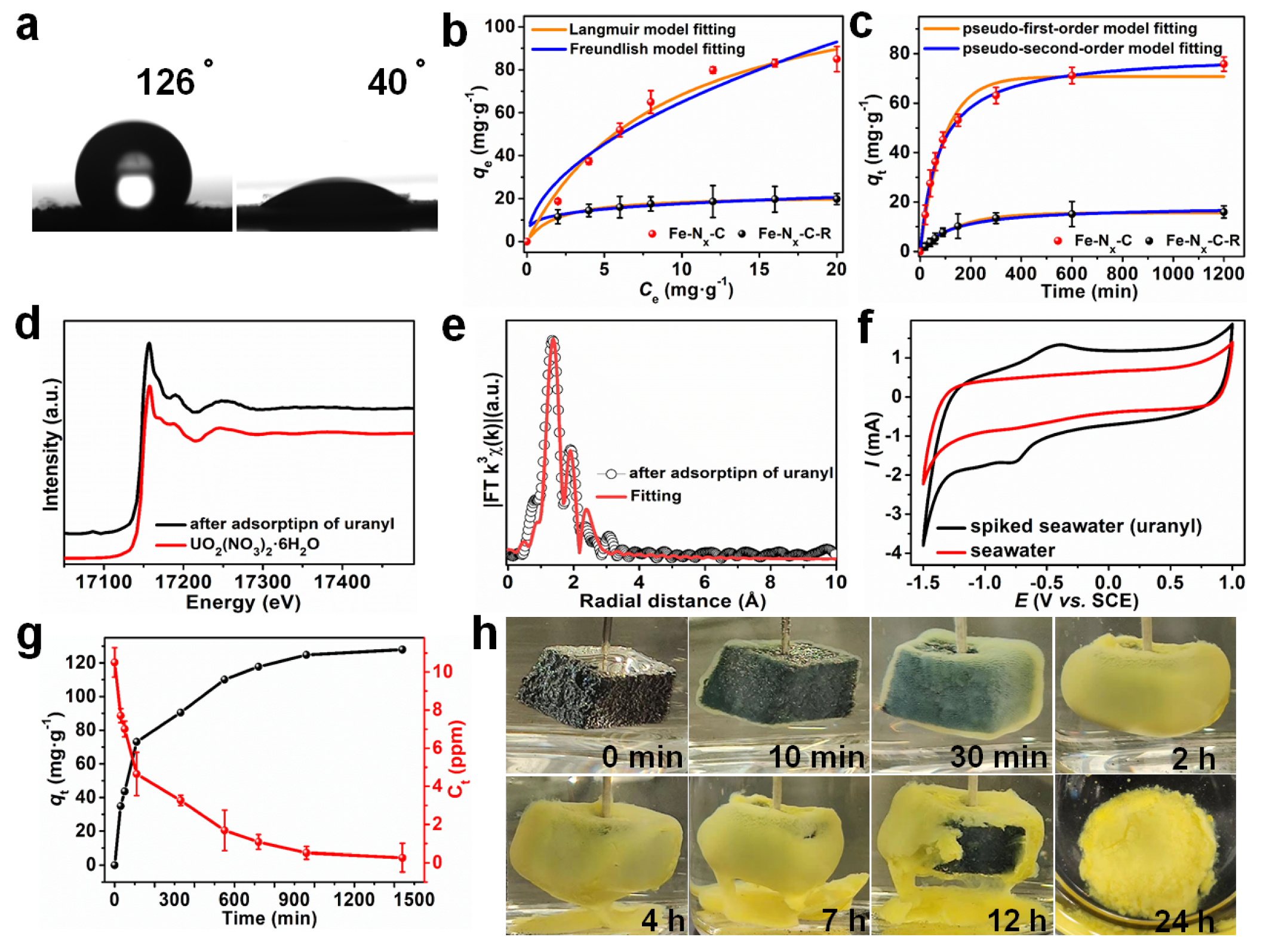
2.1. Uranium
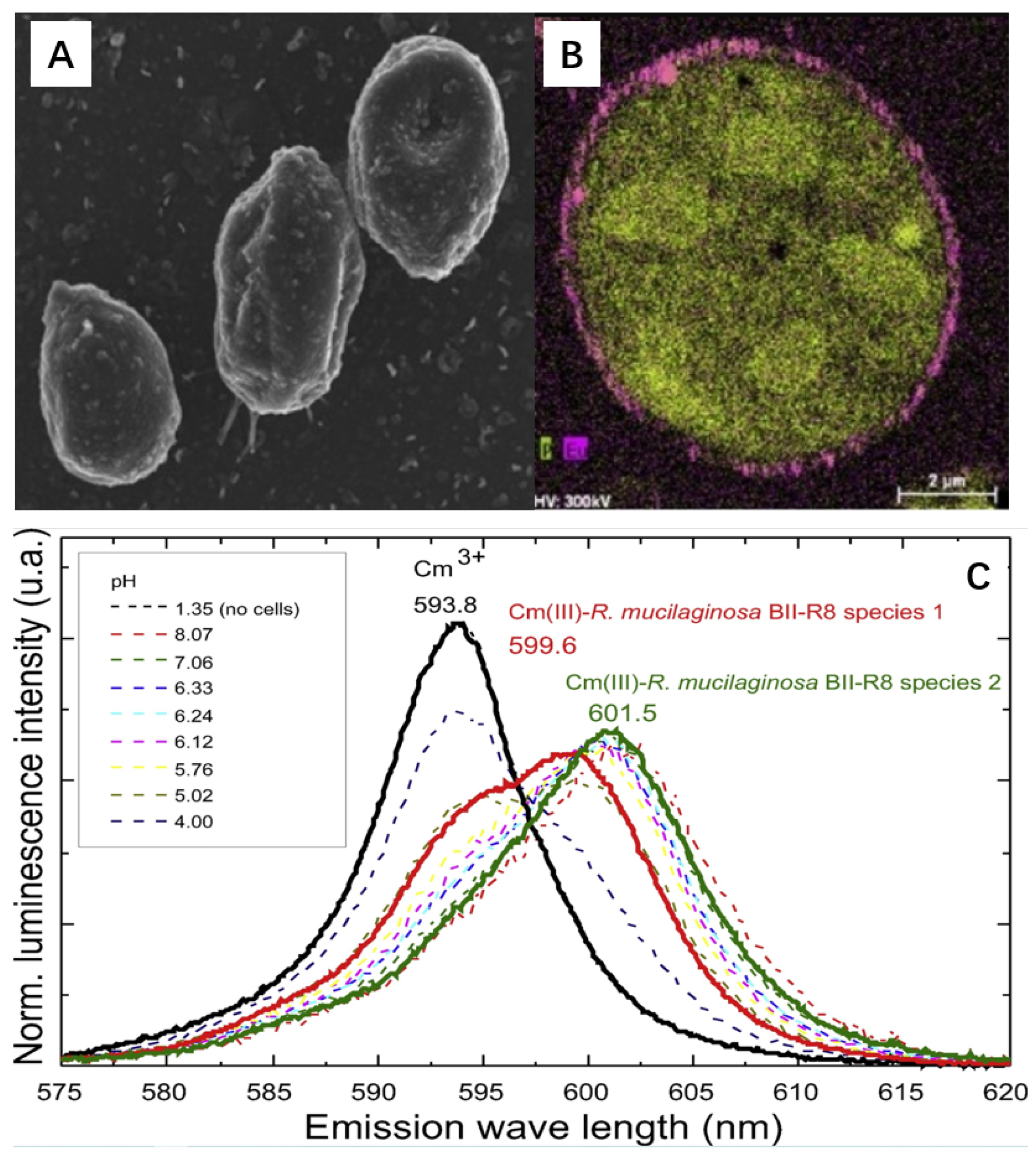
2.2. Curium
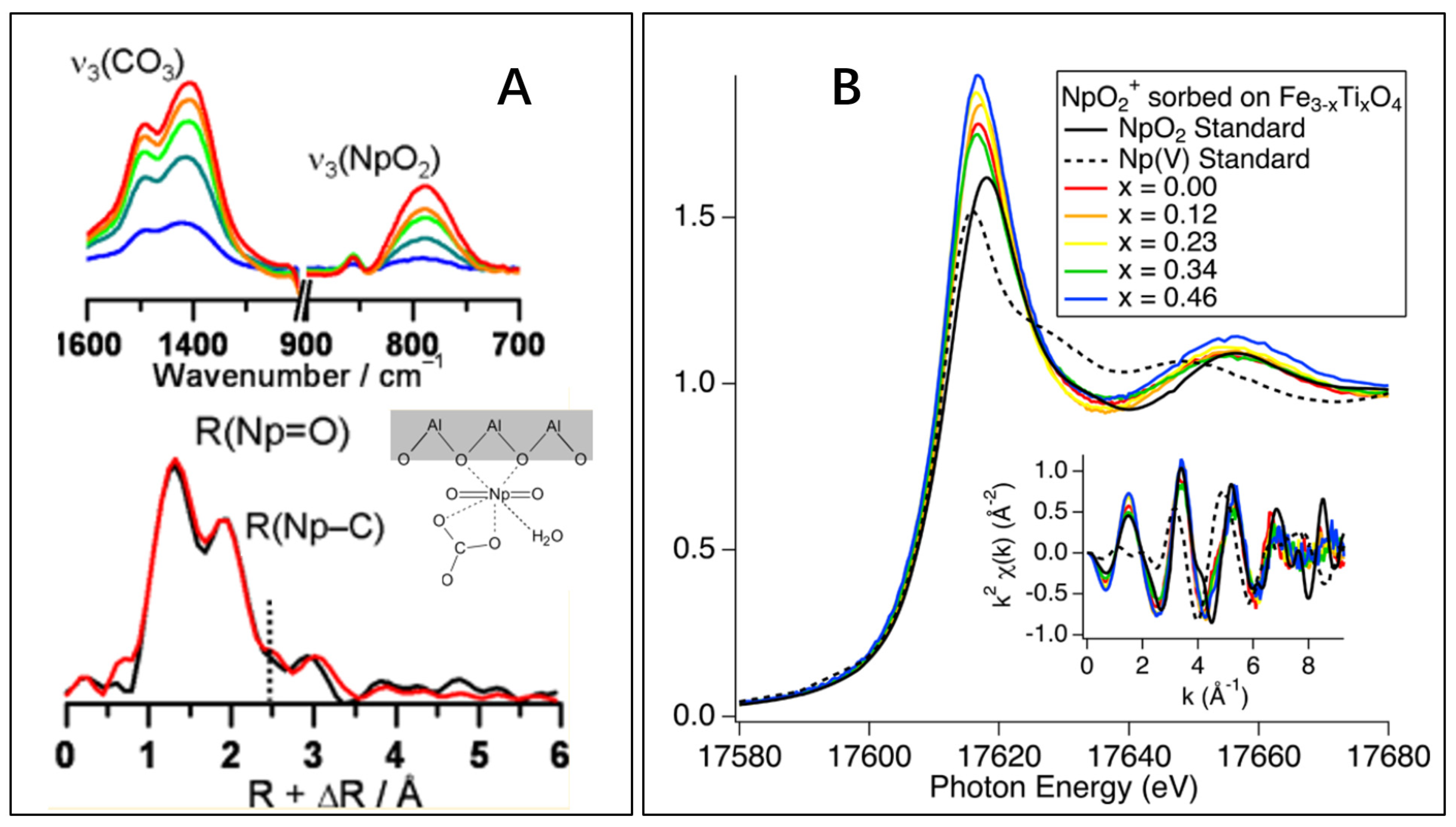
2.3. Neptunium
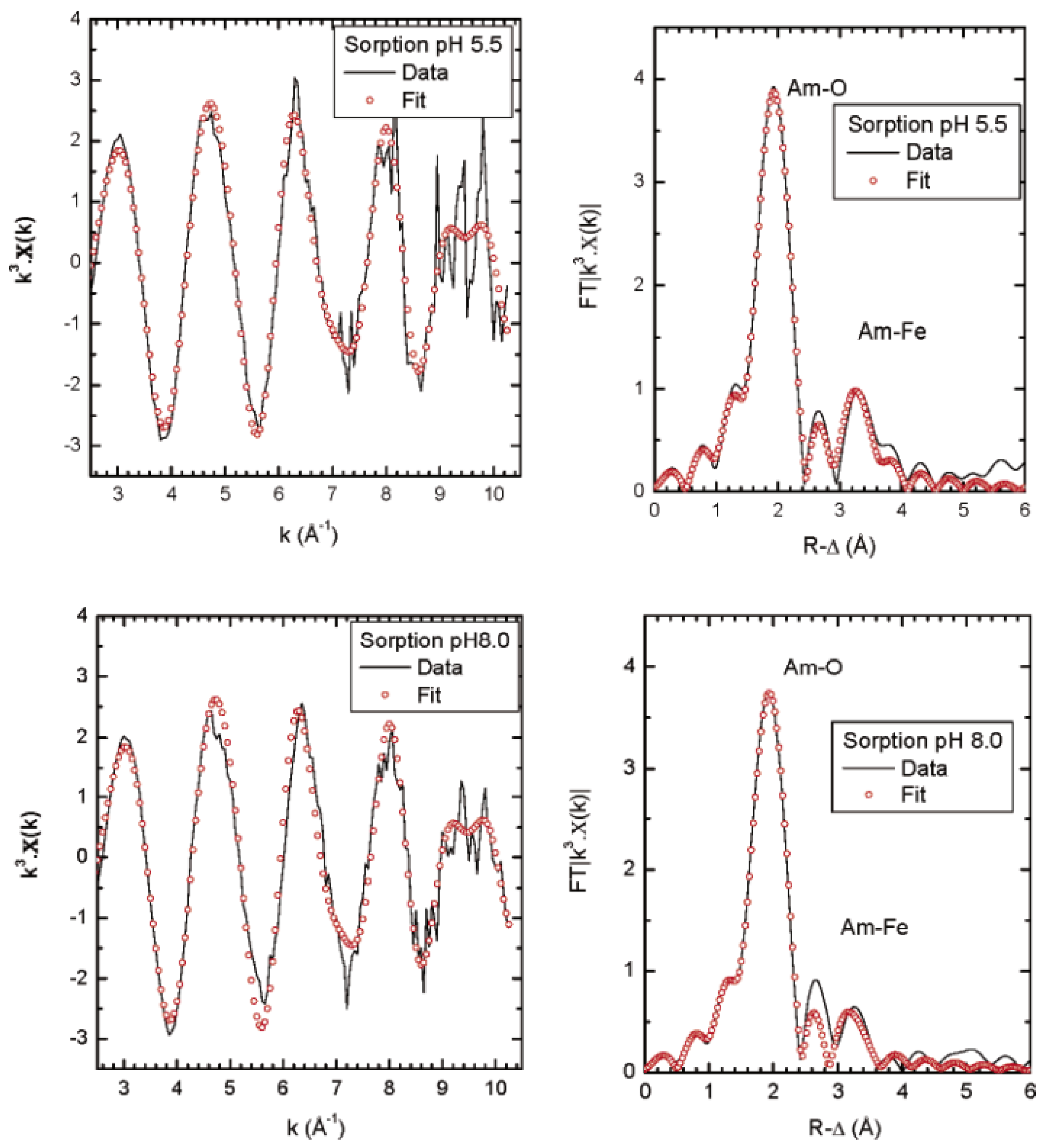
2.4. Americium
2.5. Plutonium
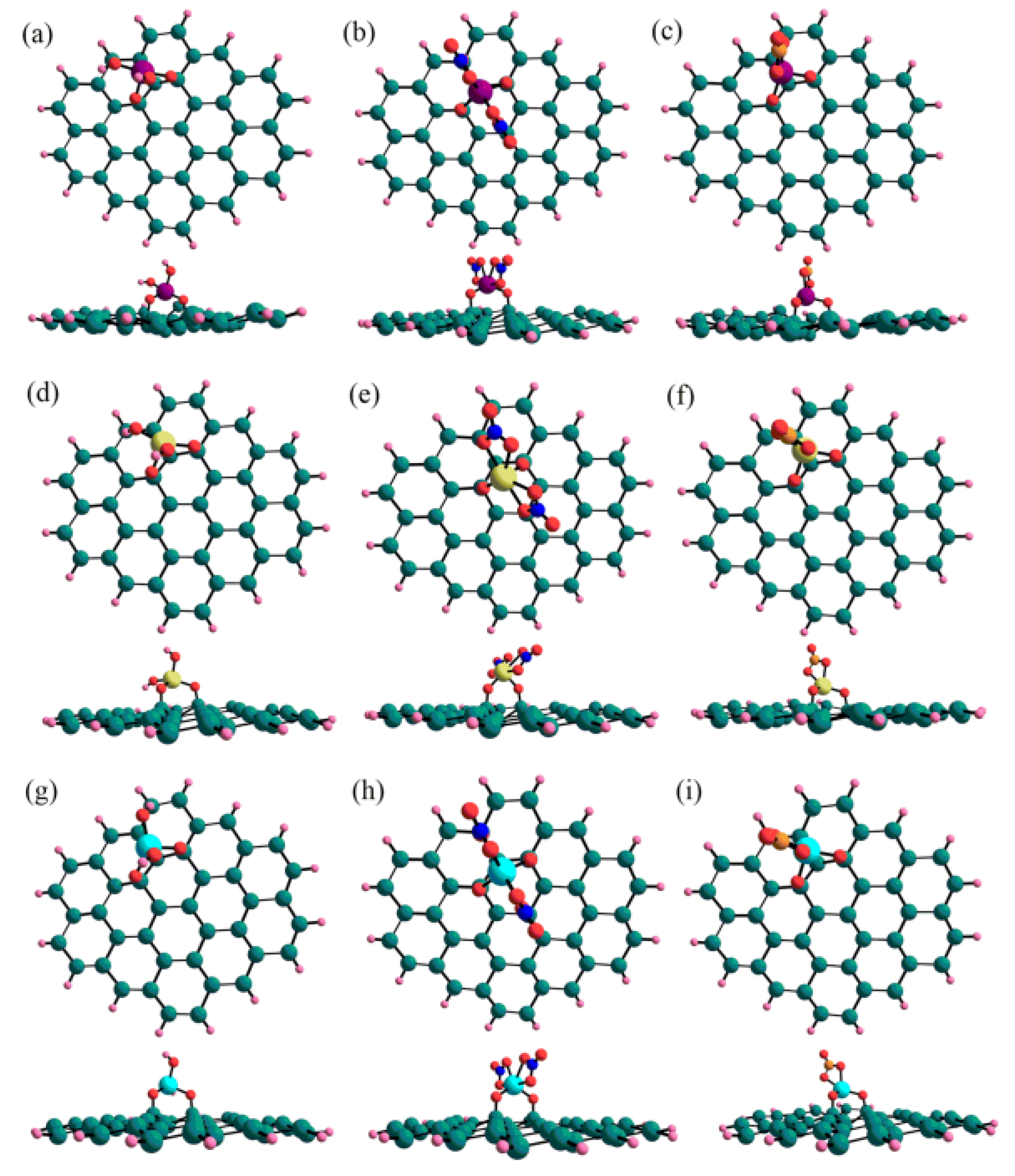
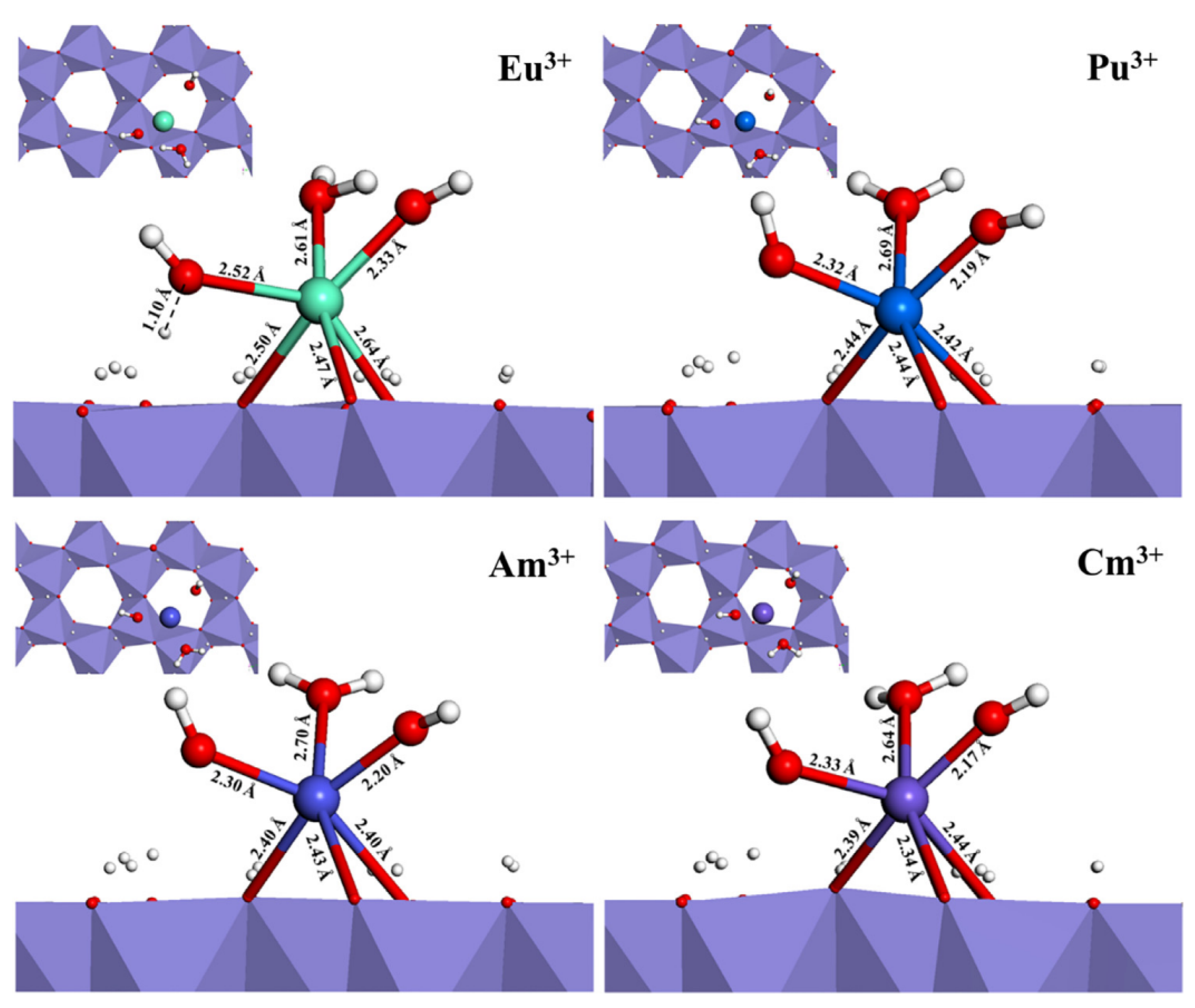
3. DFT Calculation
4. Competitive Sorption
5. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Wang, H.; Guo, H.; Zhang, N.; Chen, Z.; Hu, B.; Wang, X. Enhanced Photoreduction of U(VI) on C3N4 by Cr(VI) and Bisphenol A: ESR, XPS, and EXAFS Investigation. Environ. Sci. Technol. 2019, 53, 6454–6461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, J.; Xiong, H.; Yuan, Z.; Zhu, Y.; Hu, B. Constructing new Fe3O4@MnOx with 3D hollow structure for efficient recovery of uranium from simulated seawater. Chemosphere 2021, 283, 131241. [Google Scholar] [CrossRef]
- Liu, F.; Hua, S.; Wang, C.; Hu, B. Insight into the performance and mechanism of persimmon tannin functionalized waste paper for U(VI) and Cr(VI) removal. Chemosphere 2022, 287, 132199. [Google Scholar] [CrossRef]
- Liu, R.; Wang, H.; Han, L.; Hu, B.; Qiu, M. Reductive and adsorptive elimination of U(VI) ions in aqueous solution by SFeS@Biochar composites. Environ. Sci. Pollut. Res. 2021, 28, 55176–55185. [Google Scholar] [CrossRef]
- Hu, B.; Ai, Y.; Jin, J.; Hayat, T.; Alsaedi, A.; Zhuang, L.; Wang, X. Efficient elimination of organic and inorganic pollutants by biochar-based nanomaterials. Biochar 2020, 2, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Liang, L.; Xi, F.; Tan, W.; Meng, X.; Hu, B.; Wang, X. Review of organic pollutants and heavy metals removal by biochar and biochar-based composites. Biochar 2021, 3, 255–281. [Google Scholar] [CrossRef]
- Denecke, M.A. Actinide speciation using X-ray absorption fine structure spectroscopy. Coord. Chem. Rev. 2006, 250, 730–754. [Google Scholar] [CrossRef]
