Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membranes Preparation
2.3. Morphological and Microstructural Characterization
2.4. Ion Exchange Capacity Evaluation
2.5. Proton Conductivity Investigation
3. Results and Discussion
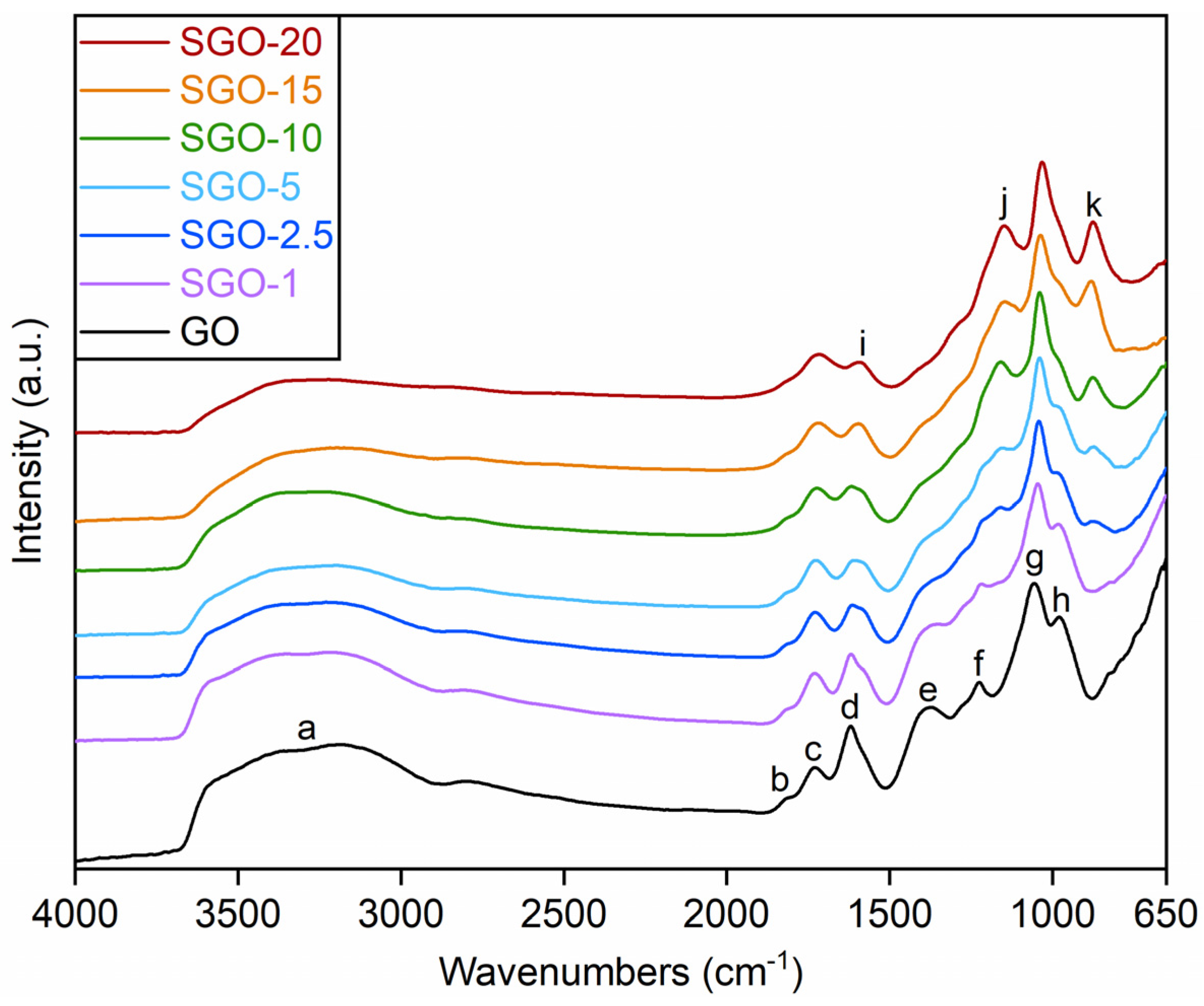
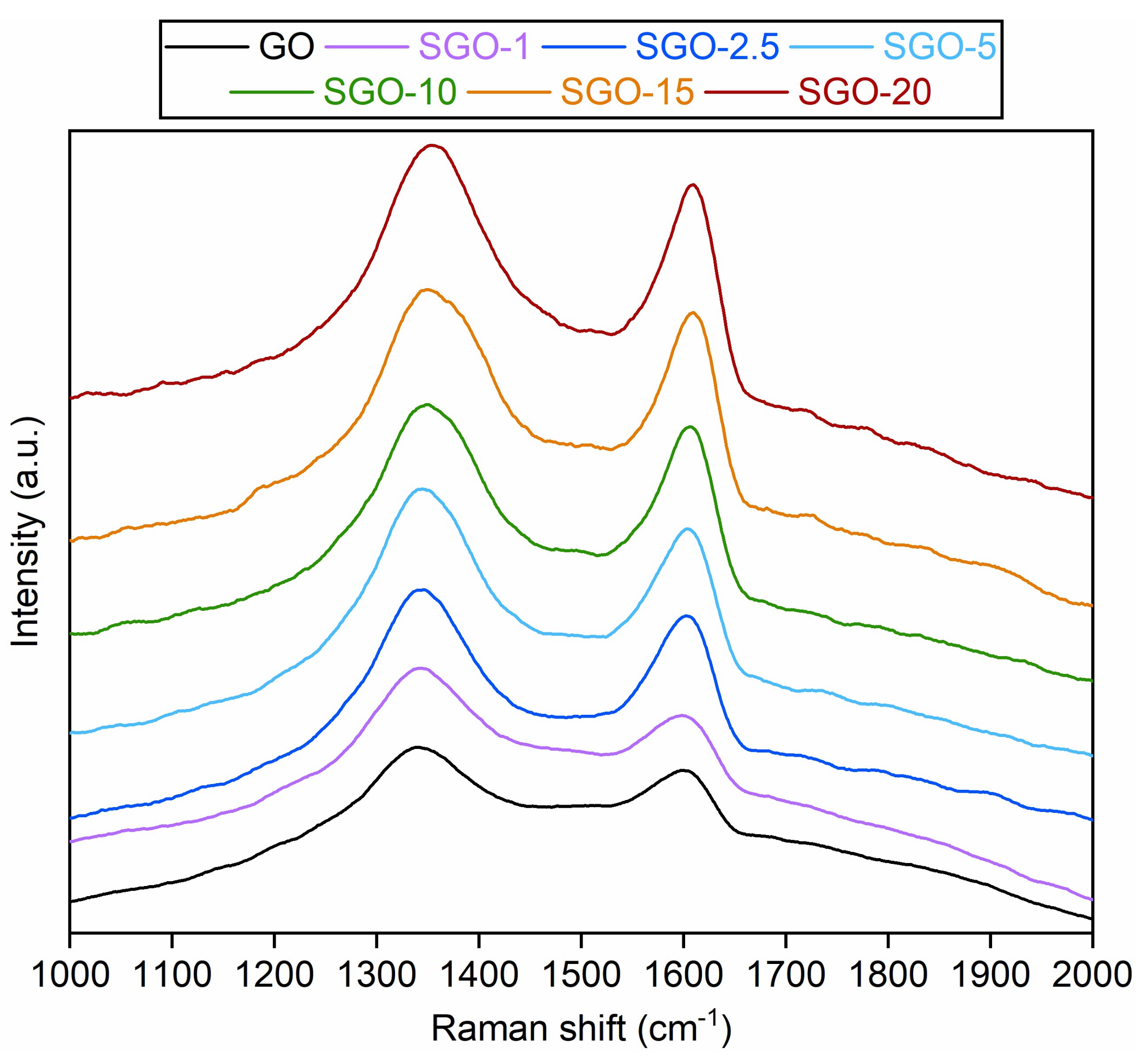
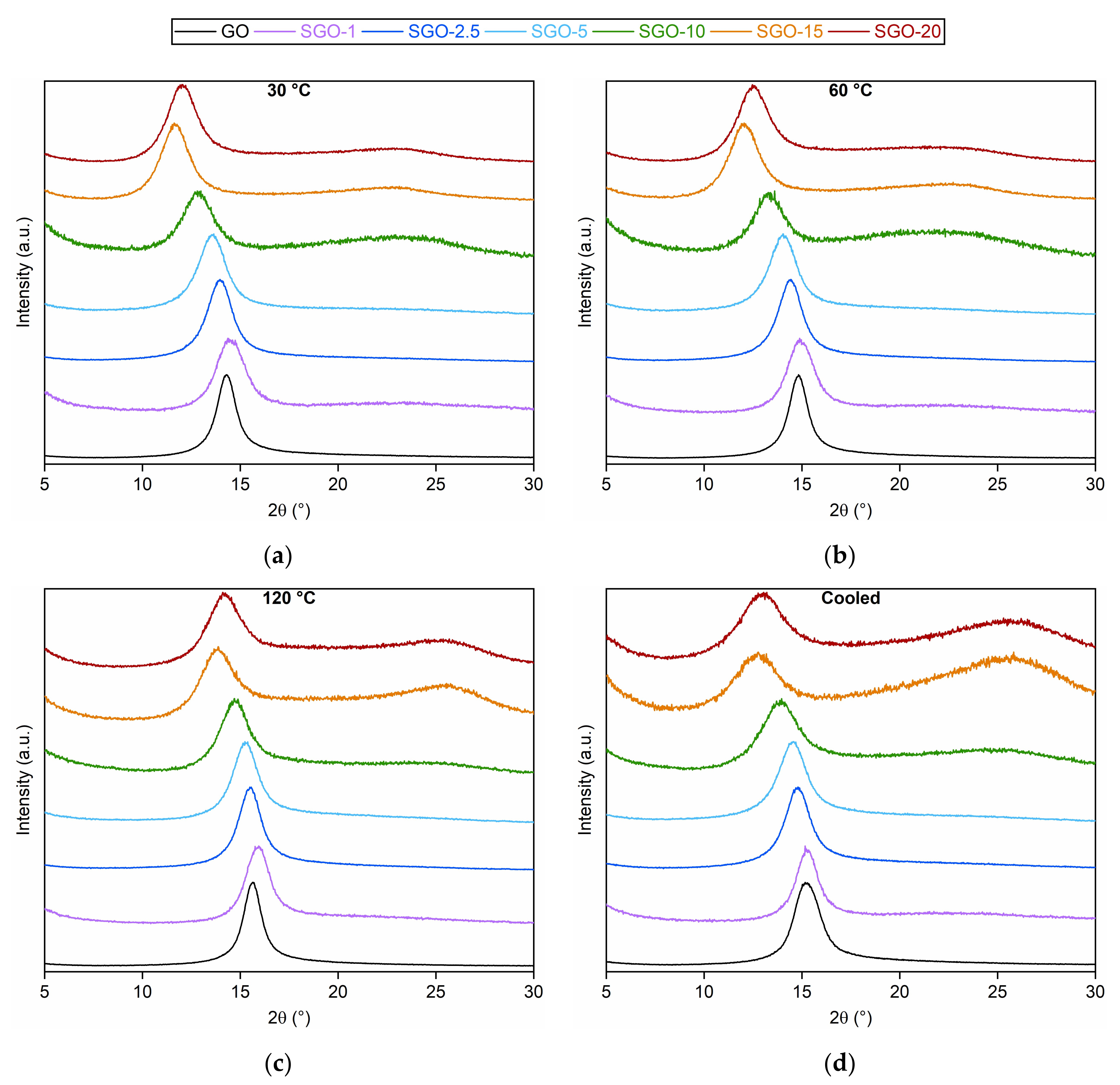
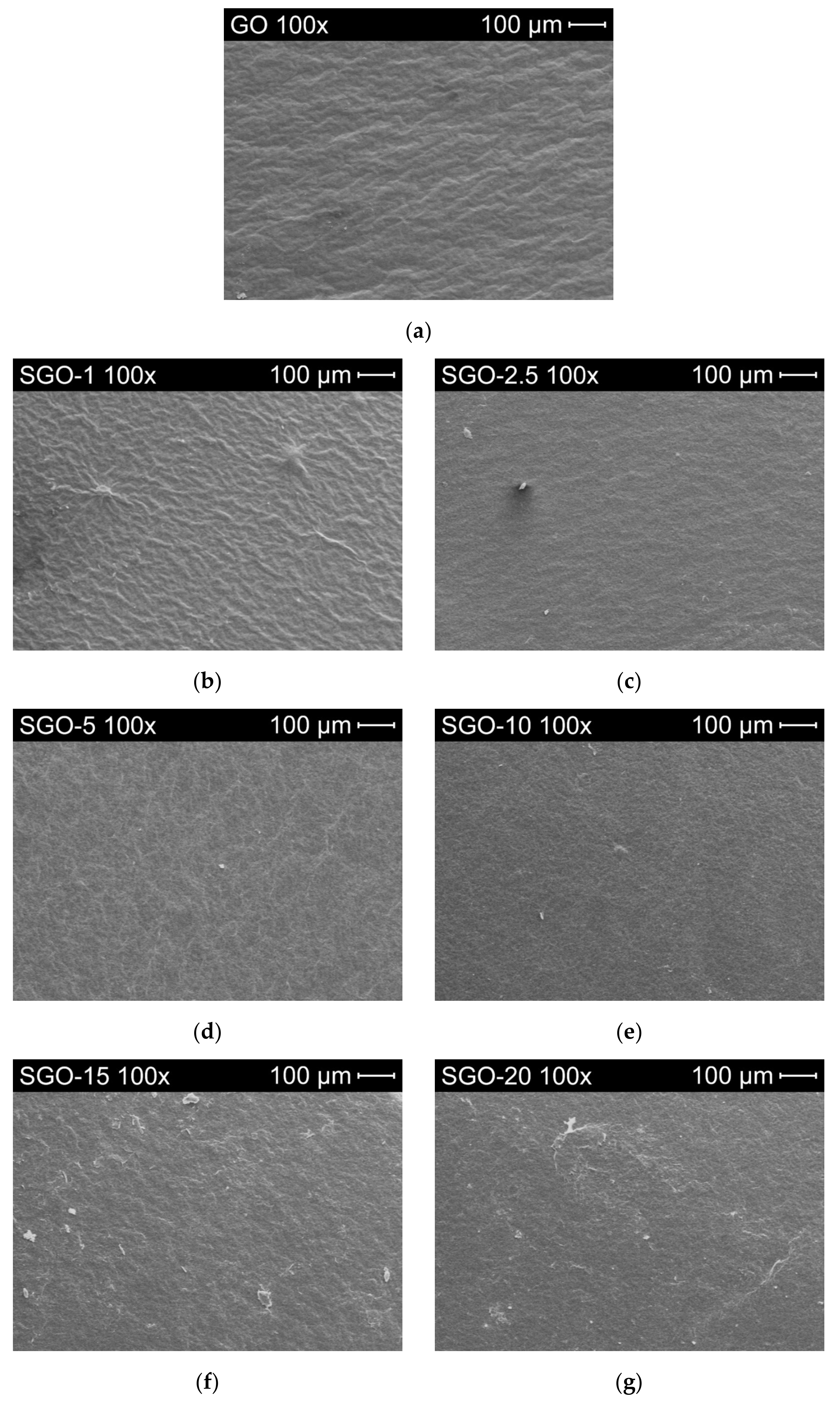
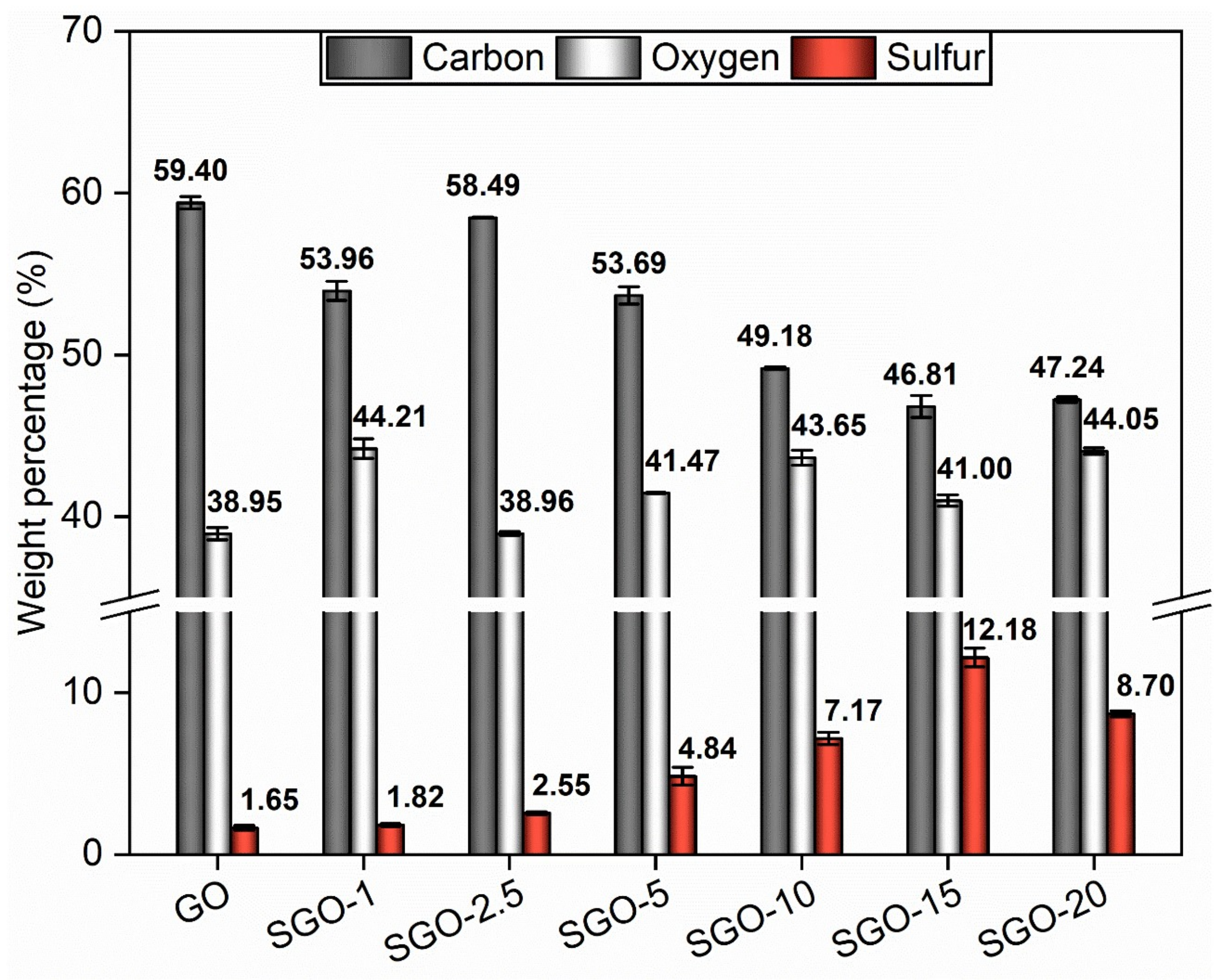
3.1. Morphological and Microstructural Characterization
3.2. Ion Exchange Capacity
3.3. Proton Conductivity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jiang, Z.; Shi, Y.; Jiang, Z.J.; Tian, X.; Luo, L.; Chen, W. High performance of a free-standing sulfonic acid functionalized holey graphene oxide paper as a proton conducting polymer electrolyte for air-breathing direct methanol fuel cells. J. Mater. Chem. A 2014, 2, 6494–6503. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The Importance of Interbands on the Interpretation of the Raman Spectrum of Graphene Oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Tian, Y.; Yu, Z.; Cao, L.; Zhang, X.L.; Sun, C.; Wang, D.-W. Graphene oxide: An emerging electromaterial for energy storage and conversion. J. Energy Chem. 2021, 55, 323–344. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Chen, Z. Graphene-related nanomaterials: Tuning properties by functionalization. Nanoscale 2013, 5, 4541–4583. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Latorrata, S.; Cristiani, C.; Basso Peressut, A.; Brambilla, L.; Bellotto, M.; Dotelli, G.; Finocchio, E.; Gallo Stampino, P.; Ramis, G. Reduced Graphene Oxide Membranes as Potential Self-Assembling Filter for Wastewater Treatment. Minerals 2020, 11, 15. [Google Scholar] [CrossRef]
