Screening and Demulsification Mechanism of Fluorinated Demulsifier Based on Molecular Dynamics Simulation
Abstract
1. Introduction
2. Experimental
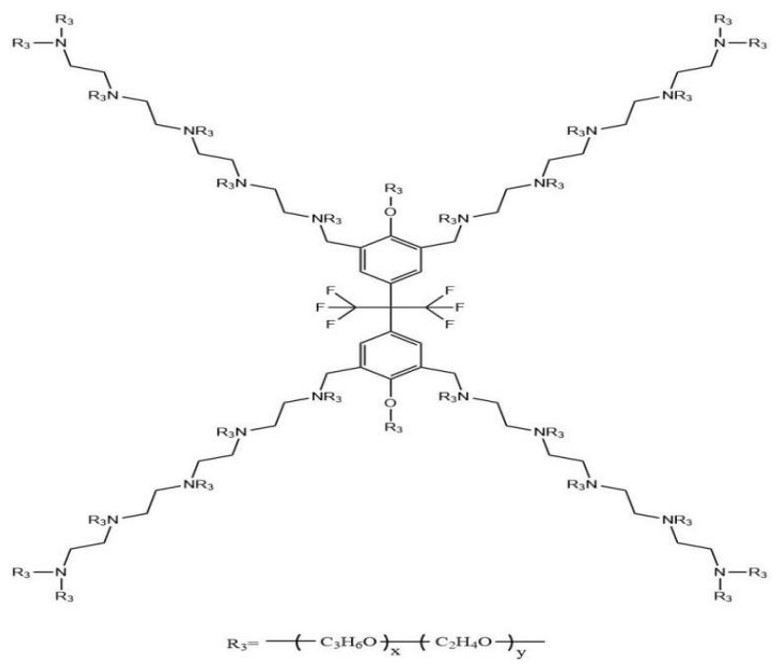
2.1. Materials
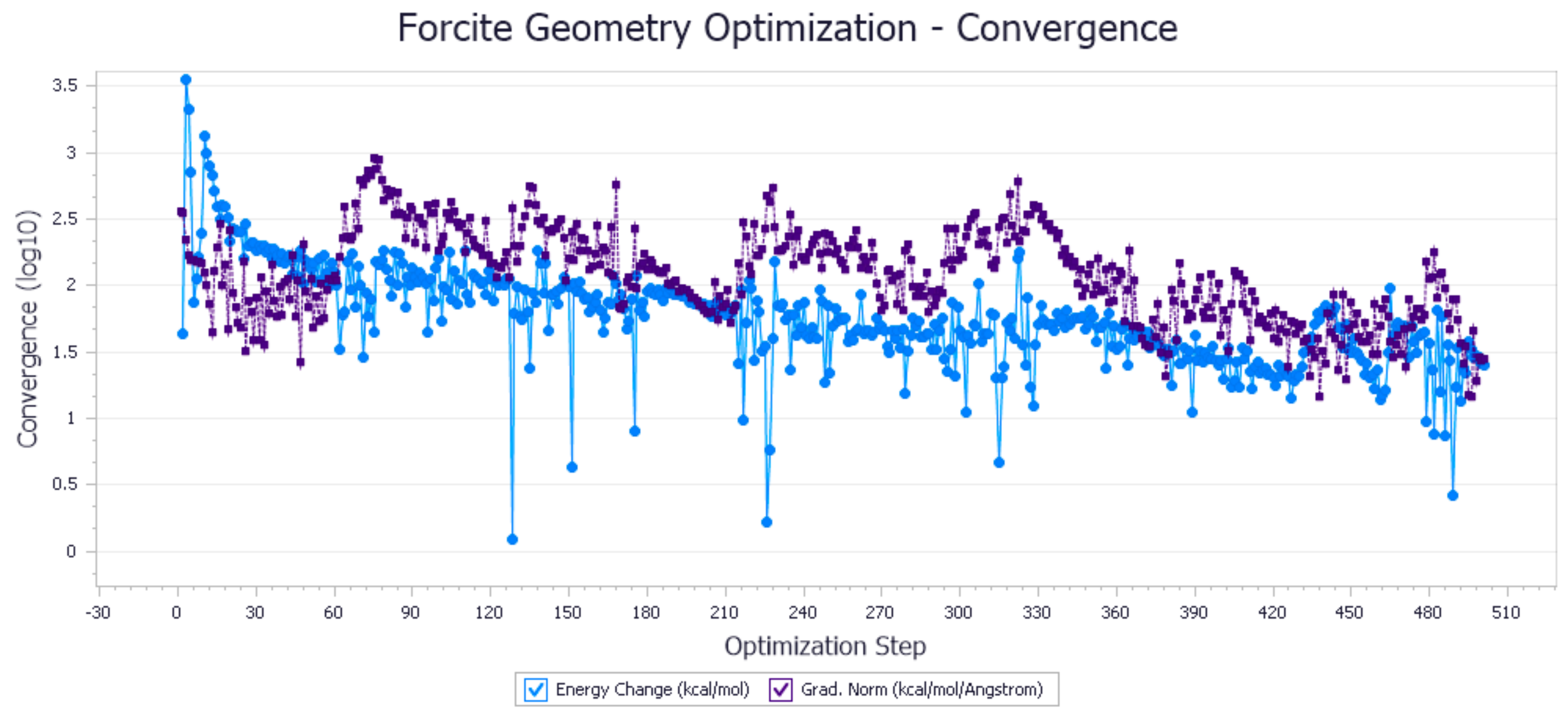
2.2. Molecular Optimization and Model Construction
2.3. Neural Network Analysis (NNA)
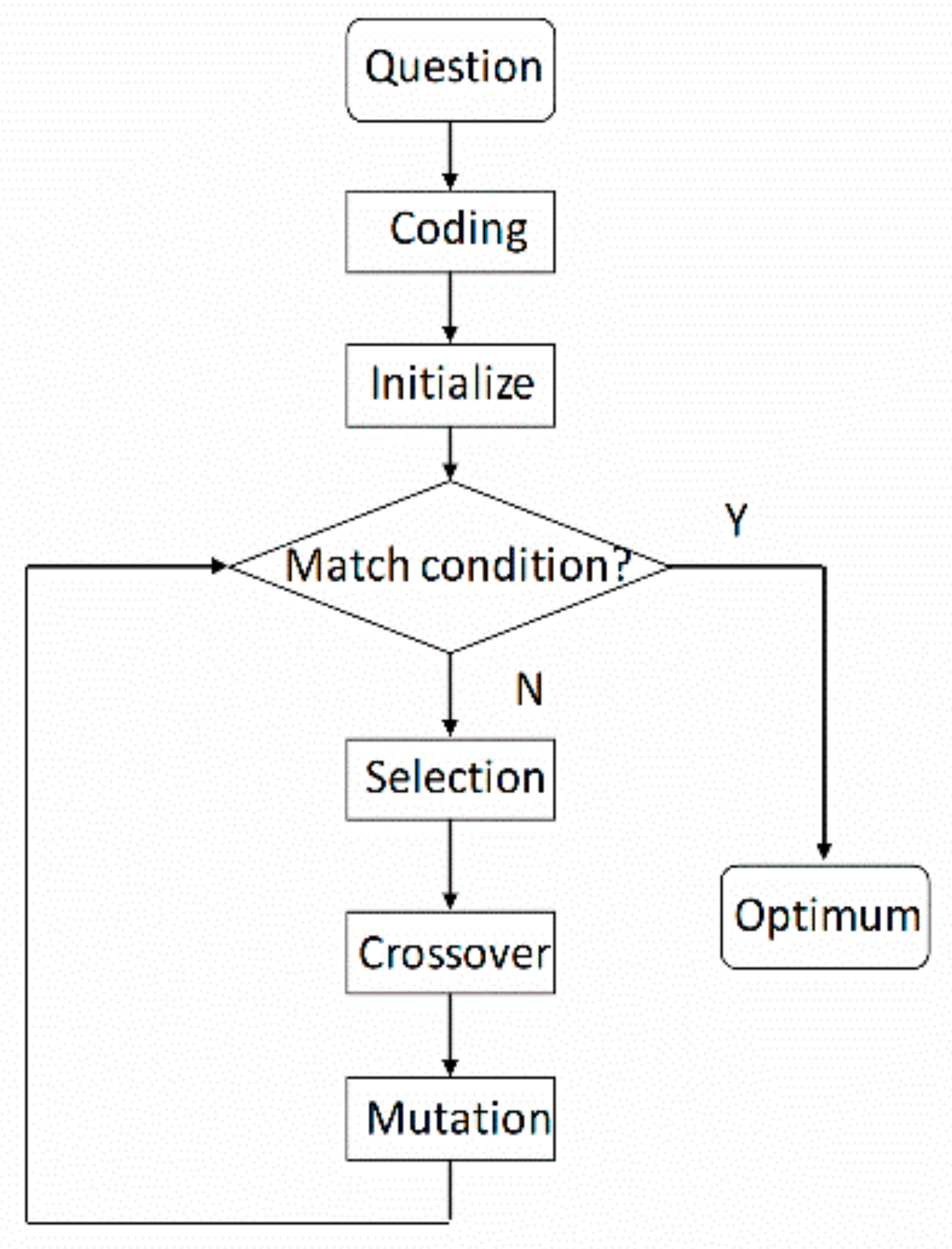
2.4. Genetic Function Approximation (GFA)
2.5. Turbidity Point and HLB Value Test
2.6. Experiment on Demulsification and Water Removal of Demulsifier
3. Results and Discussion
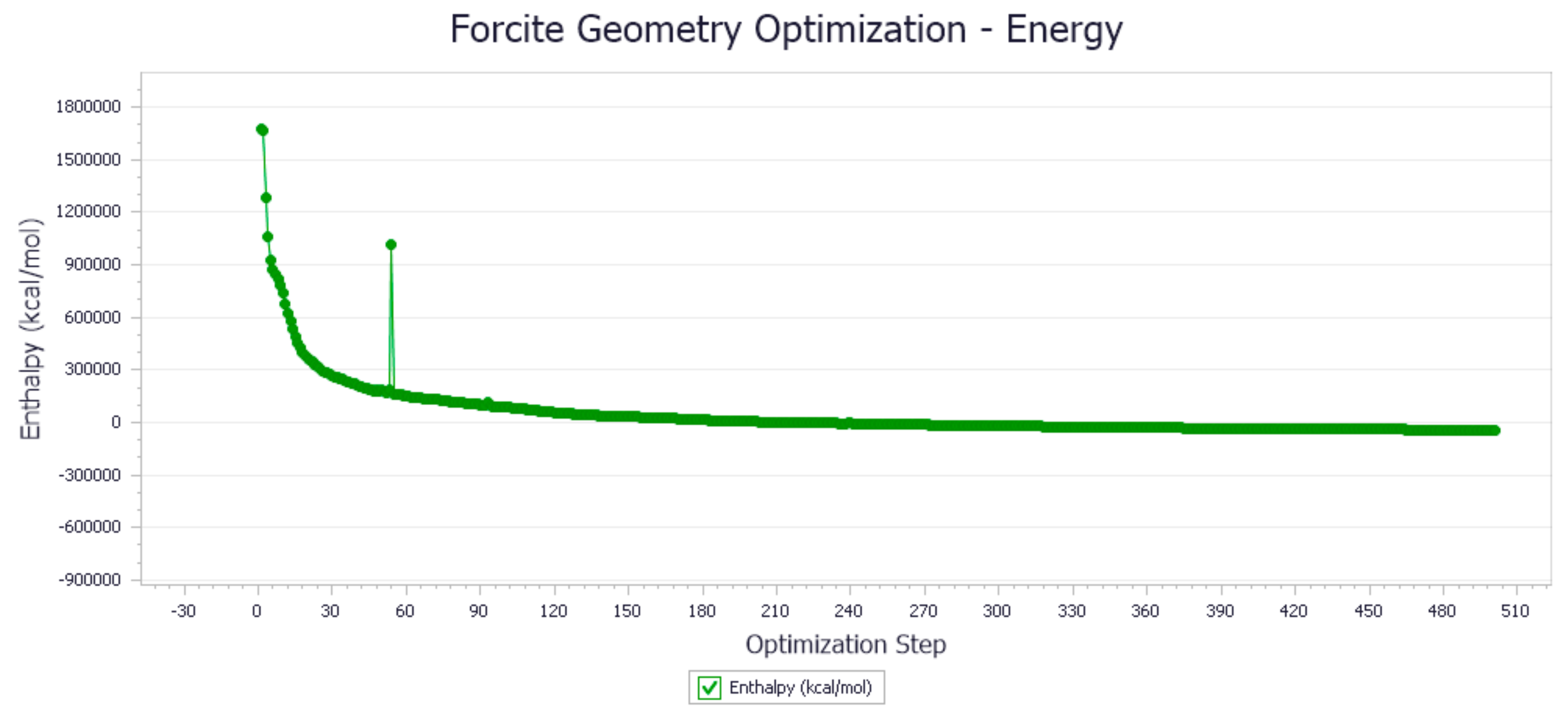
3.1. Molecular Dynamics Simulation Results
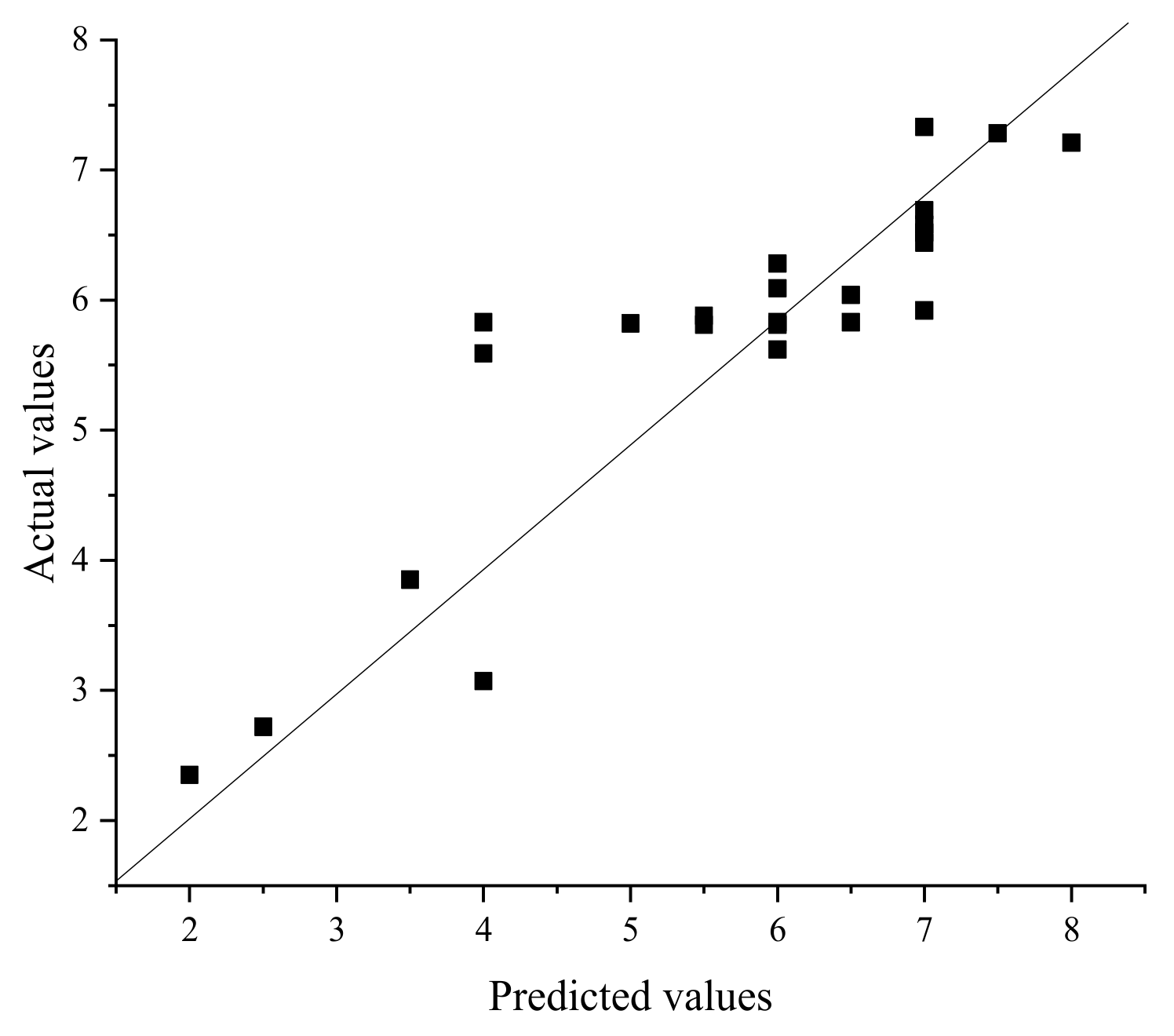
3.2. NNA and GFA Prediction Results
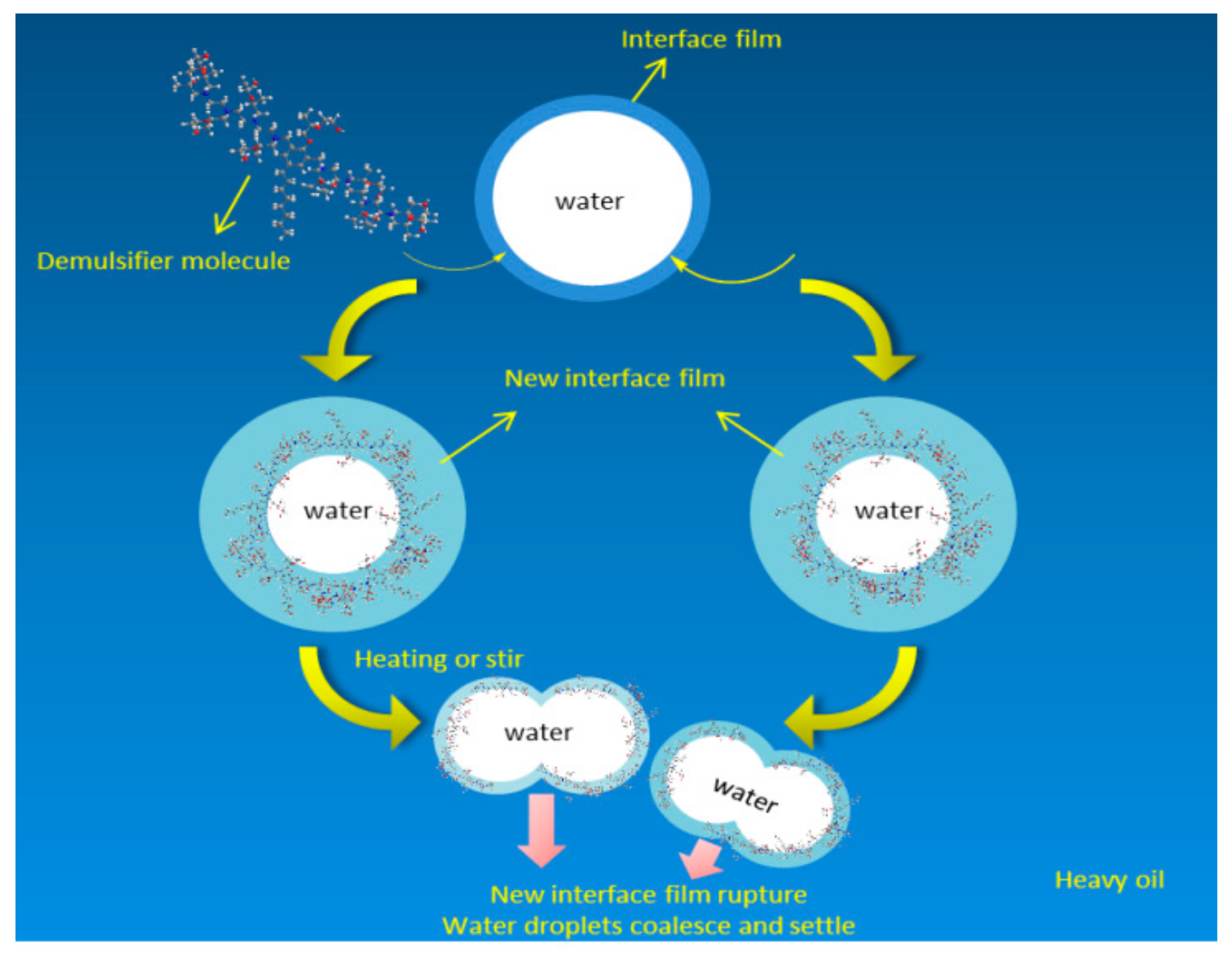
3.3. Demulsification Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhu, G.; Zhang, S.; Liu, Q.; Zhang, J.; Yang, J.; Wu, T.; Huang, Y.; Meng, S. Distribution and treatment of harmful gas from heavy oil production in the Liaohe Oilfield, Northeast China. Pet. Sci. 2010, 7, 422–427. [Google Scholar] [CrossRef][Green Version]
- Zhang, B.; Zhang, R.; Huang, D.; Shen, Y.; Gao, X.; Shi, W. Membrane fouling in microfiltration of alkali/surfactant/polymer flooding oilfield wastewater: Effect of interactions of key foulants. J. Colloid Interface Sci. 2020, 570, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, D.; Chen, Z.; Meng, X.; Gu, W.; Chen, M. Study on Basic Properties of Alkali-Surfactant-Polymer Flooding Water and Influence of Oil-Displacing Agent on Oil–Water Settlement. Arab. J. Sci. Eng. 2021, 27, 1–9. [Google Scholar] [CrossRef]
- Sun, L.; Wu, X.; Zhou, W.; Li, X.; Han, P. Technologies of enhancing oil recovery by chemical flooding in Daqing Oilfield, NE China. Pet. Explor. Dev. 2018, 45, 673–684. [Google Scholar] [CrossRef]