- Huittinen, N.; Rabung, T.; Schnurr, A.; Hakanen, M.; Lehto, J.; Geckeis, H. New insight into Cm(III) interaction with kaolinite—Influence of mineral dissolution. Geochim. Cosmochim. Acta 2012, 99, 100–109. [Google Scholar] [CrossRef]
- Neumann, J.; Qiu, C.; Eng, P.; Skanthakumar, S.; Soderholm, L.; Stumpf, T.; Schmidt, M. Effect of Background Electrolyte Composition on the Interfacial Formation of Th(IV) Nanoparticles on the Muscovite (001) Basal Plane. J. Phys. Chem. C 2021, 125, 16524–16535. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; Liu, R.; Qiu, M. Highly efficient U(VI) capture by amidoxime/carbon nitride composites: Evidence of EXAFS and modeling. Chemosphere 2021, 274, 129743. [Google Scholar] [CrossRef] [PubMed]
- Zänker, H.; Heine, K.; Weiss, S.; Brendler, V.; Husar, R.; Bernhard, G.; Gloe, K.; Henle, T.; Barkleit, A. Strong Uranium(VI) Binding onto Bovine Milk Proteins, Selected Protein Sequences, and Model Peptides. Inorg. Chem. 2019, 58, 4173–4189. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Z.-J.; Zheng, L.-R.; Wu, W.-S.; Chai, Z.-F.; Shi, W.-Q. Adsorption of Eu(III) and Th(IV) on three-dimensional graphene-based macrostructure studied by spectroscopic investigation. Environ. Pollut. 2019, 248, 82–89. [Google Scholar] [CrossRef]
- Wang, X.K.; Chen, C.L.; Hu, W.P.; Ding, A.P.; Xu, D.; Zhou, X. Sorption of 243Am(III) to Multi-wall Carbon Nanotubes. Environ. Sci. Technol. 2005, 39, 2856–2860. [Google Scholar] [CrossRef]
- Yu, S.; Pang, H.; Huang, S.; Tang, H.; Wang, S.; Qiu, M.; Chen, Z.; Yang, H.; Song, G.; Fu, D.; et al. Recent advances in metal-organic framework membranes for water treatment: A review. Sci. Total Environ. 2021, 800, 149662. [Google Scholar] [CrossRef]
- Jin, K.; Lee, B.; Park, J. Metal-organic frameworks as a versatile platform for radionuclide management. Coord. Chem. Rev. 2021, 427, 213473. [Google Scholar] [CrossRef]