- Gontrani, L.; Pulci, O.; Carbone, M.; Pizzoferrato, R.; Prosposito, P. Detection of Heavy Metals in Water Using Graphene Oxide Quantum Dots: An Experimental and Theoretical Study. Molecules 2021, 26, 5519. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.-C. Graphene-Based Nanomaterials as Heterogeneous Acid Catalysts: A Comprehensive Perspective. Molecules 2014, 19, 14582–14614. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.; Du, X.; Chen, R.; Zhou, J.; Agostini, M.; Sun, J.; Xiao, L. The Combination of 2D Layered Graphene Oxide and 3D Porous Cellulose Heterogeneous Membranes for Nanofluidic Osmotic Power Generation. Molecules 2021, 26, 5343. [Google Scholar] [CrossRef]
- Chee, W.K.; Lim, H.N.; Harrison, I.; Chong, K.F.; Zainal, Z.; Ng, C.H.; Huang, N.M. Performance of Flexible and Binderless Polypyrrole/Graphene Oxide/Zinc Oxide Supercapacitor Electrode in a Symmetrical Two-Electrode Configuration. Electrochim. Acta 2015, 157, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Cong, R.; Park, H.-H.; Jo, M.; Lee, H.; Lee, C.-S. Synthesis and Electrochemical Performance of Electrostatic Self-Assembled Nano-Silicon@N-Doped Reduced Graphene Oxide/Carbon Nanofibers Composite as Anode Material for Lithium-Ion Batteries. Molecules 2021, 26, 4831. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Hu, X.; Liu, X.; Hu, W.; Meng, R.; Li, L. Sulfonated graphene oxide with improved ionic performances. Ionics (Kiel) 2015, 21, 1919–1923. [Google Scholar] [CrossRef]
- Gagliardi, G.G.; Ibrahim, A.; Borello, D.; El-Kharouf, A. Composite Polymers Development and Application for Polymer Electrolyte Membrane Technologies—A Review. Molecules 2020, 25, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basso Peressut, A.; Latorrata, S.; Gallo Stampino, P.; Dotelli, G. Development of self-assembling sulfonated graphene oxide membranes as a potential proton conductor. Mater. Chem. Phys. 2021, 257, 123768. [Google Scholar] [CrossRef]
