In Situ XANES Studies on Extracted Copper from Scrap Cu/ITO Thin Film in an Ionic Liquid Containing Iodine/Iodide
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Wie, S.M.; Hong, C.H.; Oh, S.K.; Cheng, W.S.; Yoon, Y.J.; Kwak, J.S. Fully crystallized ultrathin ITO films deposited by sputtering with in-situ electron beam irradiation for touch-sensitive screens. Ceram. Int. 2014, 40, 11163–11169. [Google Scholar] [CrossRef]
- Erdogan, N.; Erden, F.; Astarlioglu, A.T.; Ozdemir, M.; Aygun, G.; Ozyuzer, L. ITO/Au/ITO multilayer thin films on transparent polycarbonate with enhanced EMI shielding properties. Curr. Appl. Phys. 2020, 20, 489–497. [Google Scholar] [CrossRef]
- Wang, H.L.; Tang, C.M.; Shi, Q.; Wei, M.Y.; Su, Y.F.; Lin, S.S.; Dai, M.J. Influence of Ag incorporation on the structural, optical and electrical properties of ITO/Ag/ITO multilayers for inorganic all-solid-state electrochromic devices. Ceram. Int. 2021, 47, 7666–7673. [Google Scholar] [CrossRef]
- Leppanen, K.; Augustine, B.; Saarela, J.; Myllyla, R.; Fabritius, T. Breaking mechanism of indium tin oxide and its effect on organic photovoltaic cells. Sol. Energy Mater. Sol. Cells 2013, 117, 512–518. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.L.; Shi, S.W.; Song, X.P.; Cui, J.B.A.; Sun, Z.Q. Microstructure and opto-electric properties of Cu/ITO thin films. J. Alloys Compd. 2012, 536, 231–235. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.J.; Yuan, S.H.; Wuu, D.S.; Wu, W.Y.; Zhang, S. Improvement in the figure of merit of ITO-metal-ITO sandwiched films on poly substrate by high-power impulse magnetron sputtering. Coating 2021, 11, 144. [Google Scholar] [CrossRef]
- Chae, J.H.; Kim, D. Effect of the Cu underlayer on the optoelectrical properties of ITO/Cu thin films. Renew. Energy 2010, 35, 314–317. [Google Scholar] [CrossRef]
- Trading Economics. Available online: https://tradingeconomics.com/commodity/indium (accessed on 16 February 2022).
- Trading Economics. Available online: https://tradingeconomics.com/commodity/tin (accessed on 16 February 2022).
- Dang, M.T.; Wantz, G.; Hirsch, L.; Wuest, J.D. Recycling indium tin oxide (ITO) anodes for use in organic light-emitting diodes (OLEDs). Thin Solid Film. 2017, 638, 236–243. [Google Scholar] [CrossRef]
- Xu, A.; Wang, W.; Duo, T.; Wang, Y.; Xiao, Z.; Liu, R. High-performance polyethyleneimine/sodium silicate material: One-step strategy at ambient temperature and application in removing heavy metal ion. J. Mater. Sci. 2022, 57, 4221–4238. [Google Scholar] [CrossRef]
- Wang, F.; Duo, T.; Wang, Y.; Xiao, Z.; Xu, A.; Liu, R. Novel polyethyleneimine/κ-carrageenan composite from facile one-step fabrication for the removal of copper ion from aqueous solution. J. Polym. Environ. 2022, 30, 1001–1011. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Vekariya, R.L. A review of ionic liquids: Applications towards catalytic organic transformations. J. Mol. Liq. 2017, 227, 44–60. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, Z.H.; Tzeng, L.S. Photocatalytic degradation of methyl orange on TiO2/biochar assisted by ionic liquids. MRS Commun. 2021, 11, 838–842. [Google Scholar] [CrossRef]