- Feitosa, F.X.; Alves, R.S.; De Sant’Ana, H.B. Synthesis and application of additives based on cardanol as demulsifier for water-in-oil emulsions. Fuel 2019, 245, 21–28. [Google Scholar] [CrossRef]
- Novriansyah, A.; Bae, W.; Park, C.; Permadi, A.K.; Riswati, S.S. Optimal design of alkaline–surfactant–polymer flooding under low salinity environment. Polymers 2020, 12, 626. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, J.; Cao, X.-L.; Xu, Z.-C.; Gong, Q.-T.; Zhang, L.; Zhang, L. Effect of Demulsifier Structures on the Interfacial Dilational Properties of Oil–Water Films. J. Dispers. Sci. Technol. 2015, 37, 1050–1058. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Q.; Li, X.; He, X. Novel polyether-polyquaternium copolymer as an effective reverse demulsifier for O/W emulsions: Demulsification performance and mechanism. Fuel 2020, 263, 116770. [Google Scholar] [CrossRef]
- Yi, M.; Huang, J.; Wang, L. Research on Crude Oil Demulsification Using the Combined Method of Ultrasound and Chemical Demulsifier. J. Chem. 2017, 2017, 9147926. [Google Scholar] [CrossRef]
- Pradilla, D.; Ramírez, J.; Zanetti, F.; Álvarez, O. Demulsifier Performance and Dehydration Mechanisms in Colombian Heavy Crude Oil Emulsions. Energy Fuels 2017, 31, 10369–10377. [Google Scholar] [CrossRef]
- Hjartnes, T.N.; Mhatre, S.; Gao, B.; Sørland, G.H.; Simon, S.; Sjöblom, J. Demulsification of crude oil emulsions tracked by pulsed field gradient NMR. part I: Chemical demulsification. Ind. Eng. Chem. Res. 2019, 58, 2310–2323. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T.; et al. Stabilization mechanism and chemical demulsification of water-in-oil and oil-in-water emulsions in petroleum industry: A review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Shehzad, F.; Hussein, I.A.; Kamal, M.S.; Ahmad, W.; Sultan, A.S.; Nasser, M.S. Polymeric Surfactants and Emerging Alternatives used in the Demulsification of Produced Water: A Review. Polym. Rev. 2018, 58, 63–101. [Google Scholar] [CrossRef]
