Growth and Characterization of Graphene Layers on Different Kinds of Copper Surfaces
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
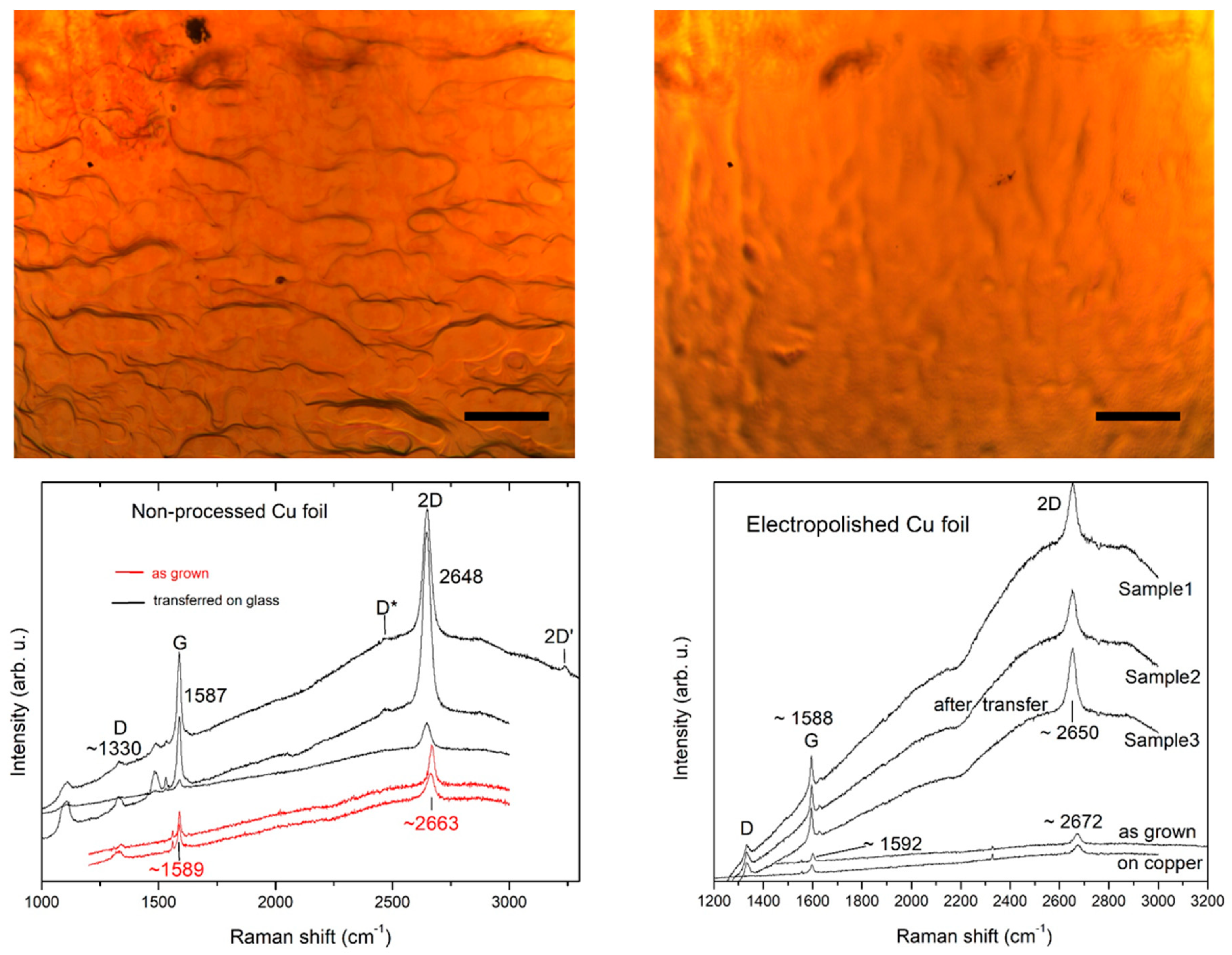
3.1. Sample Characterization with Raman and X-ray Photoelectron Spectroscopy
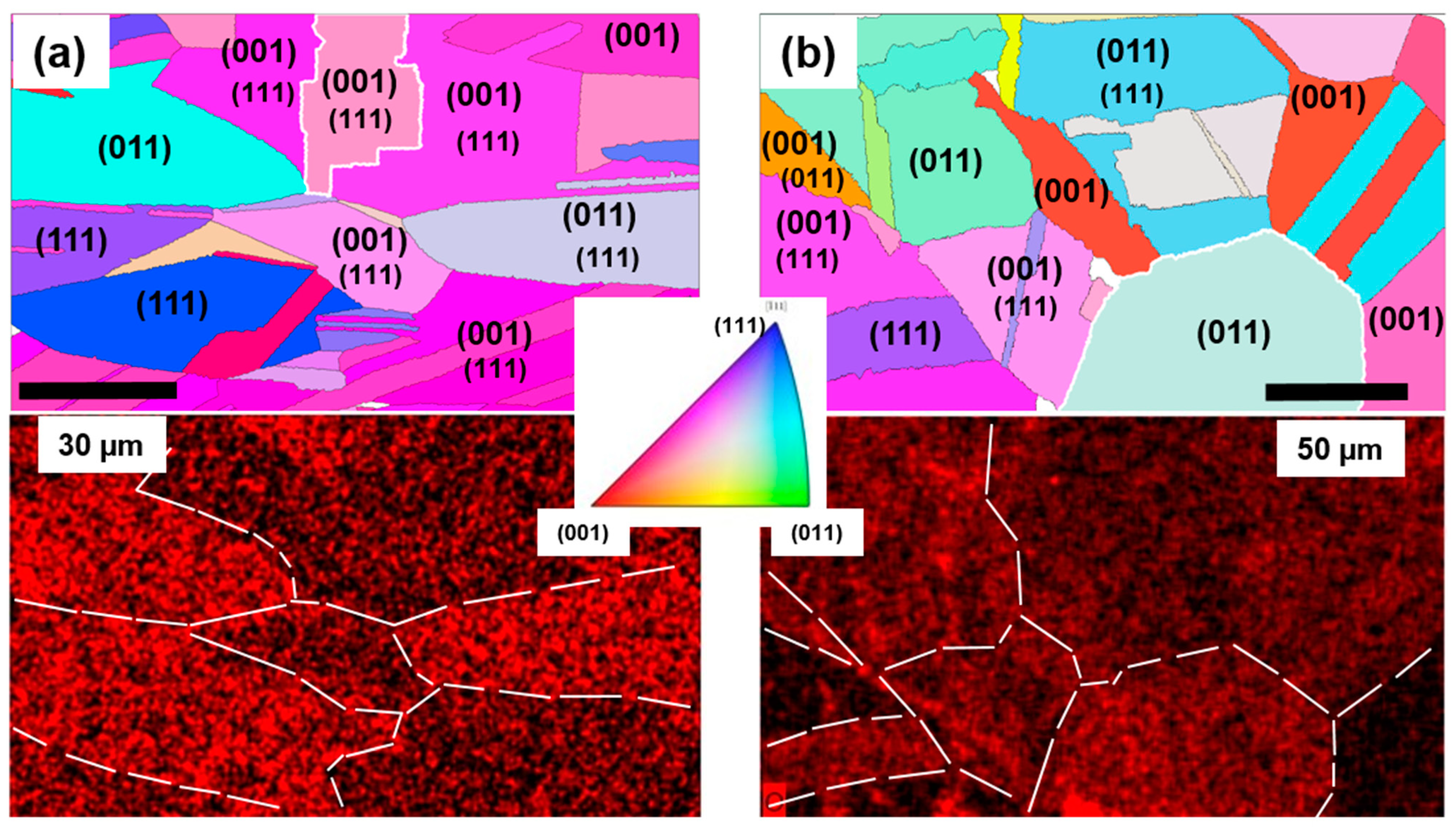
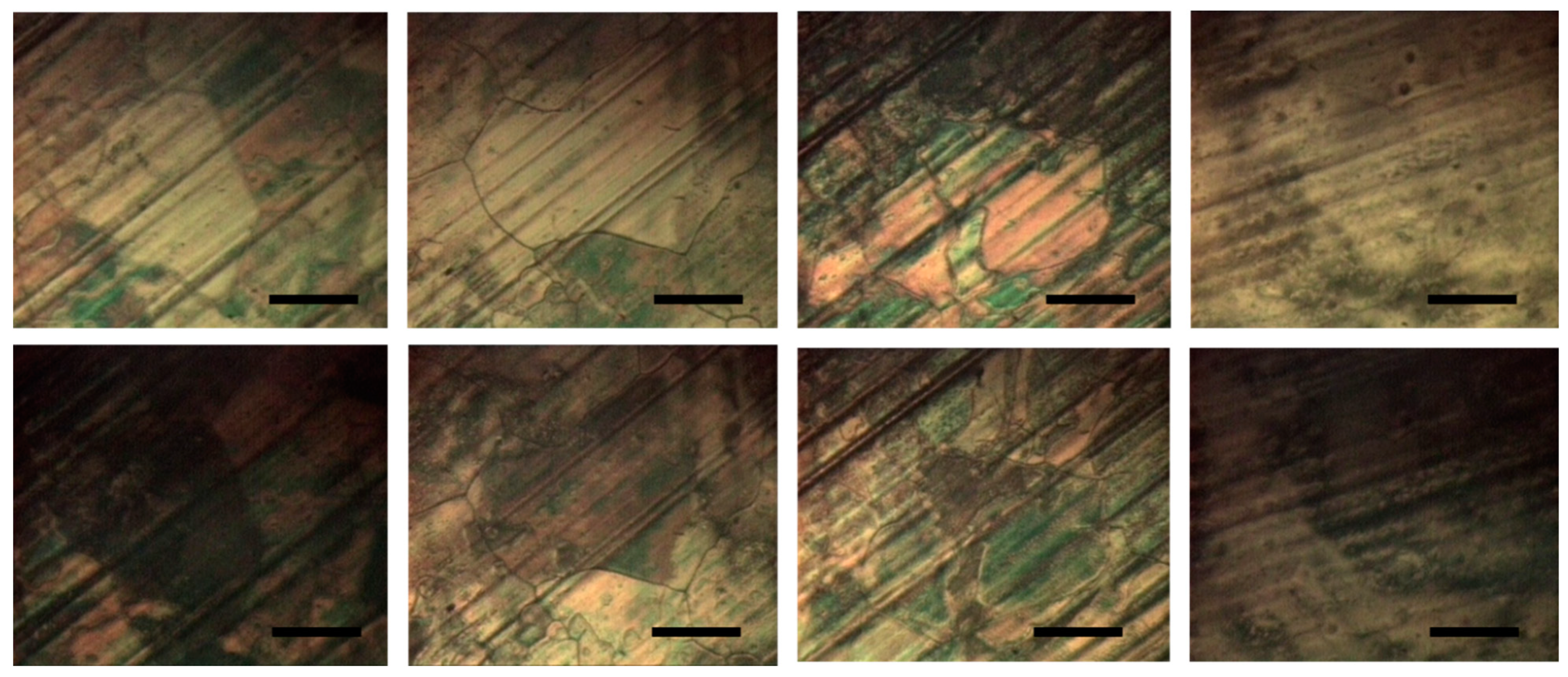
3.2. Study of the Cu Surface Oxidation with Ellipsometry and Electron Backscattering Diffraction (EBSD)
3.3. Examination of the Effects of Electropolishing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattevi, C.; Kima, H.; Chhowalla, M. A review of chemical vapour deposition of graphene on copper. J. Mater. Chem. 2011, 21, 3324. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jang, C.; Xiao, S.; Ishigami, M.; Fuhrer, M.S. Intrinsic and extrinsic performance limits of graphene devices on SiO2. Nat. Nanotechnol. 2008, 3, 206. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Skachko, I.; Barker, A.; Andrei, E.Y. Approaching ballistic transport in suspended grapheme. Nat. Nanotechnol. 2008, 3, 491. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Blake, P.; Grigorenko, A.N.; Novoselov, K.S.; Booth, T.J.; Stauber, T.; Peres, N.M.R.; Geim, A.K. Fine structure constant defines visual transparency of graphene. Science 2008, 320, 1308. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-roll production of 30-inch graphene films for transparent electrodes. Nat. Nanotechnol. 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; van der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable Atomic Membranes from Graphene Sheets. Nano Lett. 2008, 8, 2458. [Google Scholar] [CrossRef]
- Han, G.H.; Güneş, F.; Bae, J.J.; Kim, E.S.; Chae, S.J.; Shin, H.-J.; Choi, J.-Y.; Pribat, D.; Lee, Y.H. Influence of copper morphology in forming nucleation seeds for graphene growth. Nano Lett. 2011, 11, 4144–4148. [Google Scholar] [CrossRef]
- Wood, J.D.; Schmucker, S.W.; Lyons, A.S.; Pop, E.; Lyding, J.W. Effects of polycrystalline Cu substrate on graphene growth by chemical vapor deposition. Nano Lett. 2011, 11, 4547. [Google Scholar] [CrossRef]
- Schädlich, P.; Speck, F.; Bouhafs, C.; Mishra, N.; Forti, S.; Coletti, C.; Seyller, T. Stacking Relations and Substrate Interaction of Graphene on Copper Foil. Adv. Mater. Interfaces 2021, 8, 2002025. [Google Scholar] [CrossRef]
- Zhang, B.; Lee, W.H.; Piner, R.; Kholmanov, I.; Wu, Y.; Li, H.; Ji, H.; Ruoff, R.S. Low-temperature chemical vapor deposition growth of graphene from toluene on electropolished copper foils. ACS Nano 2012, 6, 2471–2476. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ahn, G.; Shim, J.; Lee, Y.S.; Ryu, S. Optical separation of mechanical strain from charge doping in graphene. Nat. Commun. 