- Zarrin, H.; Higgins, D.; Jun, Y.; Chen, Z.; Fowler, M. Functionalized graphene oxide nanocomposite membrane for low humidity and high temperature proton exchange membrane fuel cells. J. Phys. Chem. C 2011, 115, 20774–20781. [Google Scholar] [CrossRef]
- Chien, H.-C.C.; Tsai, L.-D.D.; Huang, C.-P.P.; Kang, C.Y.; Lin, J.-N.N.; Chang, F.-C.C. Sulfonated graphene oxide/Nafion composite membranes for high-performance direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 13792–13801. [Google Scholar] [CrossRef]
- Li, C.; Huang, N.; Jiang, Z.; Tian, X.; Zhao, X.; Xu, Z.L.; Yang, H.; Jiang, Z.J. Sulfonated holey graphene oxide paper with SPEEK membranes on its both sides: A sandwiched membrane with high performance for semi-passive direct methanol fuel cells. Electrochim. Acta 2017, 250, 68–76. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Kim, A.R.; Gnana Kumar, G.; Yoo, D.J. Sulfonated graphene oxide/Nafion composite membranes for high temperature and low humidity proton exchange membrane fuel cells. RSC Adv. 2018, 8, 7494–7508. [Google Scholar] [CrossRef] [Green Version]
- Graphenea Graphene Oxide, Product Datasheet. Available online: https://cdn.shopify.com/s/files/1/0191/2296/files/Graphenea_GO_4mgmL_Datasheet_202109.pdf?v=1632927913 (accessed on 10 January 2022).
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Yadav, R.; Subhash, A.; Chemmenchery, N.; Kandasubramanian, B. Graphene and Graphene Oxide for Fuel Cell Technology. Ind. Eng. Chem. Res. 2018, 57, 9333–9350. [Google Scholar] [CrossRef]
- Latorrata, S.; Basso Peressut, A.; Gallo Stampino, P.; Cristiani, C.; Dotelli, G. Preliminary study on the development of sulfonated graphene oxide membranes as potential novel electrolytes for PEM fuel cells. ECS Trans. 2018, 86, 347–356. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. Electrical fundamentals. In Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 39–93. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. Impedance and its corresponding electrochemical processes. In Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 95–138. [Google Scholar] [CrossRef]
- Yuan, X.-Z.; Song, C.; Wang, H.; Zhang, J. EIS equivalent circuits. In Electrochemical Impedance Spectroscopy in PEM Fuel Cells; Springer: London, UK, 2010; pp. 139–192. [Google Scholar] [CrossRef]
- Alharbi, T.M.D.; Harvey, D.; Alsulami, I.K.; Dehbari, N.; Duan, X.; Lamb, R.N.; Lawrance, W.D.; Raston, C.L. Shear stress mediated scrolling of graphene oxide. Carbon 2018, 137, 419–424. [Google Scholar] [CrossRef]
- Eigler, S.; Dotzer, C.; Hirsch, A.; Enzelberger, M.; Müller, P. Formation and decomposition of CO2 intercalated graphene oxide. Chem. Mater. 2012, 24, 1276–1282. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Bangal, P.R. Preparation of graphene by the rapid and mild thermal reduction of graphene oxide induced by microwaves. Carbon 2010, 48, 1146–1152. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Zhang, H.; Wu, W.; Liu, J.; Wang, J. Constructing proton-conductive highways within an ionomer membrane by embedding sulfonated polymer brush modified graphene oxide. J. Power Sources 2015, 286, 445–457. [Google Scholar] [CrossRef]
- Rattana, T.; Chaiyakun, S.; Witit-Anun, N.; Nuntawong, N.; Chindaudom, P.; Oaew, S.; Kedkeaw, C.; Limsuwan, P. Preparation and characterization of graphene oxide nanosheets. Procedia Eng. 2012, 32, 759–764. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.C.; Yang, H.N.; Park, S.H.; Kim, W.J. Nafion/graphene oxide composite membranes for low humidifying polymer electrolyte membrane fuel cell. J. Memb. Sci. 2014, 452, 20–28. [Google Scholar] [CrossRef]