- Spinelli, L.S.; Aquino, A.S.; Pires, R.V.; Barboza, E.M.; Louvisse, A.M.T.; Lucas, E.F. Influence of polymer bases on the synergistic effects obtained from mixtures of additives in the petroleum industry: Performance and residue formation. J. Pet. Sci. Eng. 2007, 58, 111–118. [Google Scholar] [CrossRef]
- Ahmadi, M.; Chen, Z. Insight into the Interfacial Behavior of Surfactants and Asphaltenes: Molecular Dynamics Simulation Study. Energy Fuels 2020, 34, 13536–13551. [Google Scholar] [CrossRef]
- Gunsteren, W.F.V.; Berendsen, H.J.C. Computer-simulation of molecular-dynamics-methodology, applications, and perspectives in chemistry. Angew. Chem. Int. Ed. 2010, 29, 992–1023. [Google Scholar] [CrossRef]
- Marquez, R.; Forgiarini, A.M.; Langevin, D.; Salager, J.-L. Breaking of Water-In-Crude Oil Emulsions. Part 9. New Interfacial Rheology Characteristics Measured Using a Spinning Drop Rheometer at Optimum Formulation. Energy Fuels 2019, 33, 8151–8164. [Google Scholar] [CrossRef]
- Ballal, D.; Srivastava, R. Modeling the interfacial properties of Poly(Ethylene oxide-Co-Propylene oxide) polymers at water-toluene interface. Fluid Phase Equilibria 2016, 427, 209–218. [Google Scholar] [CrossRef]
- Zhang, L.; Ying, H.; Yan, S.; Zhan, N.; Guo, Y.; Fang, W. Hyperbranched poly(amido amine) as an effective demulsifier for oil-in-water emulsions of microdroplets. Fuel 2018, 211, 197–205. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, L.; Chao, M.; Jia, X.; Liu, C.; Shi, L. Synthesis and Study of a New Type of Nonanionic Demulsifier for Chemical Flooding Emulsion Demulsification. ACS Omega 2021, 6, 17709–17719. [Google Scholar] [CrossRef]