2012, 3, 1024. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, C.; Reich, S. Double Resonant Raman Scattering in Graphite. Phys. Rev. Lett. 2000, 85, 5214–5217. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.A.Y.; Wei, S.Y.; Wu, C.Y.; Hernandez, Y.; Chen, T.Y.; Liu, T.H.; Pao, C.W.; Chen, F.R.; Li, L.J.; Juang, Z.Y. Decoupling of CVD graphene by controlled oxidation of recrystallized Cu. RSC Adv. 2012, 2, 3008–3013. [Google Scholar] [CrossRef]
- Yin, X.; Li, Y.; Ke, F.; Lin, C.; Zhao, H.; Gan, L.; Luo, Z.; Zhao, R.; Heinz, T.F.; Hu, Z. Evolution of the Raman spectrum of graphene grown on copper upon oxidation of the substrate. Nano Res. 2014, 7, 1613. [Google Scholar] [CrossRef]
- Cazzanelli, E.; De Luca, O.; Vuono, D.; Policicchio, A.; Castriota, M.; Desiderio, G.; De Santo, M.P.; Aloise, A.; Fasanella, A.; Rugiero, T.; et al. Characterization of graphene grown on copper foil by chemical vapor deposition (CVD) at ambient pressure conditions. J. Raman Spectrosc. 2018, 49, 1006–1014. [Google Scholar] [CrossRef]
- Bartolomé, J.; Álvarez-Fraga, L.; Aguilar-Pujol, M.X.; Cortijo, S.; Cremades, A.; Prieto, C.; de Andrés, A. Grain selective Cu oxidation and anomalous shift of graphene 2D Raman peak in the graphene-Cu system. 2D Mater. 2019, 6, 015023. [Google Scholar] [CrossRef]
