Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation
Abstract
:1. Introduction
2. Experiential Section
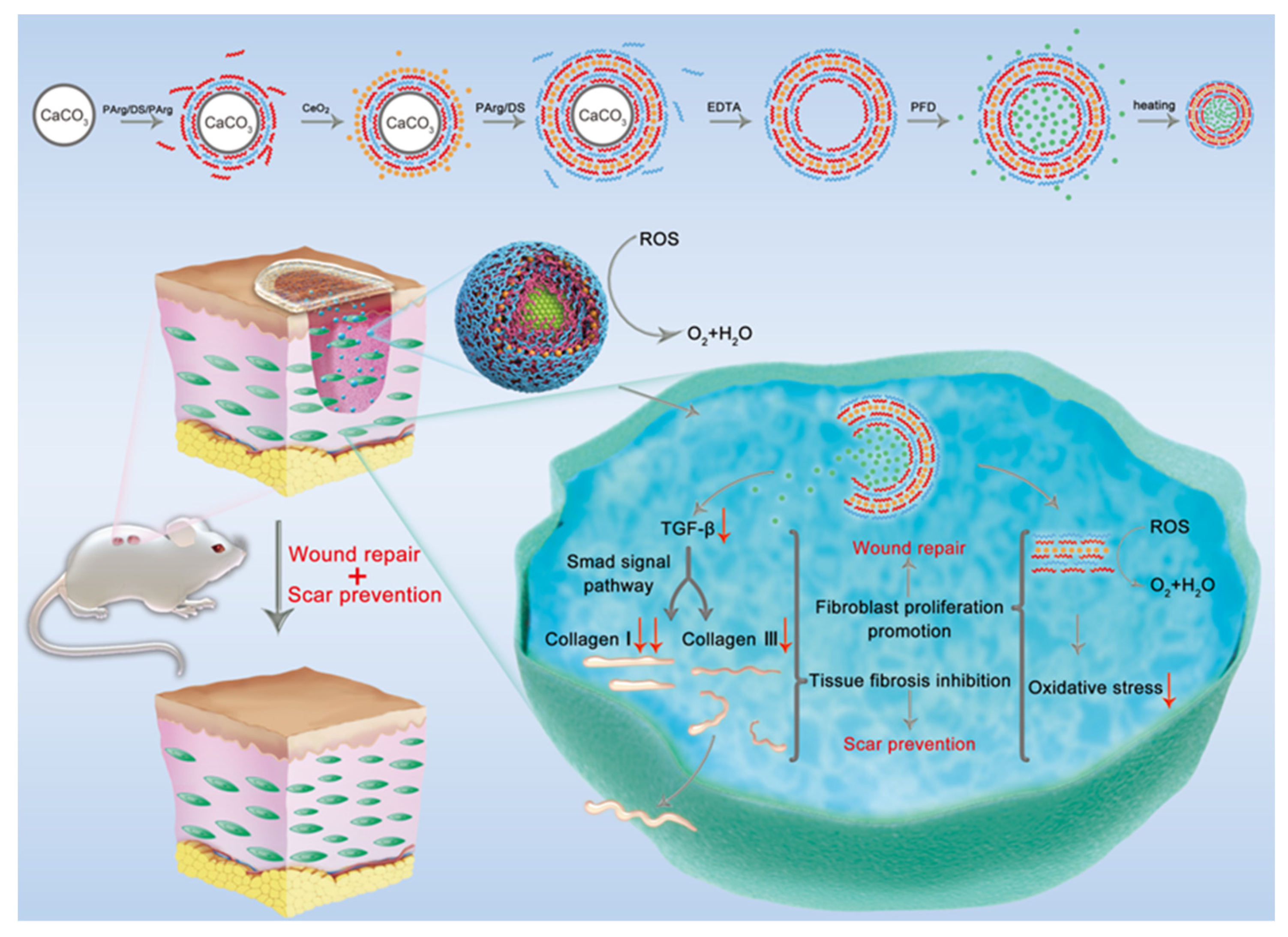
2.1. Synthesis of PFD/CeO2 NCs
2.2. Characterization of the NCs
2.3. Cell Culture
2.4. CCK-8 Assay
2.5. Cellular Uptake Assay
2.6. Live/Dead Assay
2.7. ROS Assay
2.8. Scratch Assay
2.9. Synthesis and Characterization of Capsule-Loaded PLA Dressing
2.10. Mouse Wound-Healing Model
2.11. Evaluation of Wound Closure
2.12. Histological Staining
2.12.1. ROS Assay
2.12.2. TGF-β Assay
2.12.3. Collagen Assay
2.13. Statistical Analysis
3. Results and Discussion
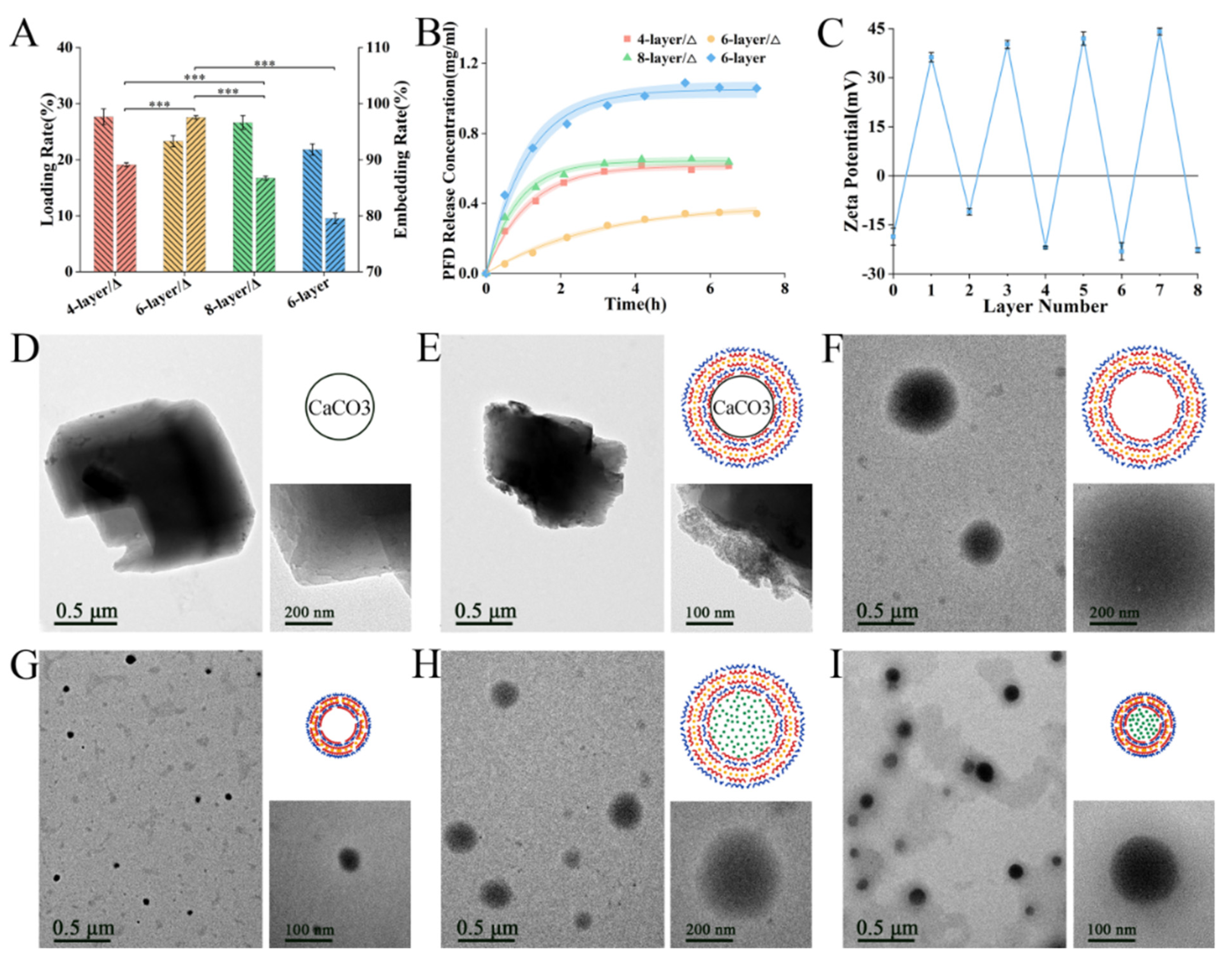
3.1. Preparation and Characterization of PFD/CeO2 NCs
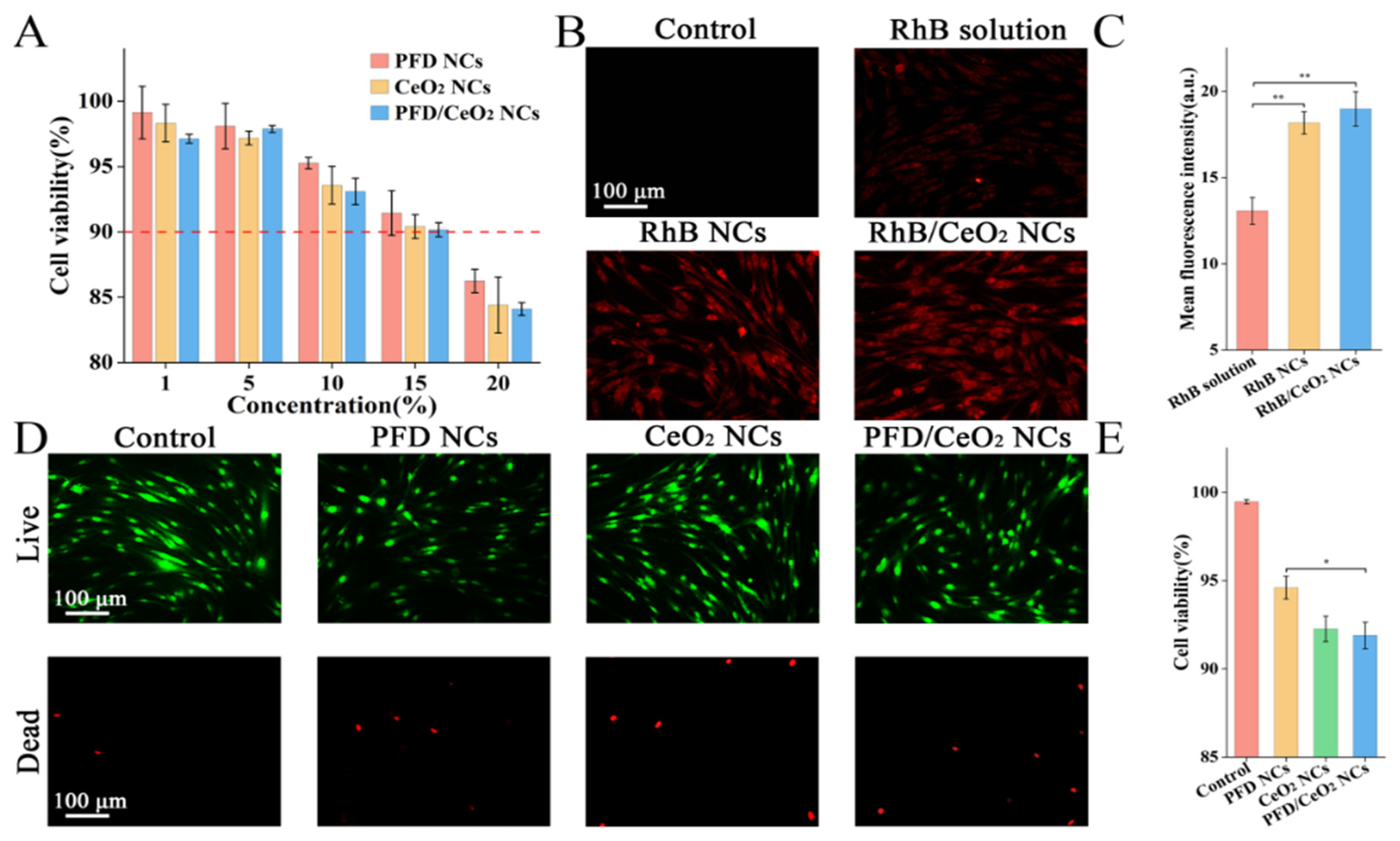
3.2. CCK-8 Assay
3.3. Cellular Uptake Assay
3.4. Live/Dead Assay
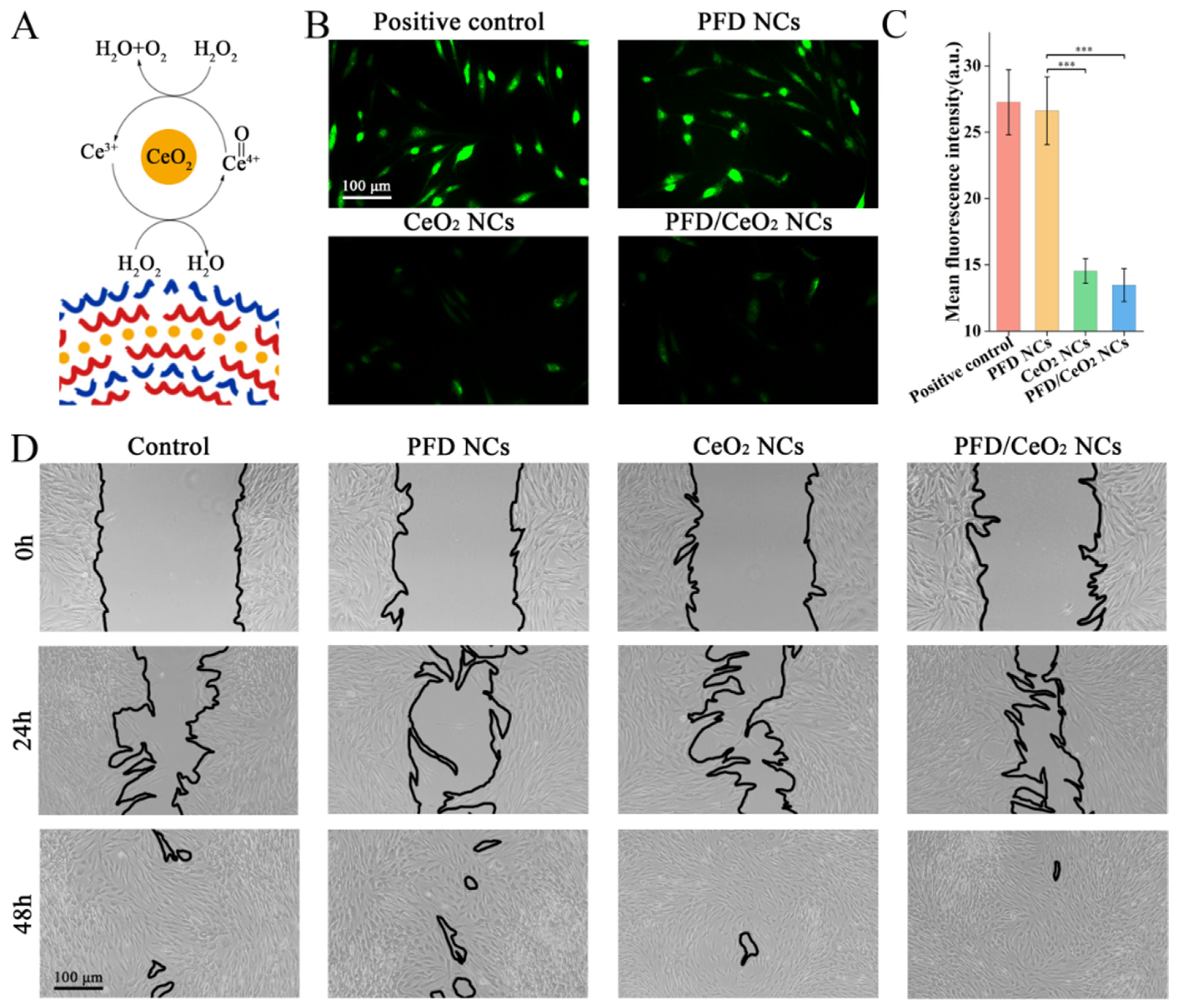
3.5. ROS Assay
3.6. Scratch Assay
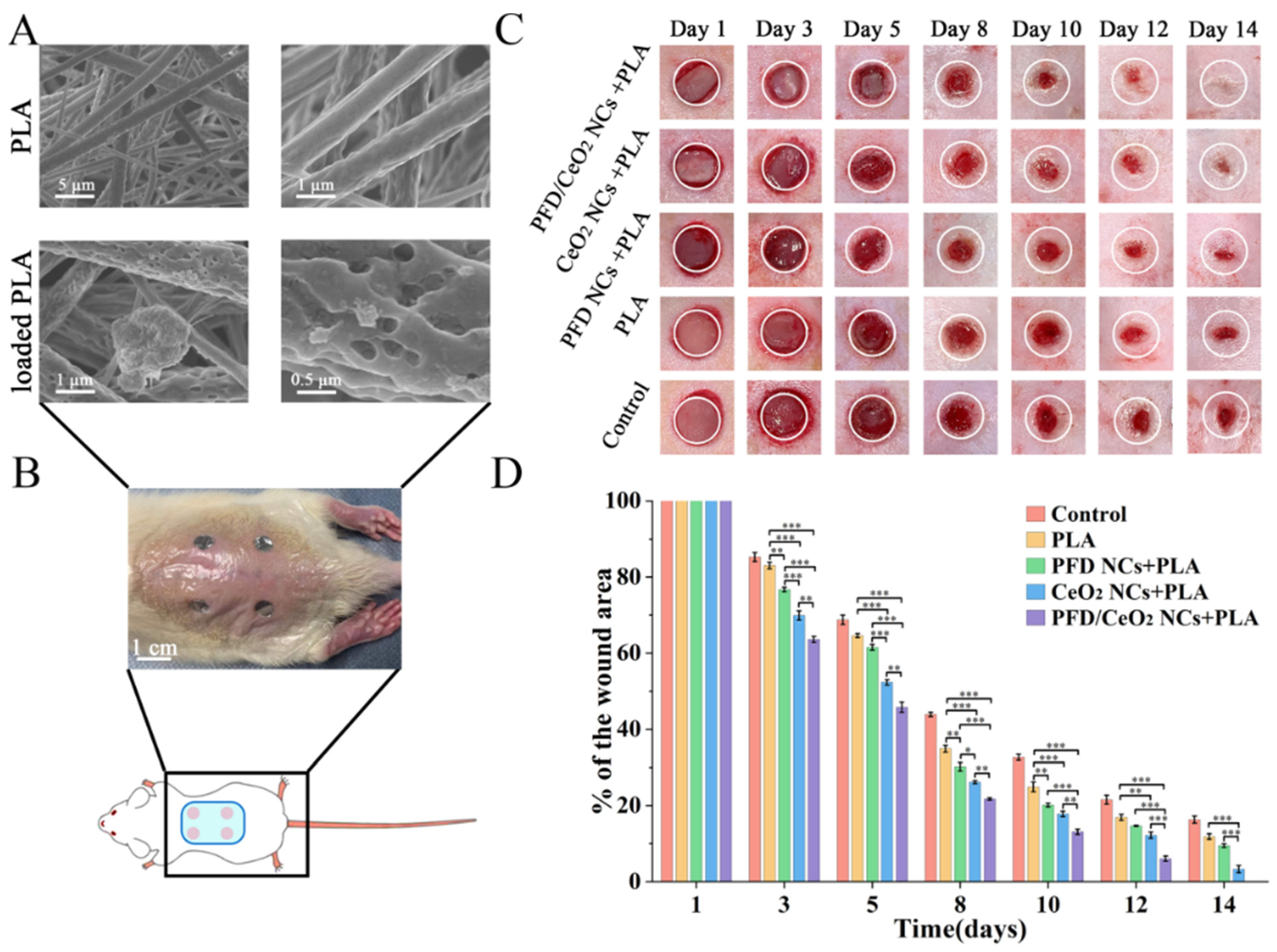
3.7. Synthesis and Characterization of Capsule-Loaded PLA Dressing
3.8. Evaluation of Wound Closure
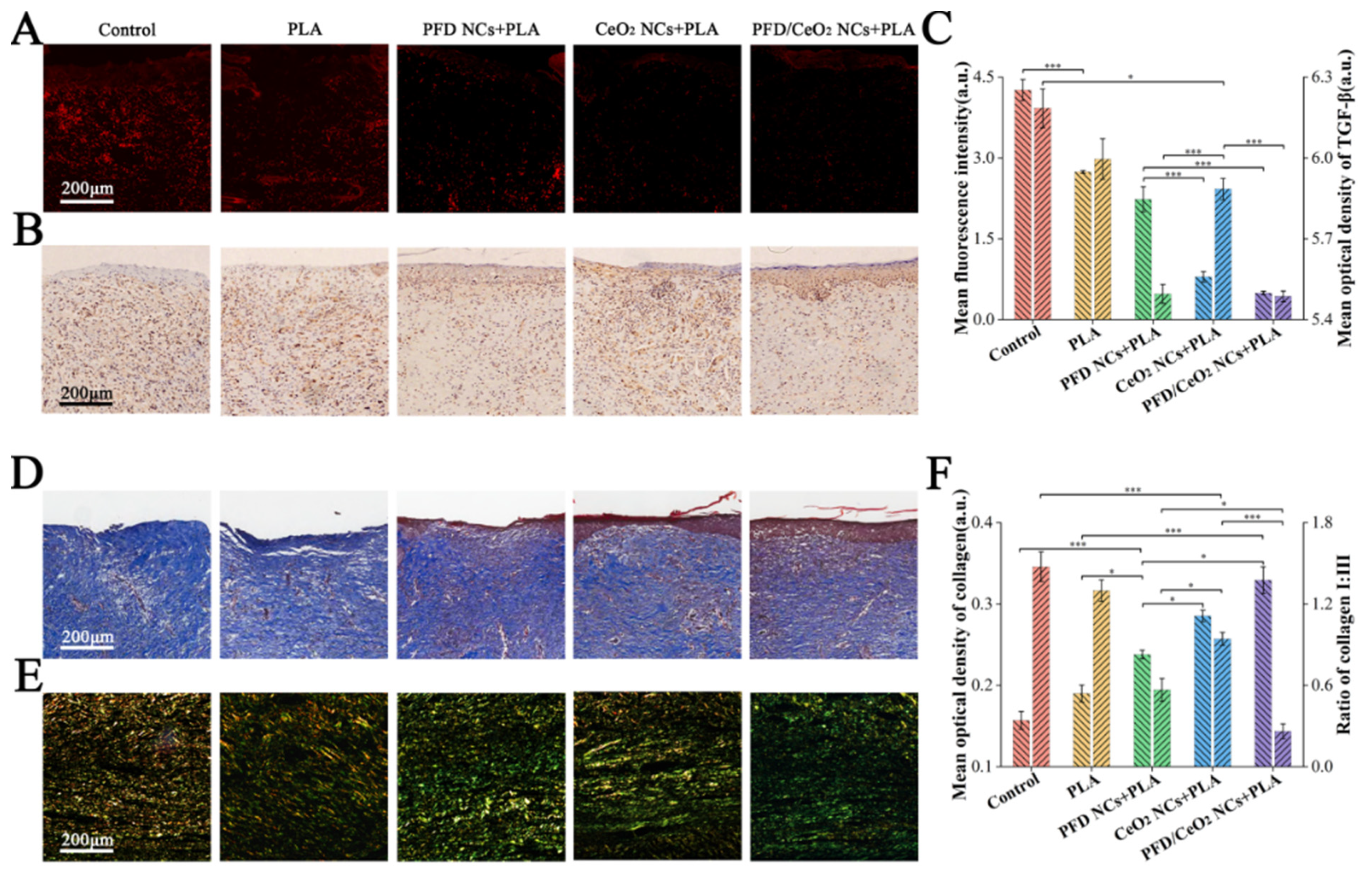
3.9. Histological Staining