- Ji, S.; Xu, W.; Yang, M.; Yu, K. 3D Convolutional Neural Networks for Human Action Recognition. IEEE Trans. Pattern Anal. Mach. Intell. 2013, 35, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Smyser, C.D.; Inder, T.E.; Shimony, J.S.; Hill, J.E.; Degnan, A.; Snyder, A.Z.; Neil, J.J. Longitudinal Analysis of Neural Network Development in Preterm Infants. Cereb. Cortex 2010, 20, 2852–2862. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Roy, K. Exploring QSTR modeling and toxicophore mapping for identification of important molecular features contributing to the chemical toxicity in Escherichia coli. Toxicol. Vitr. 2014, 28, 265–272. [Google Scholar] [CrossRef]
- Al-Hajri, M.T.; Abido, M.A.; Darwish, M.K. Assessment of genetic algorithm selection, crossover and mutation techniques in power loss optimization for a hydrocarbon facility. In Proceedings of the 2015 50th International Universities Power Engineering Conference (UPEC), Trent, UK, 1–4 September 2015; pp. 1–6. [Google Scholar]
- Sastry, K.; Goldberg, D.E.; Kendall, G. Genetic Algorithms; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Hanif, N.M.; Adnan, S.N.N.; Latif, M.T.; Zakaria, Z.; Othman, M.R. The composition of surfactants in river water and its influence to the amount of surfactants in drinking water. World Appl. Sci. J. 2012, 17, 970–975. [Google Scholar]
- Wang, Z.-Y.; Gang, H.-Z.; He, X.-L.; Bao, X.-N.; Ye, R.-Q.; Yang, S.-Z.; Li, Y.-C.; Mu, B.-Z. The middle phenyl-group at the hydrophobic tails of bio-based zwitterionic surfactants induced waved monolayers and more hydrated status on the surface of water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 622, 126655. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Effect of particle size, pressure and temperature on the activation process of hydrogen absorption in TiVZrHfNb high entropy alloy. J. Alloys Compd. 2021, 861, 158615. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, J.; Li, H.; Zhong, R.; Lin, M. Effect of heavy oil constituents on the oil-water interfacial properties and emulsion stability. J. Petrochem. Univ. 2016, 29, 32–38. [Google Scholar]