- Kwon, G.D.; Young, W.K.; Moyen, E.; Keum, D.H.; Lee, Y.H.; Baik, S.; Pribat, D. Controlled electropolishing of copper foils at elevated temperature. Appl. Surf. Sci. 2014, 307, 731–735. [Google Scholar] [CrossRef]
- Park, K.T.; Kim, T.; and Park, C.R. Overlook of current chemical vapor deposition-grown large single-crystal graphene domains. Carbon Lett. 2014, 15, 151–161. [Google Scholar] [CrossRef][Green Version]
- Bhaviripudi, S.; Jia, X.; Dresselhaus, M.S.; Kong, J. Role of kinetic factors in chemical vapor deposition synthesis of uniform large area graphene using copper catalyst. Nano Lett. 2010, 10, 4128–4133. [Google Scholar] [CrossRef]
- Debbichi, L.; de Lucas, M.C.M.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu4O3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Kidambi, P.R.; Bayer, B.C.; Blume, R.; Wang, Z.-J.; Baehtz, C.; Weatherup, R.S.; Willinger, M.-G.; Schloegl, R.; Hofmann, S. Observing Graphene Grow: Catalyst−Graphene Interactions during Scalable Graphene Growth on Polycrystalline Copper. Nano Lett. 2013, 13, 4769–4778. [Google Scholar] [CrossRef] [PubMed]
- Blume, R.; Kidambi, P.R.; Bayer, B.C.; Weatherup, R.S.; Wang, Z.-J.; Weinberg, G.; Willinger, M.-G.; Greiner, M.; Hofmann, S.; Knop-Gericke, A.; et al. The influence of intercalated oxygen on the properties of graphene on polycrystalline Cu under various environmental conditions. Phys. Chem. Chem. Phys. 2014, 16, 25989–26003. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, C.R.; Walker, M.; McConville, C.F. Reaction of atomic oxygen with a Pt(111) surface: Chemical and structural determination using XPS, CAICISS and LEED. Surf. Sci. 2003, 545, 19–33. [Google Scholar] [CrossRef]
- Puglia, C.; Nilsson, A.; Hernniis, B.; Karis, O.; Bennich, P.; Martensson, N. Physisorbed, chemisorbed and dissociated O2 on Pt (111) studied by different core level spectroscopy methods. Surf. Sci. 1995, 342, 119–133. [Google Scholar] [CrossRef]
- Álvarez-Fraga, L.; Rubio-Zuazo, J.; Jiménez-Villacorta, F.; Climent-Pascual, E.; Ramírez-Jiménez, R.; Prieto, C.; de Andrés, A. Oxidation Mechanisms of Copper under Graphene: The Role of Oxygen Encapsulation. Chem. Mater. 2017, 29, 3257–3264. [Google Scholar] [CrossRef]
- Choi, J.; Koo, S.; Song, M.; Jung, D.; Choi, S.; Ryu, S. Varying electronic coupling at graphene-copper interfaces probed with Raman spectroscopy. 2D Mater. 2020, 7, 025006. [Google Scholar] [CrossRef]
- Zhou, R.; Yasuda, S.; Minamimoto, H.; Murakoshi, K. Sensitive Raman Probe of Electronic Interactions between Monolayer Graphene and Substrate under Electrochemical Potential Control. ACS Omega 2018, 3, 2322–2328. [Google Scholar] [CrossRef]