- Iwan, A.; Caballero-Briones, F.; Malinowski, M.; Filapek, M.; Tazbir, I.; Guerrero-Contreras, J.; Kamaraj, S.-K. Graphene oxide influence on selected properties of polymer fuel cells based on Nafion. Int. J. Hydrogen Energy 2017, 42, 15359–15369. [Google Scholar] [CrossRef]
- Acik, M.; Mattevi, C.; Gong, C.; Lee, G.; Cho, K.; Chhowalla, M.; Chabal, Y.J. The role of intercalated water in multilayered graphene oxide. ACS Nano 2010, 4, 5861–5868. [Google Scholar] [CrossRef]
- Reynosa-Martínez, A.C.; Gómez-Chayres, E.; Villaurrutia, R.; López-Honorato, E. Controlled reduction of graphene oxide using sulfuric acid. Materials 2021, 14, 59. [Google Scholar] [CrossRef]
- Rahmanian, A.; Naji, L.; Javanbakht, M. The influence of sulfonation level on the electrochemical characteristics of Pt/rSGO as electrocatalyst for proton exchange membrane fuels cells. Solid State Ion. 2018, 326, 27–36. [Google Scholar] [CrossRef]
- Fernandes, A.C.; Ticianelli, E.A. A performance and degradation study of Nafion 212 membrane for proton exchange membrane fuel cells. J. Power Sources 2009, 193, 547–554. [Google Scholar] [CrossRef]
- Cançado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhães-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- López-Díaz, D.; López Holgado, M.; García-Fierro, J.L.; Velázquez, M.M. Evolution of the Raman Spectrum with the Chemical Composition of Graphene Oxide. J. Phys. Chem. C 2017, 121, 20489–20497. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bayer, T.; Cunning, B.V.; Selyanchyn, R.; Daio, T.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Alkaline anion exchange membranes based on KOH-treated multilayer graphene oxide. J. Memb. Sci. 2016, 508, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, J.; Zhang, H.; Ma, C.; Liu, J.; Cao, S.; Zhang, X. Enhancement of proton conductivity of chitosan membrane enabled by sulfonated graphene oxide under both hydrated and anhydrous conditions. J. Power Sources 2014, 269, 898–911. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Stobinski, L.; Lesiak, B.; Malolepszy, A.; Mazurkiewicz, M.; Mierzwa, B.; Zemek, J.; Jiricek, P.; Bieloshapka, I. Graphene oxide and reduced graphene oxide studied by the XRD, TEM and electron spectroscopy methods. J. Electron Spectros. Relat. Phenom. 2014, 195, 145–154. [Google Scholar] [CrossRef]
- Mkhoyan, K.A.; Contryman, A.W.; Silcox, J.; Stewart, D.A.; Eda, G.; Mattevi, C.; Miller, S.; Chhowalla, M. Atomic and electronic structure of graphene-oxide. Nano Lett. 2009, 9, 1058–1063. [Google Scholar] [CrossRef] [Green Version]
- Araújo, M.P.; Soares, O.S.G.P.; Fernandes, A.J.S.; Pereira, M.F.R.; Freire, C. Tuning the surface chemistry of graphene flakes: New strategies for selective oxidation. RSC Adv. 2017, 7, 14290–14301. [Google Scholar] [CrossRef] [Green Version]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Eda, G.; Chhowalla, M. Chemically derived graphene oxide: Towards large-area thin-film electronics and optoelectronics. Adv. Mater. 2010, 22, 2392–2415. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Zhang, H.; Luo, Q.; Zhang, Y.; Bi, C. Properties and fuel cell performance of proton exchange membranes prepared from disulfonated poly(sulfide sulfone). J. Power Sources 2008, 185, 19–25. [Google Scholar] [CrossRef]
- Yuan, D.; Qin, Y.; Li, S.; Du, S.; Xu, Y.; Weng, Q.; Chen, P.; Chen, X.; An, Z. Enhanced performance of proton-conducting poly(arylene ether sulfone)s via multiple alkylsulfonated side-chains and block copolymer structures. J. Memb. Sci. 2021, 621, 118932. [Google Scholar] [CrossRef]