- Ma, L.; Zhang, C.; Luo, J. Investigation of the film formation mechanism of oil-in-water (O/W) emulsions. Soft Matter 2011, 7, 4207–4213. [Google Scholar] [CrossRef]
- Frömel, T.; Knepper, T.P. Biodegradation of fluorinated alkyl substances. Rev. Environ. Contam. Toxicol. 2010, 208, 161–177. [Google Scholar] [PubMed]
- Reiner, J.L.; Blaine, A.C.; Higgins, C.P.; Huset, C.; Jenkins, T.M.; Kwadijk, C.J.A.F.; Lange, C.; Muir, D.; Reagen, W.K.; Rich, C.; et al. Polyfluorinated substances in abiotic standard reference materials. Anal. Bioanal. Chem. 2015, 407, 2975–2983. [Google Scholar] [CrossRef] [PubMed]
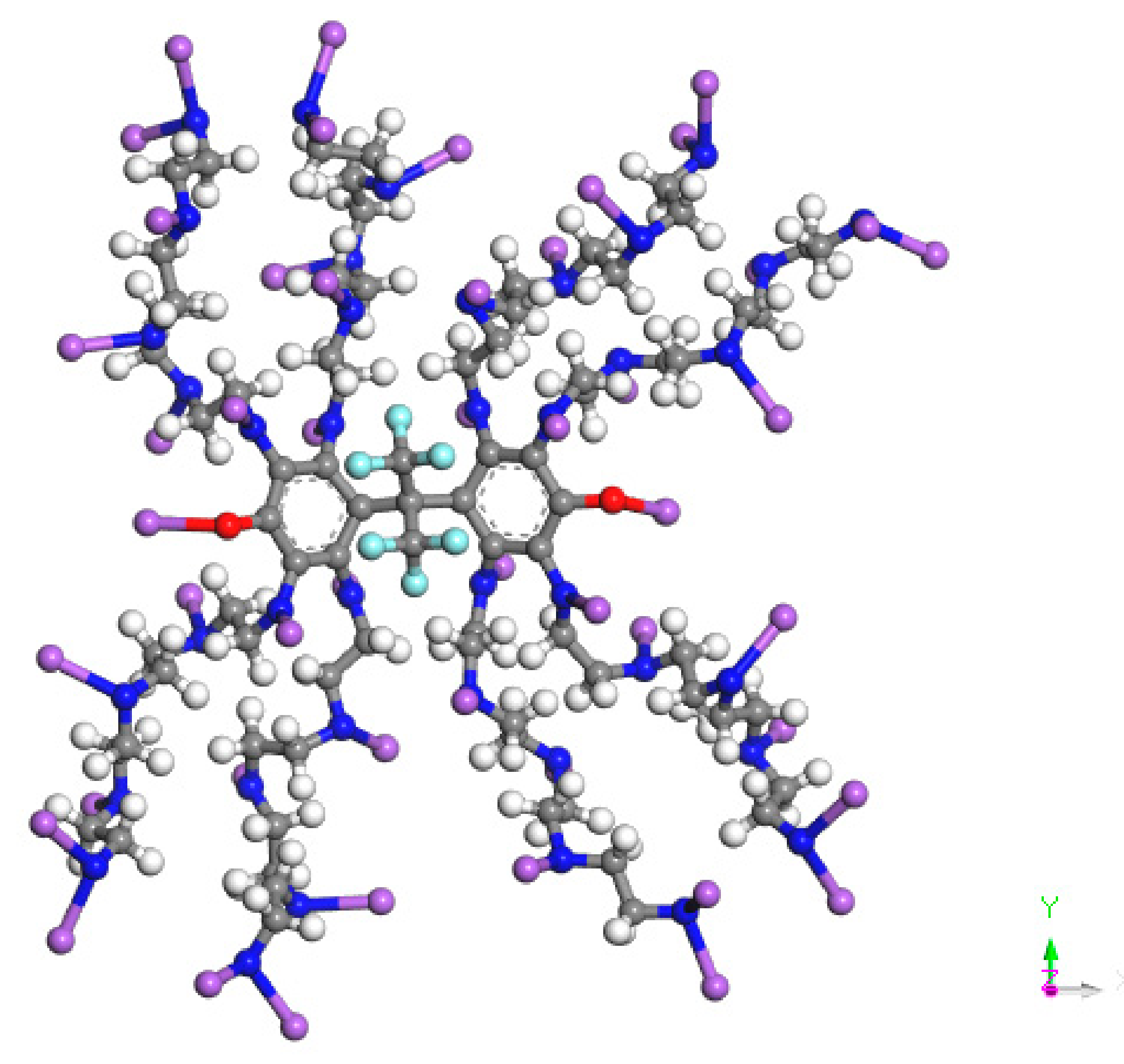
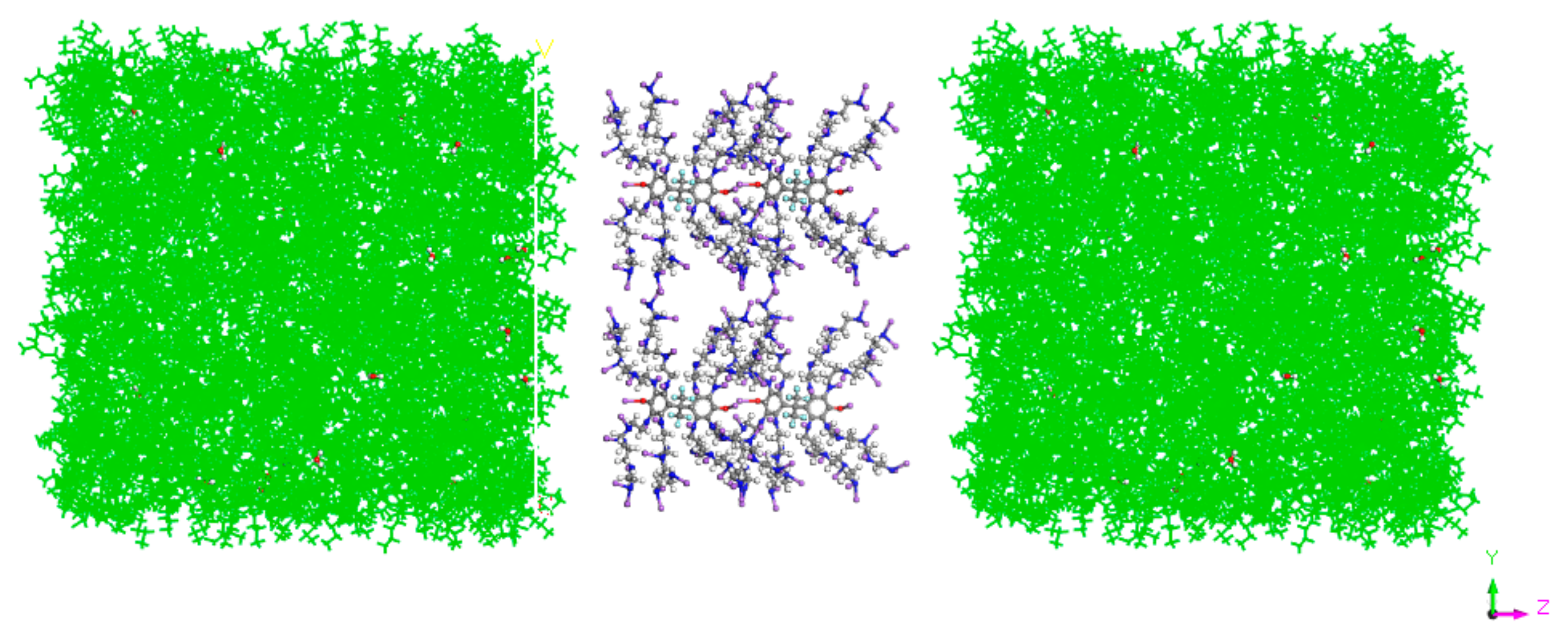






| Density kg·m−3 | Dynamic Viscosity (50 °C) mPa·s | Gum % | Asphaltene % | Acid Value mgKOH·g−1 | Pour Point °C | Moisture Content % | Saturate % | Aromatic % | Resin % |
|---|---|---|---|---|---|---|---|---|---|
| 943.0 | 180.2 | 24.93 | 12.75 | 2.45 | 17.3 | 17.02 | 46.63 | 15.69 | 32.83 |
| Number | Determine of x,y | Water Removal/mL | Etotal/ (Kcal/mol) | IFE/ (Kcal/mol) |
|---|---|---|---|---|
| 1# | SDJ6920: x = 31, y = 20 | 3.5 | −47,819.55 | −209.31 |
| 2# | SDJ6927: x = 31, y = 15 | 4 | −47,869.13 | −214.28 |
| 3# | SDJ6930: x = 31, y = 13 | 2.5 | −47,002.77 | −127.64 |
| 4# | SDJ6937: x = 31, y = 11 | 2 | −46,933.29 | −120.69 |
| 5# | SDJ9920: x = 44, y = 29 | 7 | −51,139.1 | −541.27 |
| 6# | SDJ9927: x = 44, y = 21 | 8 | −52,131.12 | −640.48 |
| 7# | SDJ9930: x = 44, y = 19 | 7.5 | −51,943.12 | −621.68 |
| 8# | SDJ9937: x = 44, y = 16 | 7 | −51,611.02 | −588.47 |
| 9# | SDJ15920: x = 71, y = 47 | 7 | −51,002.13 | −527.58 |
| 10# | SDJ15927: x = 71, y = 35 | 4 | −47,133.09 | −140.67 |
| 11# | SDJ15930: x = 71, y = 31 | 6 | −50,169.33 | −444.30 |