- Marsden, A.J.; Asensio, M.-C.; Avila, J.; Dudin, P.; Barinov, A.; Moras, P.; Sheverdyaeva, P.M.; White, T.W.; Maskery, I.; Costantini, G.; et al. Is graphene on copper doped? Phys. Status Solidi (RRL) Rapid Res. Lett. 2013, 7, 643–646. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Y.; Lacey, S.D.; Xu, L.; Xie, H.; Li, T.; Danner, V.A.; Hu, L. Reduced graphene oxide film with record-high conductivity and mobility. Mater. Today 2018, 21, 186–192. [Google Scholar] [CrossRef]
- Losurdo, M.; Giangregorio, M.M.; Capezzuto, P.; Bruno, G. Ellipsometry as a Real-Time Optical Tool for Monitoring and Understanding Graphene Growth on Metals. J. Phys. Chem. C 2011, 115, 21804–21812. [Google Scholar] [CrossRef]
- Albrektsen, O.; Eriksen, R.L.; Novikov, S.M.; Schall, D.; Karl, M.; Bozhevolnyi, S.I.; Simonsen, A.C. High resolution imaging of few-layer graphene. J. Appl. Phys. 2012, 111, 064305. [Google Scholar] [CrossRef]
- Castriota, M.; Politano, G.G.; Vena, C.; De Santo, M.P.; Desiderio, G.; Davoli, M.; Cazzanelli, E.; Versace, C. Variable Angle Spectroscopic Ellipsometry investigation of CVD-grown monolayer grapheme. Appl. Surf. Sci. 2019, 467–468, 213–220. [Google Scholar] [CrossRef]
- Palik, E. Handbook of Optical Constants of Solids, 1st ed.; Elsevier: Amsterdam, The Netherland, 1997. [Google Scholar]