- Gao, M.; Liu, G.; Gao, Y.; Chen, G.; Huang, X.; Xu, X.; Wang, J.; Yang, X.; Xu, D. Recent advances in metal-organic frameworks/membranes for adsorption and removal of metal ions. TrAC Trends Anal. Chem. 2021, 137, 116226. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Liu, X.; Pang, H.; Liu, X.; Li, Q.; Zhang, N.; Mao, L.; Qiu, M.; Hu, B.; Yang, H.; Wang, X. Orderly Porous Covalent Organic Frameworks-based Materials: Superior Adsorbents for Pollutants Removal from Aqueous Solutions. Innovation 2021, 2, 100076. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Lu, Z.P.; Liang, W.; Guo, X.J.; Hu, B.W. The fabrication of 3D hierarchical flower-like delta-MnO2@COF nanocomposites for the efficient and ultra-fast removal of UO22+ ions from aqueous solution. Environ. Sci. Nano 2020, 7, 3303–3317. [Google Scholar] [CrossRef]
- Zhao, B.; Yuan, L.; Wang, Y.; Duan, T.; Shi, W. Carboxylated UiO-66 Tailored for U(VI) and Eu(III) Trapping: From Batch Adsorption to Dynamic Column Separation. ACS Appl. Mater. Interfaces 2021, 13, 16300–16308. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, X.; Hao, M.; Xie, Y.; Wang, X.; Tian, H.; Waterhouse, G.I.N.; Kruger, P.E.; Telfer, S.G.; Ma, S. Functionalized Iron−Nitrogen−Carbon Electrocatalyst Provides a Reversible Electron Transfer Platform for Efficient Uranium Extraction from Seawater. Adv. Mater. 2021, 2106621. [Google Scholar] [CrossRef]
- Qiu, M.; Hu, B.; Chen, Z.; Yang, H.; Zhuang, L.; Wang, X. Challenges of organic pollutant photocatalysis by biochar-based catalysts. Biochar 2021, 3, 117–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.; Zhang, S.; Cai, Y.; Lv, Z.; Fang, M.; Tan, X.; Wang, X. Highly efficient removal of U(VI) by the photoreduction of SnO2/CdCO3/CdS nanocomposite under visible light irradiation. Appl. Catal. B Environ. 2020, 279, 119390. [Google Scholar] [CrossRef]