| 12# | SDJ15937: x = 71, y = 25 | 6 | −50,129.32 | −440.30 |
| 13# | SDJ19920: x = 89, y = 59 | 6 | −50,196.02 | −447.00 |
| 14# | SDJ19927: x = 89, y = 43 | 7 | −51,584.18 | −585.78 |
| 15# | SDJ19930: x = 89, y = 39 | 7 | −50,113.22 | −438.69 |
| 16# | SDJ19937: x = 89, y = 32 | 6.5 | −51,008.12 | −528.18 |
| 17# | SDJ29920: x = 134, y = 88 | 4 | −47,003.72 | −127.74 |
| 18# | SDJ29927: x = 134, y = 65 | 5.5 | −48,121.12 | −239.48 |
| 19# | SDJ29930: x = 134, y = 59 | 7 | −51,341.91 | −561.55 |
| 20# | SDJ29937: x = 134, y = 48 | 6.5 | −51,620.12 | −589.38 |
| 21# | SDJ39920: x = 178, y = 118 | 5.5 | −49,723.18 | −399.68 |
| 22# | SDJ39927: x = 179, y = 87 | 6 | −50,339.33 | −461.30 |
| 23# | SDJ39930: x = 179, y = 79 | 5 | −48,013.13 | −228.68 |
| 24# | SDJ39937: x = 17, y = 64 | 6 | −50,632.44 | −490.61 |
| Number | Determination of x, y Values | Cloud Point/°C | HLB Value |
|---|---|---|---|
| 1# | SDJ6920: x = 31, y = 20 | 53.2 | 9.23 |
| 2# | SDJ6927: x = 31, y = 15 | 52.5 | 9.17 |
| 3# | SDJ6930: x = 31, y = 13 | 52.1 | 9.13 |
| 4# | SDJ6937: x = 31, y = 11 | 51.8 | 9.10 |
| 5# | SDJ9920: x = 44, y = 29 | 47.8 | 8.70 |
| 6# | SDJ9927: x = 44, y = 21 | 47.3 | 8.66 |
| 7# | SDJ9930: x = 44, y = 19 | 46.9 | 8.62 |
| 8# | SDJ9937: x = 44, y = 16 | 46.5 | 8.58 |
| 9# | SDJ15920: x = 71, y = 47 | 41.3 | 8.07 |
| 10# | SDJ15927: x = 71, y = 35 | 40.7 | 8.01 |
| 11# | SDJ15930: x = 71, y = 31 | 40.4 | 7.98 |
| 12# | SDJ15937: x = 71, y = 25 | 40.0 | 7.94 |
| 13# | SDJ19920: x = 89, y = 59 | 36.2 | 7.57 |
| 14# | SDJ19927: x = 89, y = 43 | 35.4 | 7.49 |
| 15# | SDJ19930: x = 89, y = 39 | 35.0 | 7.45 |
| 16# | SDJ19937: x = 89, y = 32 | 34.7 | 7.42 |
| 17# | SDJ29920: x = 134, y = 88 | 31.2 | 7.08 |
| 18# | SDJ29927: x = 134, y = 65 | 30.8 | 7.04 |
| 19# | SDJ29930: x = 134, y = 59 | 30.3 | 6.99 |
| 20# | SDJ29937: x = 134, y = 48 | 30.1 | 6.97 |