- Bachmann, F.; Hielscher, R.; Schaeben, H. Texture Analysis with MTEX – Free and Open Source Software Toolbox. Solid State Phenom. 2010, 160, 63–68. [Google Scholar] [CrossRef]
- Hu, J.; Xu, J.; Zhao, Y.; Shi, L.; Li, Q.; Liu, F.; Ullah, Z.; Li, W.; Guo, Y.; Liu, L. Roles of Oxygen and Hydrogen in Crystal Orientation Transition of Copper Foils for High-Quality Graphene Growth. Sci. Rep. 2017, 7, 45358. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Y.; Chang, H.; Stehle, R.; Xu, J.; Gao, L.; Zhang, W.; Jia, Y.; Qing, F.; Li, X. Preparation of Ultra-Smooth Cu Surface for High-Quality Graphene Synthesis. Nanoscale Res. Lett. 2018, 13, 340. [Google Scholar] [CrossRef]
- Kondrashov, I.; Komlenok, M.; Pivovarov, P.; Savin, S.; Obraztsova, E.; Rybin, M. Preparation of Copper Surface for the Synthesis of Single-Layer Graphene. Nanomaterials 2021, 11, 1071. [Google Scholar] [CrossRef]
- Basu, R.; Kinnamon, D.; Garvey, A. Graphene and liquid crystal mediated interactions. Liq. Cryst. 2016, 43, 2375–2390. [Google Scholar] [CrossRef]

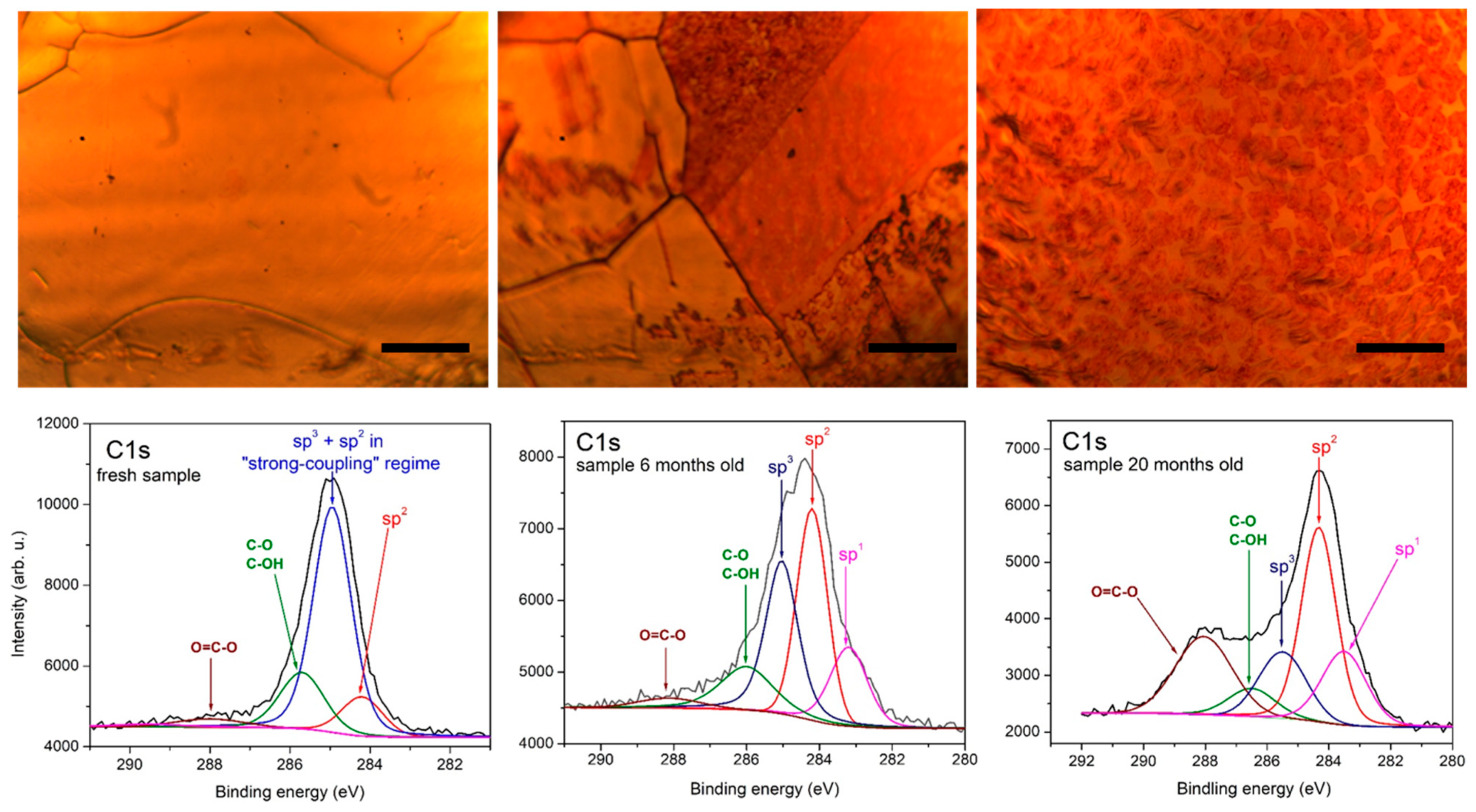
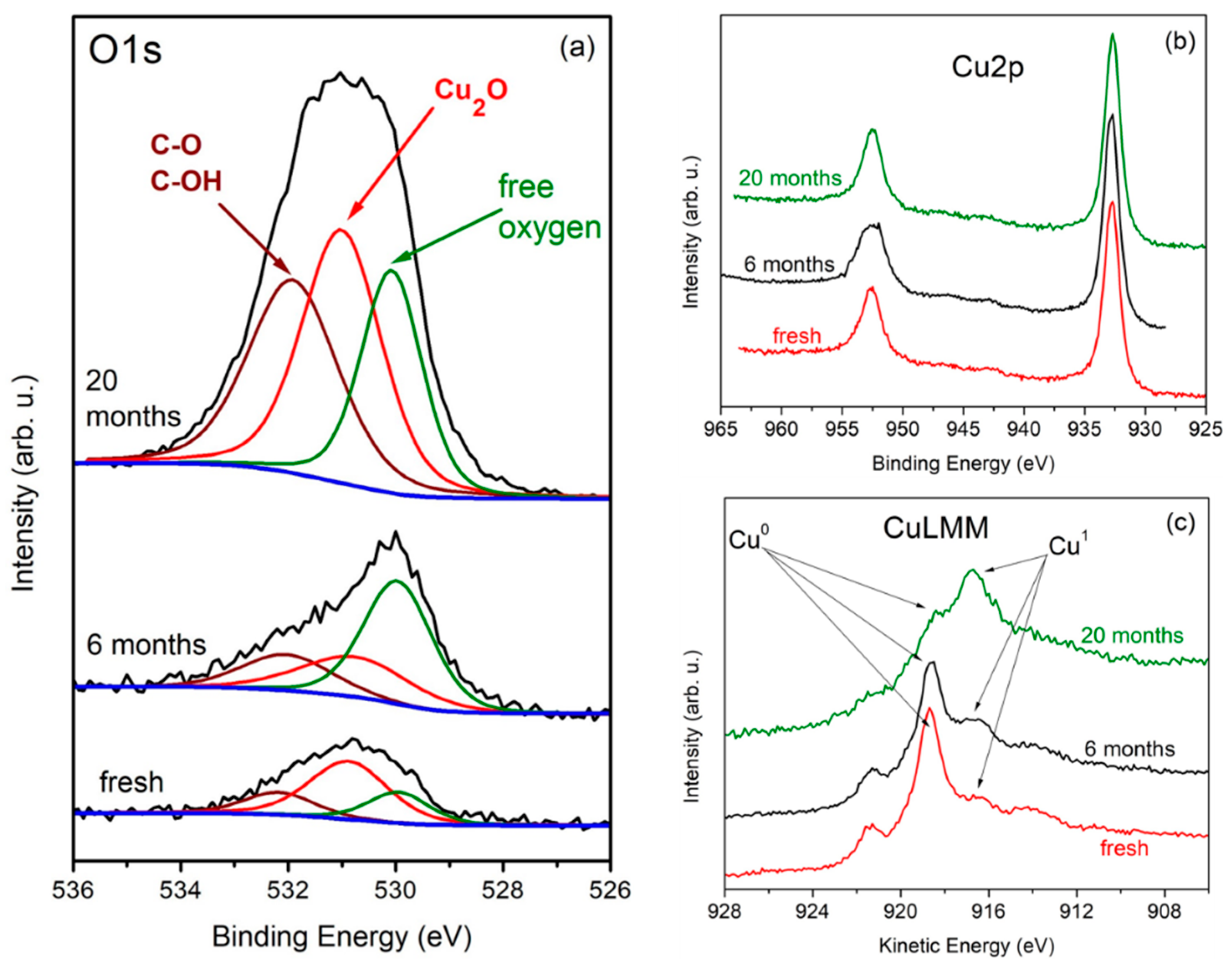
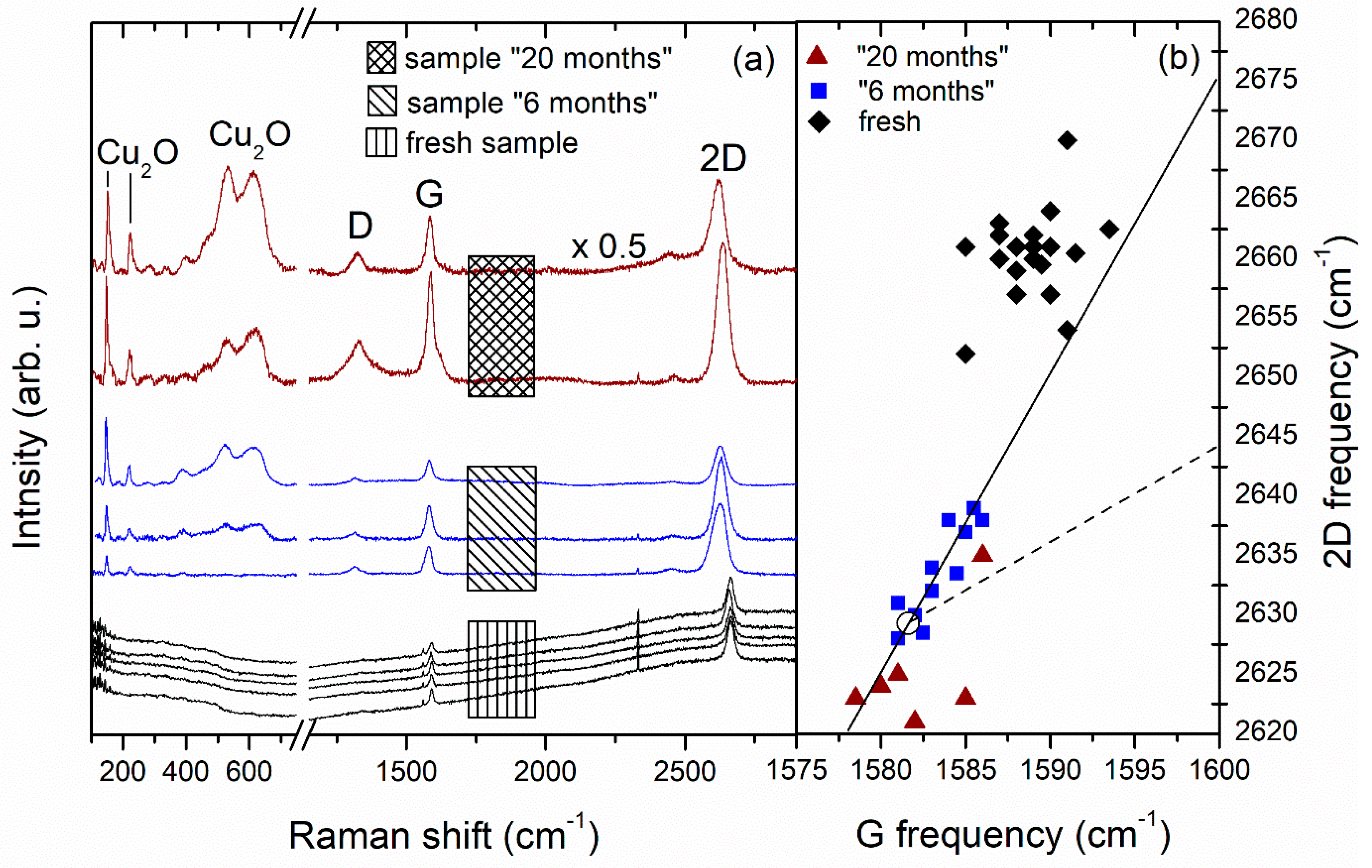
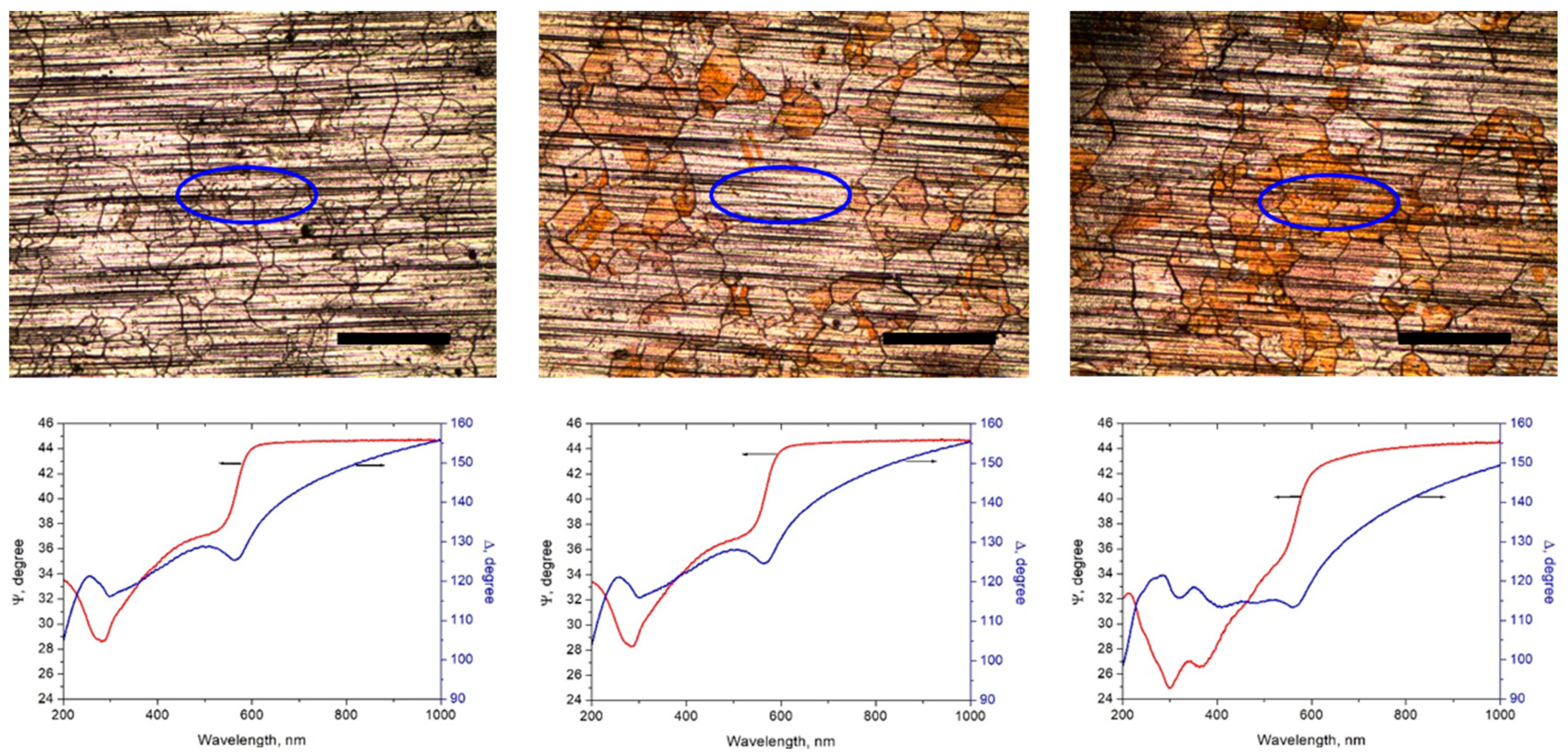
| Sample | Time Passed after CVD Growth | O, at% |
|---|---|---|
| “fresh” | 1–2 days | 9.54 |
| “6 months” | 6 months | 18.32 |