- Qiu, M.Q.; Liu, Z.X.; Wang, S.Q.; Hu, B.W. The photocatalytic reduction of U(VI) into U(IV) by ZIF-8/g-C3N4 composites at visible light. Environ. Res. 2021, 196, 110349. [Google Scholar] [CrossRef]
- Li, S.; Hu, Y.; Shen, Z.; Cai, Y.; Ji, Z.; Tan, X.; Liu, Z.; Zhao, G.; Hu, S.; Wang, X. Rapid and selective uranium extraction from aqueous solution under visible light in the absence of solid photocatalyst. Sci. China Ser. B Chem. 2021, 64, 1323–1331. [Google Scholar] [CrossRef]
- Kar, A.S.; Kumar, S.; Tomar, B.; Manchanda, V. Sorption of curium by silica colloids: Effect of humic acid. J. Hazard. Mater. 2011, 186, 1961–1965. [Google Scholar] [CrossRef] [PubMed]
- Moll, H.; Stumpf, T.; Merroun, M.; Rossberg, A.; Selenska-Pobell, S.; Bernhard, G. Time-Resolved Laser Fluorescence Spectroscopy Study on the Interaction of Curium(III) with Desulfovibrio äspöensis DSM 10631T. Environ. Sci. Technol. 2004, 38, 1455–1459. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fernandez, M.; Moll, H.; Merroun, M.L. Reversible pH-dependent Curium(III) biosorption by the bentonite yeast isolate Rhodotorula mucilaginosa BII-R8. J. Hazard. Mater. 2019, 370, 156–163. [Google Scholar] [CrossRef]
- Gückel, K.; Rossberg, A.; Müller, K.; Brendler, V.; Bernhard, G.; Foerstendorf, H. Spectroscopic Identification of Binary and Ternary Surface Complexes of Np(V) on Gibbsite. Environ. Sci. Technol. 2013, 47, 14418–14425. [Google Scholar] [CrossRef]
- Wylie, E.M.; Olive, D.T.; Powell, B.A. Effects of Titanium Doping in Titanomagnetite on Neptunium Sorption and Speciation. Environ. Sci. Technol. 2016, 50, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Gröschel, A.; Rossberg, A.; Bok, F.; Franzen, C.; Brendler, V.; Foerstendorf, H. In situ Spectroscopic Identification of Neptunium(V) Inner-Sphere Complexes on the Hematite–Water Interface. Environ. Sci. Technol. 