| 21# | SDJ39920: x = 179, y = 118 | 28.4 | 6.80 |
| 22# | SDJ39927: x = 179, y = 87 | 27.9 | 6.75 |
| 23# | SDJ39930: x = 179, y = 79 | 27.5 | 6.72 |
| 24# | SDJ39937: x = 179, y = 64 | 27.0 | 6.67 |
| Number | Determination of x, y Values | Water Removal Amount/mL | Demulsification Rate/% |
|---|---|---|---|
| 1# | SDJ6920: x = 31, y = 20 | 3.5 | 41.13 |
| 2# | SDJ6927: x = 31, y = 15 | 4 | 47.00 |
| 3# | SDJ6930: x = 31, y = 13 | 2.5 | 29.38 |
| 4# | SDJ6937: x = 31, y = 11 | 2 | 23.50 |
| 5# | SDJ9920: x = 44, y = 29 | 7 | 82.26 |
| 6# | SDJ9927: x = 44, y = 21 | 8 | 94.01 |
| 7# | SDJ9930: x = 44, y = 19 | 7.5 | 88.13 |
| 8# | SDJ9937: x = 44, y = 16 | 7 | 82.26 |
| 9# | SDJ15920: x = 71, y = 47 | 7 | 82.26 |
| 10# | SDJ15927: x = 71, y = 35 | 4 | 47.00 |
| 11# | SDJ15930: x = 71, y = 31 | 6 | 70.51 |
| 12# | SDJ15937: x = 71, y = 25 | 6 | 70.51 |
| 13# | SDJ19920: x = 89, y = 59 | 6 | 70.51 |
| 14# | SDJ19927: x = 89, y = 43 | 7 | 82.26 |
| 15# | SDJ19930: x = 89, y = 39 | 7 | 82.26 |
| 16# | SDJ19937: x = 89, y = 32 | 6.5 | 76.38 |
| 17# | SDJ29920: x = 134, y = 88 | 4 | 47.00 |
| 18# | SDJ29927: x = 134, y = 65 | 5.5 | 64.63 |
| 19# | SDJ29930: x = 134, y = 59 | 7 | 82.26 |
| 20# | SDJ29937: x = 134, y = 48 | 6.5 | 76.38 |
| 21# | SDJ39920: x = 179, y = 118 | 5.5 | 64.63 |
| 22# | SDJ39927: x = 179, y = 87 | 6 | 70.51 |
| 23# | SDJ39930: x = 179, y = 79 | 5 | 58.75 |
| 24# | SDJ39937: x = 179, y = 64 | 6 | 70.51 |
| Black | No demulsifier | 0.3 | 3.53 |
| Number | Actual Values | NNA Prediction | Differential Value | GFA Prediction | Differential Value |
|---|---|---|---|---|---|
| 1# | 3.5 | 3.85 | −0.35 | 3.45 | 0.04 |
| 2# | 4 | 3.07 | 0.93 | 3.03 | 0.97 |
| 3# | 2.5 | 2.72 | −0.22 | 2.84 | −0.34 |
| 4# | 2 | 2.35 | −0.35 | 2.630 | −0.630 |
| 5# | 7 | 6.69 | 0.31 | 7.92 | −0.92 |
| 6# | 8 | 7.21 | 0.79 | 7.41 | 0.59 |
| 7# | 7.5 | 7.28 | 0.22 | 7.25 | 0.25 |