- Ureña, N.; Pérez-Prior, M.T.; Levenfeld, B.; García-Salaberri, P.A. On the conductivity of proton-exchange membranes based on multiblock copolymers of sulfonated polysulfone and polyphenylsulfone: An experimental and modeling study. Polymers 2021, 13, 363. [Google Scholar] [CrossRef]
- Sun, X.; Simonsen, S.C.; Norby, T.; Chatzitakis, A. Composite membranes for high temperature PEM fuel cells and electrolysers: A critical review. Membranes 2019, 9, 83. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Guiver, M.D. Fuel Cell Performance of Poly(arylene ether nitrile)s Containing Pendant Sulfonic Acid Groups under Reduced RH Conditions. In Electrochemical Society Meeting Abstracts 215; The Electrochemical Society, Inc.: Honolulu, HI, USA, 2009. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, H.; Cao, L.; He, X.; Shi, B.; Li, Y.; Xu, M.; Jiang, Z. Enhanced Proton Conductivity of Sulfonated Polysulfone Membranes under Low Humidity via the Incorporation of Multifunctional Graphene Oxide. ACS Appl. Nano Mater. 2019, 2, 4734–4743. [Google Scholar] [CrossRef]
- Zhai, L.; Li, H. Polyoxometalate-Polymer Hybrid Materials as Proton Exchange Membranes for Fuel Cell Applications. Molecules 2019, 24, 3425. [Google Scholar] [CrossRef] [Green Version]
- Vilela, S.M.F.; Salcedo-Abraira, P.; Gómez-Peña, A.; Trens, P.; Várez, A.; Salles, F.; Horcajada, P. Proton Conductive Zr-Phosphonate UPG-1—Aminoacid Insertion as Proton Carrier Stabilizer. Molecules 2020, 25, 3519. [Google Scholar] [CrossRef]
- Doyle, M.; Rajendran, G. Perfluorinated membranes. In Handbook of Fuel Cells; John Wiley & Sons, Ltd.: New York, NY, USA, 2010; pp. 1–45. [Google Scholar] [CrossRef]



| Sample | Acid-to-GO Molar Ratio | Moles of Acid (mol) | Volume of Acid (mL) |
|---|---|---|---|
| SGO-1 | 1 | 0.017 | 0.90 |
| SGO-2.5 | 2.5 | 0.042 | 2.25 |
| SGO-5 | 5 | 0.085 | 4.50 |
| SGO-10 | 10 | 0.170 | 9.00 |
| SGO-15 | 15 | 0.255 | 13.50 |
| SGO-20 | 20 | 0.340 | 18.00 |