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Mesenchymal stem cell therapy for attenuation of scar formation during wound healing. Stem Cell Res. Ther. 2012, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hollestein, L.M.; Nijsten, T. An insight into the global burden of skin diseases. J. Investig. Dermatol. 2014, 134, 1499–1501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greaves, N.S.; Ashcroft, K.J.; Baguneid, M.; Bayat, A. Current understanding of molecular and cellular mechanisms in fibroplasia and angiogenesis during acute wound healing. J. Dermatol. Sci. 2013, 72, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.J.; Rosenthal, N. Preparing the ground for tissue regeneration: From mechanism to therapy. Nat. Med. 2014, 20, 857–869. [Google Scholar] [CrossRef]
- Hu, M.S.; Rennert, R.C.; McArdle, A.; Chung, M.T.; Walmsley, G.G.; Longaker, M.T.; Lorenz, H.P. The Role of Stem Cells During Scarless Skin Wound Healing. Adv. Wound Care 2014, 3, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Li, F.; Wang, S.; Lu, J.; Li, J.; Du, Y.; Sun, X.; Chen, X.; Gao, J.; Ling, D. Ceria nanocrystals decorated mesoporous silica nanoparticle based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials 2018, 151, 66–77. [Google Scholar] [CrossRef]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef]
- Carney, B.C.; Chen, J.H.; Kent, R.A.; Rummani, M.; Alkhalil, A.; Moffatt, L.T.; Rosenthal, D.S.; Shupp, J.W. Reactive Oxygen Species Scavenging Potential Contributes to Hypertrophic Scar Formation. J. Surg. Res. 2019, 244, 312–323. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Ni, D.; Rosenkrans, Z.T.; Cai, W. Scavenging of reactive oxygen and nitrogen species with nanomaterials. Nano Res. 2018, 11, 4955–4984. [Google Scholar] [CrossRef]
- Patel, V.; Singh, M.; Mayes, E.L.H.; Martinez, A.; Shutthanandan, V.; Bansal, V.; Singh, S.; Karakoti, A.S. Ligand-mediated reversal of the oxidation state dependent ROS scavenging and enzyme mimicking activity of ceria nanoparticles. Chem. Commun. 2018, 54, 13973–13976. [Google Scholar] [CrossRef]
- Kavok, N.; Grygorova, G.; Klochkov, V.; Yefimova, S. The role of serum proteins in the stabilization of colloidal LnVO4:Eu3+ (Ln = La, Gd, Y) and CeO2 nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 594–599. [Google Scholar] [CrossRef]
- Raţă, D.M.; Chailan, J.-F.; Peptu, C.A.; Costuleanu, M.; Popa, M. Chitosan: Poly(N-vinylpyrrolidone-alt-itaconic anhydride) nanocapsules—a promising alternative for the lung cancer treatment. J. Nanoparticle Res. 2015, 17, 316. [Google Scholar] [CrossRef]