| 8# | 7 | 7.33 | −0.33 | 6.99 | 0.01 |
| 9# | 7 | 6.52 | 0.48 | 6.36 | 0.64 |
| 10# | 4 | 5.59 | −1.59 | 6.16 | −2.16 |
| 11# | 6 | 5.62 | 0.38 | 6.00 | −0.00 |
| 12# | 6 | 6.09 | −0.09 | 5.67 | 0.33 |
| 13# | 6 | 6.28 | −0.28 | 6.29 | −0.29 |
| 14# | 7 | 6.58 | 0.42 | 6.50 | 0.50 |
| 15# | 7 | 6.44 | 0.56 | 6.44 | 0.56 |
| 16# | 6.5 | 5.83 | 0.67 | 6.21 | 0.29 |
| 17# | 4 | 5.83 | −1.83 | 5.11 | −1.11 |
| 18# | 5.5 | 5.88 | −0.38 | 6.02 | −0.52 |
| 19# | 7 | 5.92 | 1.07 | 6.29 | 0.71 |
| 20# | 6.5 | 6.04 | 0.46 | 6.52 | −0.020 |
| 21# | 5.5 | 5.81 | −0.31 | 5.11 | 0.39 |
| 22# | 6 | 5.81 | 0.18 | 5.11 | 0.89 |
| 23# | 5 | 5.82 | −0.82 | 5.11 | −0.11 |
| 24# | 6 | 5.83 | 0.17 | 6.070 | −0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geng, X.; Li, C.; Zhang, L.; Guo, H.; Shan, C.; Jia, X.; Wei, L.; Cai, Y.; Han, L. Screening and Demulsification Mechanism of Fluorinated Demulsifier Based on Molecular Dynamics Simulation. Molecules 2022, 27, 1799. https://doi.org/10.3390/molecules27061799
Geng X, Li C, Zhang L, Guo H, Shan C, Jia X, Wei L, Cai Y, Han L. Screening and Demulsification Mechanism of Fluorinated Demulsifier Based on Molecular Dynamics Simulation. Molecules. 2022; 27(6):1799. https://doi.org/10.3390/molecules27061799
Chicago/Turabian StyleGeng, Xiaoheng, Changjun Li, Lin Zhang, Haiying Guo, Changqing Shan, Xinlei Jia, Lixin Wei, Yinghui Cai, and Lixia Han. 2022. "Screening and Demulsification Mechanism of Fluorinated Demulsifier Based on Molecular Dynamics Simulation" Molecules 27, no. 6: 1799. https://doi.org/10.3390/molecules27061799
APA StyleGeng, X., Li, C., Zhang, L., Guo, H., Shan, C., Jia, X., Wei, L., Cai, Y., & Han, L. (2022). Screening and Demulsification Mechanism of Fluorinated Demulsifier Based on Molecular Dynamics Simulation. Molecules, 27(6), 1799. https://doi.org/10.3390/molecules27061799