2015, 49, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Scheinost, A.C.; Steudtner, R.; Hübner, R.; Weiss, S.; Bok, F. Neptunium V Retention by Siderite under Anoxic Conditions: Precipitation of NpO2–Like Nanoparticles and of NpIV Pentacarbonate. Environ. Sci. Technol. 2016, 50, 10413–10420. [Google Scholar] [CrossRef]
- Veeramani, H.; Scheinost, A.; Monsegue, N.; Qafoku, N.P.; Kukkadapu, R.; Newville, M.; Lanzirotti, A.; Pruden, A.; Murayama, M.; Hochella, M.F. Abiotic Reductive Immobilization of U(VI) by Biogenic Mackinawite. Environ. Sci. Technol. 2013, 47, 2361–2369. [Google Scholar] [CrossRef] [PubMed]
- Latta, D.E.; Gorski, C.A.; Boyanov, M.I.; O’Loughlin, E.J.; Kemner, K.M.; Scherer, M.M. Influence of Magnetite Stoichiometry on U(VI) Reduction. Environ. Sci. Technol. 2012, 46, 778–786. [Google Scholar] [CrossRef]
- Banik, N.L.; Marsac, R.; Lützenkirchen, J.; Marquardt, C.M.; Dardenne, K.; Rothe, J.; Bender, K.; Geckeis, H. Neptunium sorption and redox speciation at the illite surface under highly saline conditions. Geochim. Cosmochim. Acta 2017, 215, 421–431. [Google Scholar] [CrossRef] [Green Version]
- Butorin, S.M.; Shuh, D.K.; Kvashnina, K.O.; Guo, J.; Werme, L.; Nordgren, J. Chemical Reduction of Actinides Probed by Resonant Inelastic X-ray Scattering. Anal. Chem. 2013, 85, 11196–11200. [Google Scholar] [CrossRef]
- Lloyd, J.R.; Yong, A.P.; Macaskie, L.E. Biological Reduction and Removal of Np(V) by Two Microorganisms. Environ. Sci. Technol. 2000, 34, 1297–1301. [Google Scholar] [CrossRef]
- Rittmann, B.E.; Banaszak, J.E.; Reed, D.T. Reduction of Np(V) and precipitation of Np(IV) by an anaerobic microbial consortium. Biodegradation 2002, 13, 329–342. [Google Scholar] [CrossRef]