- Raţă, D.M.; Popa, M.; Chailan, J.-F.; Zamfir, C.L.; Peptu, C.A. Biomaterial properties evaluation of poly(vinyl acetate-alt-maleic anhydride)/chitosan nanocapsules. J. Nanoparticle Res. 2014, 16, 2569. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.R.; Tarakina, N.V.; Ivanova, O.S.; Ermakov, A.M.; Ivanov, V.K.; Sukhorukov, G.B. Intracellular Delivery of Antioxidant CeO2 Nanoparticles via Polyelectrolyte Microcapsules. ACS Biomater. Sci. Eng. 2018, 4, 2453–2462. [Google Scholar] [CrossRef]
- Wells, A.R.; Leung, K.P. Pirfenidone attenuates the profibrotic contractile phenotype of differentiated human dermal myofibroblasts. Biochem. Biophys. Res. Commun. 2020, 521, 646–651. [Google Scholar] [CrossRef]
- Popov, A.L.; Popova, N.; Gould, D.J.; Shcherbakov, A.B.; Sukhorukov, G.B.; Ivanov, V.K. Ceria Nanoparticles-Decorated Microcapsules as a Smart Drug Delivery/Protective System: Protection of Encapsulated P. pyralis Luciferase. ACS Appl. Mater. Interfaces 2018, 10, 14367–14377. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef]
- Hall, C.L.; Wells, A.R.; Leung, K.P. Pirfenidone reduces profibrotic responses in human dermal myofibroblasts, in vitro. Lab. Investig. 2018, 98, 640–655. [Google Scholar] [CrossRef]