| “20 months” | 20 months | 33.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafailov, P.M.; Sveshtarov, P.K.; Mehandzhiev, V.B.; Avramova, I.; Terziyska, P.; Petrov, M.; Katranchev, B.; Naradikian, H.; Boyadjiev, S.I.; Cserháti, C.; et al. Growth and Characterization of Graphene Layers on Different Kinds of Copper Surfaces. Molecules 2022, 27, 1789. https://doi.org/10.3390/molecules27061789
Rafailov PM, Sveshtarov PK, Mehandzhiev VB, Avramova I, Terziyska P, Petrov M, Katranchev B, Naradikian H, Boyadjiev SI, Cserháti C, et al. Growth and Characterization of Graphene Layers on Different Kinds of Copper Surfaces. Molecules. 2022; 27(6):1789. https://doi.org/10.3390/molecules27061789
Chicago/Turabian StyleRafailov, Peter M., Peter K. Sveshtarov, Vladimir B. Mehandzhiev, Ivalina Avramova, Penka Terziyska, Minko Petrov, Boyko Katranchev, Haritun Naradikian, Stefan I. Boyadjiev, Csaba Cserháti, and et al. 2022. "Growth and Characterization of Graphene Layers on Different Kinds of Copper Surfaces" Molecules 27, no. 6: 1789. https://doi.org/10.3390/molecules27061789
APA StyleRafailov, P. M., Sveshtarov, P. K., Mehandzhiev, V. B., Avramova, I., Terziyska, P., Petrov, M., Katranchev, B., Naradikian, H., Boyadjiev, S. I., Cserháti, C., Erdélyi, Z., & Szilágyi, I. M. (2022). Growth and Characterization of Graphene Layers on Different Kinds of Copper Surfaces. Molecules, 27(6), 1789. https://doi.org/10.3390/molecules27061789