- Icopini, G.A.; Boukhalfa, H.; Neu, M.P. Biological Reduction of Np(V) and Np(V) Citrate by Metal-Reducing Bacteria. Environ. Sci. Technol. 2007, 41, 2764–2769. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Finck, N.; Dardenne, K.; Geckeis, H. Am(III) coprecipitation with and adsorption on the smectite hectorite. Chem. Geol. 2015, 409, 12–19. [Google Scholar] [CrossRef]
- Stumpf, S.; Stumpf, T.; Dardenne, K.; Hennig, C.; Foerstendorf, H.; Klenze, R.; Fanghänel, T. Sorption of Am(III) onto 6-Line-Ferrihydrite and Its Alteration Products: Investigations by EXAFS. Environ. Sci. Technol. 2006, 40, 3522–3528. [Google Scholar] [CrossRef] [Green Version]
- Finck, N.; Nedel, S.; Dideriksen, K.; Schlegel, M.L. Trivalent Actinide Uptake by Iron (Hydr)oxides. Environ. Sci. Technol. 2016, 50, 10428–10436. [Google Scholar] [CrossRef] [PubMed]
- Perminova, I.; Karpiouk, L.; Shcherbina, N.; Ponomarenko, S.; Kalmykov, S.; Hatfield, K. Preparation and use of humic coatings covalently bound to silica gel for Np(V) and Pu(V) sequestration. J. Alloys Compd. 2007, 444–445, 512–517. [Google Scholar] [CrossRef]
- Blinova, O.; Novikov, A.; Perminova, I.; Goryachenkova, T.; Haire, R. Redox interactions of Pu(V) in solutions containing different humic substances. J. Alloys Compd. 2007, 444–445, 486–490. [Google Scholar] [CrossRef]
- Paul, S.; Pandey, A.; Kumar, P.; Kaity, S.; Aggarwal, S.K. Tailored Bifunctional Polymer for Plutonium Monitoring. Anal. Chem. 2014, 86, 6254–6261. [Google Scholar] [CrossRef]
- Romanchuk, A.Y.; Kalmykov, S.N.; Egorov, A.V.; Zubavichus, Y.V.; Shiryaev, A.A.; Batuk, O.N.; Conradson, S.D.; Pankratov, D.A.; Presnyakov, I.A. Formation of crystalline PuO2+x nH2O nanoparticles upon sorption of Pu(V/VI) onto hematite. Geochim. Cosmochim. Acta 2013, 121, 29–40. [Google Scholar] [CrossRef]
- Gujar, R.B.; Ansari, S.; Verboom, W.; Mohapatra, P.K. Highly Efficient Extraction Chromatography Resins Containing Dendrimers with DGA Groups in Ionic Liquid for Actinide Uptake. Ind. Eng. Chem. Res. 2018, 57, 13226–13234. [Google Scholar] [CrossRef]