- Medina, J.L.; Sebastian, E.A.; Fourcaudot, A.B.; Dorati, R.; Leung, K.P. Pirfenidone Ointment Modulates the Burn Wound Bed in C57BL/6 Mice by Suppressing Inflammatory Responses. Inflammation 2019, 42, 45–53. [Google Scholar] [CrossRef]
- Shi, K.; Wang, F.Z.; Xia, J.H.; Zuo, B.L.; Wang, Z.H.; Cao, X.J. Pirfenidone inhibits epidural scar fibroblast proliferation and differentiation by regulating TGF-beta 1-induced Smad-dependent and -independent pathways. Am. J. Transl. Res. 2019, 11, 1593–1604. [Google Scholar]
- Dorati, R.; Medina, J.L.; DeLuca, P.P.; Leung, K.P. Development of a Topical 48-H Release Formulation as an Anti-scarring Treatment for Deep Partial-Thickness Burns. AAPS PharmSciTech 2018, 19, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Katta, K.; Busko, D.; Avlasevich, Y.; Landfester, K.; Baluschev, S.; Muñoz-Espí, R. Ceria/polymer nanocontainers for high-performance encapsulation of fluorophores. Beilstein J. Nanotechnol. 2019, 10, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; Feng, T.; Li, B.; Han, Y. In Vitro and In Vivo Comparison Study of Electrospun PLA and PLA/PVA/SA Fiber Membranes for Wound Healing. Polymers 2020, 12, 839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, J.R.; Granja, P.L.; Bártolo, P.J. Advances in electrospun skin substitutes. Prog. Mater. Sci. 2016, 84, 314–334. [Google Scholar] [CrossRef]
- Zafar, M.; Najeeb, S.; Khurshid, Z.; Vazirzadeh, M.; Zohaib, S.; Najeeb, B.; Sefat, F. Potential of Electrospun Nanofibers for Biomedical and Dental Applications. Materials 2016, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Miguel, S.P.; Sequeira, R.S.; Moreira, A.F.; Cabral, C.S.D.; Mendonca, A.G.; Ferreira, P.; Correia, I.J. An overview of electrospun membranes loaded with bioactive molecules for improving the wound healing process. Eur. J. Pharm. Biopharm. 2019, 139, 1–22. [Google Scholar] [CrossRef]
- Ivanova, O.S.; Shekunova, T.O.; Ivanov, V.K.; Shcherbakov, A.B.; Popov, A.L.; Davydova, G.A.; Selezneva, I.I.; Kopitsa, G.P.; Tret’yakov, Y.D. One-stage synthesis of ceria colloid solutions for biomedical use. Dokl. Chem. 2011, 437, 103–106. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, X.; Zhu, M.; Qu, M.; Bogari, M.; Lin, L.; Mar Aung, Z.; Chen, W.; Chen, X.; Chai, G.; et al. Expansion of CD26 positive fibroblast population promotes keloid progression. Exp. Cell Res. 2017, 356, 104–113. [Google Scholar]