| Sample | D Band Position (cm−1) | G Band Position (cm−1) | D/G Intensity Ratio |
|---|---|---|---|
| GO | 1340 | 1599 | 1.53 ± 0.06 |
| SGO-1 | 1344 | 1598 | 1.60 ± 0.03 |
| SGO-2.5 | 1347 | 1602 | 1.36 ± 0.08 |
| SGO-5 | 1345 | 1604 | 1.41 ± 0.02 |
| SGO-10 | 1350 | 1606 | 1.08 ± 0.01 |
| SGO-15 | 1349 | 1609 | 0.98 ± 0.03 |
| SGO-20 | 1353 | 1609 | 1.29 ± 0.13 |
| Sample | Temperature (°C) | |||
|---|---|---|---|---|
| 30 | 60 | 120 | 30 (Cooled) | |
| Interplanar Distance (nm) | ||||
| GO | 0.72 | 0.69 | 0.66 | 0.68 |
| SGO-1 | 0.71 | 0.69 | 0.65 | 0.68 |
| SGO-2.5 | 0.74 | 0.71 | 0.67 | 0.70 |
| SGO-5 | 0.75 | 0.73 | 0.68 | 0.71 |
| SGO-10 | 0.79 | 0.76 | 0.70 | 0.74 |
| SGO-15 | 0.88 | 0.85 | 0.74 | 0.81 |
| SGO-20 | 0.85 | 0.83 | 0.73 | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basso Peressut, A.; Di Virgilio, M.; Bombino, A.; Latorrata, S.; Muurinen, E.; Keiski, R.L.; Dotelli, G. Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes. Molecules 2022, 27, 1507. https://doi.org/10.3390/molecules27051507
Basso Peressut A, Di Virgilio M, Bombino A, Latorrata S, Muurinen E, Keiski RL, Dotelli G. Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes. Molecules. 2022; 27(5):1507. https://doi.org/10.3390/molecules27051507
Chicago/Turabian StyleBasso Peressut, Andrea, Matteo Di Virgilio, Antonella Bombino, Saverio Latorrata, Esa Muurinen, Riitta L. Keiski, and Giovanni Dotelli. 2022. "Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes" Molecules 27, no. 5: 1507. https://doi.org/10.3390/molecules27051507
APA StyleBasso Peressut, A., Di Virgilio, M., Bombino, A., Latorrata, S., Muurinen, E., Keiski, R. L., & Dotelli, G. (2022). Investigation of Sulfonated Graphene Oxide as the Base Material for Novel Proton Exchange Membranes. Molecules, 27(5), 1507. https://doi.org/10.3390/molecules27051507