- Graser, C.-H.; Banik, N.L.; Bender, K.A.; Lagos, M.; Marquardt, C.M.; Marsac, R.; Montoya, V.; Geckeis, H. Sensitive Redox Speciation of Iron, Neptunium, and Plutonium by Capillary Electrophoresis Hyphenated to Inductively Coupled Plasma Sector Field Mass Spectrometry. Anal. Chem. 2015, 87, 9786–9794. [Google Scholar] [CrossRef]
- Grover, P.; Ferch, L.S.; Schreckenbach, G. Adsorption of Actinide (U–Pu) Complexes on the Silicene and Germanene Surface: A Theoretical Study. J. Phys. Chem. A 2020, 124, 1522–1534. [Google Scholar] [CrossRef]
- Yuan, K.; Taylor, S.; Powell, B.A.; Becker, U. An ab initio study of the adsorption of Eu3+, Pu3+, Am3+, and Cm3+ hydroxide complexes on hematite (001) surface: Role of magnetism on adsorption. Surf. Sci. 2017, 664, 120–128. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Zheng, M.; Schreckenbach, G.; Pan, Q.-J.; Schreckenbach, H.G. Interfacial Interaction of Titania Nanoparticles and Ligated Uranyl Species: A Relativistic DFT Investigation. Inorg. Chem. 2017, 56, 2763–2776. [Google Scholar] [CrossRef]
- Yuan, L.-Y.; Zhu, L.; Xiao, C.-L.; Wu, Q.-Y.; Zhang, N.; Yu, J.-P.; Chai, Z.-F.; Shi, W.-Q. Large-Pore 3D Cubic Mesoporous (KIT-6) Hybrid Bearing a Hard–Soft Donor Combined Ligand for Enhancing U(VI) Capture: An Experimental and Theoretical Investigation. ACS Appl. Mater. Interfaces 2017, 9, 3774–3784. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Jiang, G. Adsorption of actinide ion complexes on C60O: An all-electron ZORA-DFT-D3 study. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 223, 117375. [Google Scholar] [CrossRef]
- Zhou, L.; Jia, Y.; Peng, J.; Liu, Z.; Al-Zaini, E. Competitive adsorption of uranium(VI) and thorium(IV) ions from aqueous solution using triphosphate-crosslinked magnetic chitosan resins. J. Radioanal. Nucl. Chem. 2014, 302, 331–340. [Google Scholar] [CrossRef]