- Cui, J.; van Koeverden, M.P.; Mullner, M.; Kempe, K.; Caruso, F. Emerging methods for the fabrication of polymer capsules. Adv. Colloid Interface Sci. 2014, 207, 14–31. [Google Scholar] [CrossRef] [Green Version]
- Popova, N.R.; Popov, A.L.; Ermakov, A.M.; Reukov, V.V.; Ivanov, V.K. Ceria-Containing Hybrid Multilayered Microcapsules for Enhanced Cellular Internalisation with High Radioprotection Efficiency. Molecules 2020, 25, 2957. [Google Scholar] [CrossRef]
- McAloney, R.A.; Sinyor, M.; Dudnik, V.; Goh, M.C. Atomic force microscopy studies of salt effects on polyelectrolyte multilayer film morphology. Langmuir 2001, 17, 6655–6663. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Borodina, T.N.; Belova, D.D.; Belyakov, S.; Antipina, M.N. Heat-driven size reduction of biodegradable polyelectrolyte multilayer hollow capsules assembled on CaCO3 template. Colloids Surf. B Biointerfaces 2018, 170, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Gravina, N.; Maghni, K.; Welman, M.; Yahia, L.; Mbeh, D.A.; Messina, P.V. Protective role against hydrogen peroxide and fibroblast stimulation via Ce-doped TiO2 nanostructured materials. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Andre-Levigne, D.; Modarressi, A.; Pepper, M.S.; Pittet-Cuenod, B. Reactive Oxygen Species and NOX Enzymes Are Emerging as Key Players in Cutaneous Wound Repair. Int. J. Mol. Sci 2017, 18, 2149. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, J.; Meng, X.; Meng, C.; Zhao, J.; Chen, Y.; Zhang, Z.; Zhang, Y. Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation. Molecules 2022, 27, 1830. https://doi.org/10.3390/molecules27061830
He J, Meng X, Meng C, Zhao J, Chen Y, Zhang Z, Zhang Y. Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation. Molecules. 2022; 27(6):1830. https://doi.org/10.3390/molecules27061830
Chicago/Turabian StyleHe, Junwei, Xinxian Meng, Chen Meng, Jiayu Zhao, Yunsheng Chen, Zheng Zhang, and Yixin Zhang. 2022. "Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation" Molecules 27, no. 6: 1830. https://doi.org/10.3390/molecules27061830
APA StyleHe, J., Meng, X., Meng, C., Zhao, J., Chen, Y., Zhang, Z., & Zhang, Y. (2022). Layer-by-Layer Pirfenidone/Cerium Oxide Nanocapsule Dressing Promotes Wound Repair and Prevents Scar Formation. Molecules, 27(6), 1830. https://doi.org/10.3390/molecules27061830