- Virtanen, S.; Meriläinen, S.; Eibl, M.; Rabung, T.; Lehto, J.; Huittinen, N. Sorption competition and kinetics of trivalent cations (Eu, Y and Cm) on corundum (α-Al2O3): A batch sorption and TRLFS study. Appl. Geochem. 2018, 92, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Luca, V.; Sizgek, D.G.; Sizgek, E.; Arrachart, G.; Rey, C.; Scales, N.; Aly, Z.; Drisko, G.L. Actinide and Lanthanide Adsorption onto Hierarchically Porous Carbons Beads: A High Surface Affinity for Pu. Nanomaterials 2019, 9, 1464. [Google Scholar] [CrossRef] [Green Version]
- Falaise, C.; Volkringer, C.; Giovine, R.; Prelot, B.; Huve, M.; Loiseau, T. Capture of actinides (Th4+, [UO2]2+) and surrogating lanthanide (Nd3+) in porous metal–organic framework MIL-100(Al) from water: Selectivity and imaging of embedded nanoparticles. Dalton Trans. 2017, 46, 12010–12014. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, L.; Cai, Y.; Liu, Z.; Li, B.; Bian, Q.; Hu, B.; Wang, X. High Sorption and Selective Extraction of Actinides from Aqueous Solutions. Molecules 2021, 26, 7101. https://doi.org/10.3390/molecules26237101
Bao L, Cai Y, Liu Z, Li B, Bian Q, Hu B, Wang X. High Sorption and Selective Extraction of Actinides from Aqueous Solutions. Molecules. 2021; 26(23):7101. https://doi.org/10.3390/molecules26237101
Chicago/Turabian StyleBao, Linfa, Yawen Cai, Zhixin Liu, Bingfeng Li, Qi Bian, Baowei Hu, and Xiangke Wang. 2021. "High Sorption and Selective Extraction of Actinides from Aqueous Solutions" Molecules 26, no. 23: 7101. https://doi.org/10.3390/molecules26237101
APA StyleBao, L., Cai, Y., Liu, Z., Li, B., Bian, Q., Hu, B., & Wang, X. (2021). High Sorption and Selective Extraction of Actinides from Aqueous Solutions. Molecules, 26(23), 7101. https://doi.org/10.3390/molecules26237101