- Krishnan, A.; Gopinath, K.P.; Vo, D.V.N.; Malolan, R.; Nagarajan, V.M.; Arun, J. Ionic liquids, deep eutectic solvents and liquid polymers as green solvents in carbon capture technologies: A review. Environ. Chem. Lett. 2020, 18, 2031–2054. [Google Scholar] [CrossRef]
- Huang, H.L.; Lin, P.C.; Wang, H.H.; Wu, C.H. Ionic liquid extraction behavior of Cr(VI) absorbed on humic acid–vermiculite. Molecules 2021, 26, 7478. [Google Scholar] [CrossRef]
- Alguacil, F.J. Liquid-liquid extraction of indium(III) from the HCl medium by ionic liquid A327H+Cl− and its use in a supported liquid membrane system. Molecules 2020, 25, 5238. [Google Scholar] [CrossRef]
- Castillo, J.; Toro, N.; Hernandez, P.; Navarro, P.; Vargas, C.; Galvez, E.; Sepulveda, R. Extraction of Cu(II), Fe(III), Zn(II), and Mn(II) from Aqueous Solutions with Ionic Liquid R4NCy. Metals 2021, 11, 1585. [Google Scholar] [CrossRef]
- Boudesocque, S.; Mohamadou, A.; Dupont, L.; Martinez, A.; Dechamps, I. Use of dicyanamide ionic liquids for extraction of metal ions. RSC Adv. 2016, 6, 107894–107904. [Google Scholar] [CrossRef]
- Janssen, C.H.C.; Macias-Ruvalcaba, N.A.; Aguilar-Martinez, M.; Kobrak, M.N. Copper extraction using protic ionic liquids: Evidence of the Hofmeister effect. Sep. Purif. Technol. 2016, 168, 275–283. [Google Scholar] [CrossRef]
- Huang, H.L.; Huang, H.H.; Wei, Y.J. Chemical structure of extracted copper from scrap Cu/ITO thin films in a room temperature ionic liquid containing iodine/iodide. Chem. Phys. Lett. 2016, 652, 195–198. [Google Scholar] [CrossRef]
- Hatakeyama, Y.; Judai, K.; Onishi, K.; Takahashi, S.; Kimura, S.; Nishikawa, K. Anion and cation effects on the size control of Au nanoparticles prepared by sputter deposition in imidazolium-based ionic liquids. Phys. Chem. Chem. Phys. 2016, 18, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.L.; Wang, H.P.; Wei, G.T.; Sun, I.W.; Huang, J.F.; Yang, Y.W. Extraction of nanosize copper pollutants with an ionic liquid. Environ. Sci. Technol. 2006, 40, 4761–4764. [Google Scholar] [CrossRef] [PubMed]
- Stern, E.A.; Newville, M.; Ravel, B.; Haskel, D.; Yacoby, Y. The UWXAFS analysis package: Philosophy and details. Phys. B Condens. Matter 1995, 208, 117–120. [Google Scholar] [CrossRef]
- Guinier, A.; Fournet, G. Small-Angle Scattering of X-rays; Wiley: New York, NY, USA, 1955. [Google Scholar]
- Aragon, S.R.; Pecora, R. Theory of dynamic light-scattering from polydisperse system. J. Chem. Phys. 1975, 64, 2395–2404. [Google Scholar] [CrossRef]
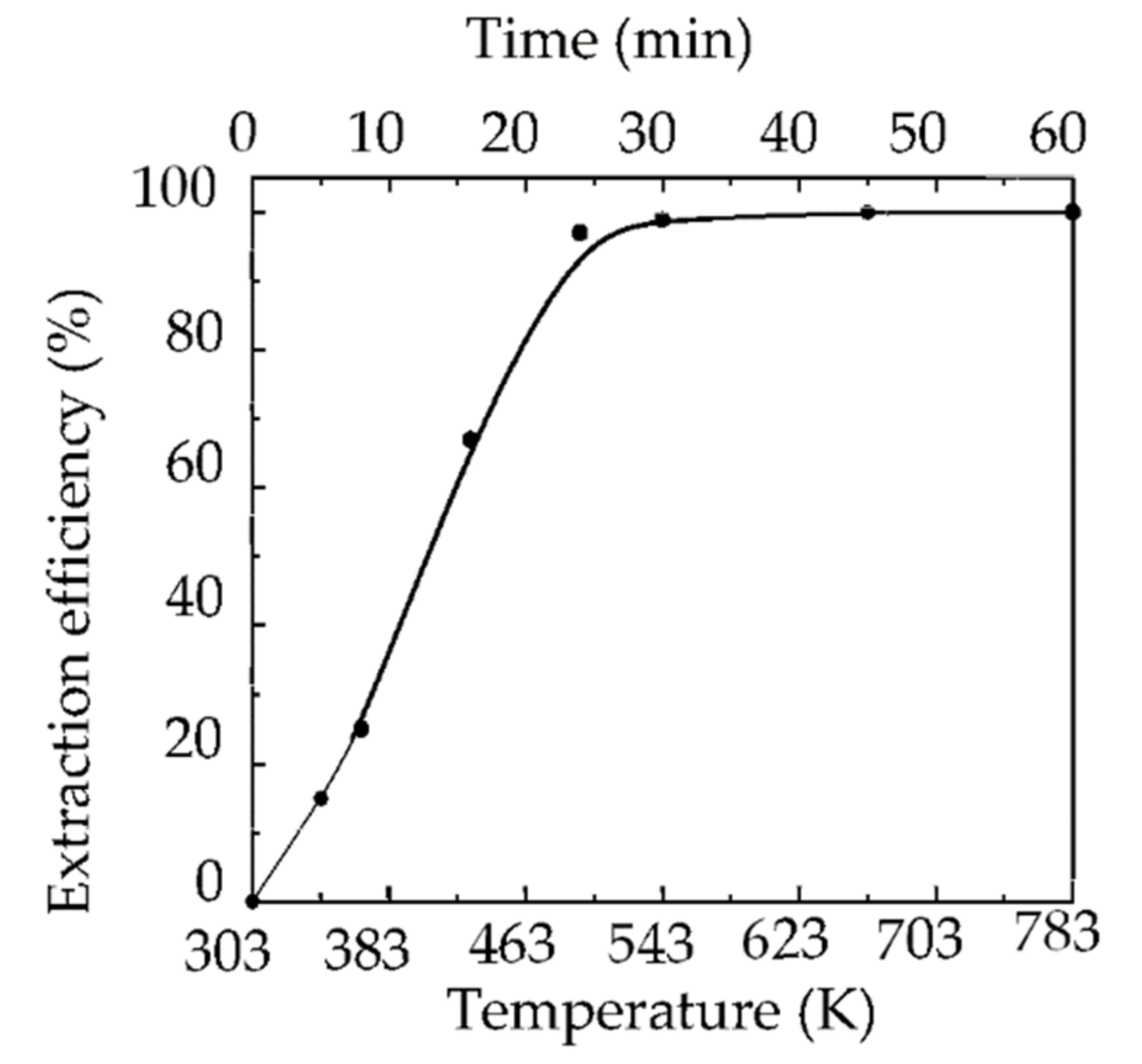



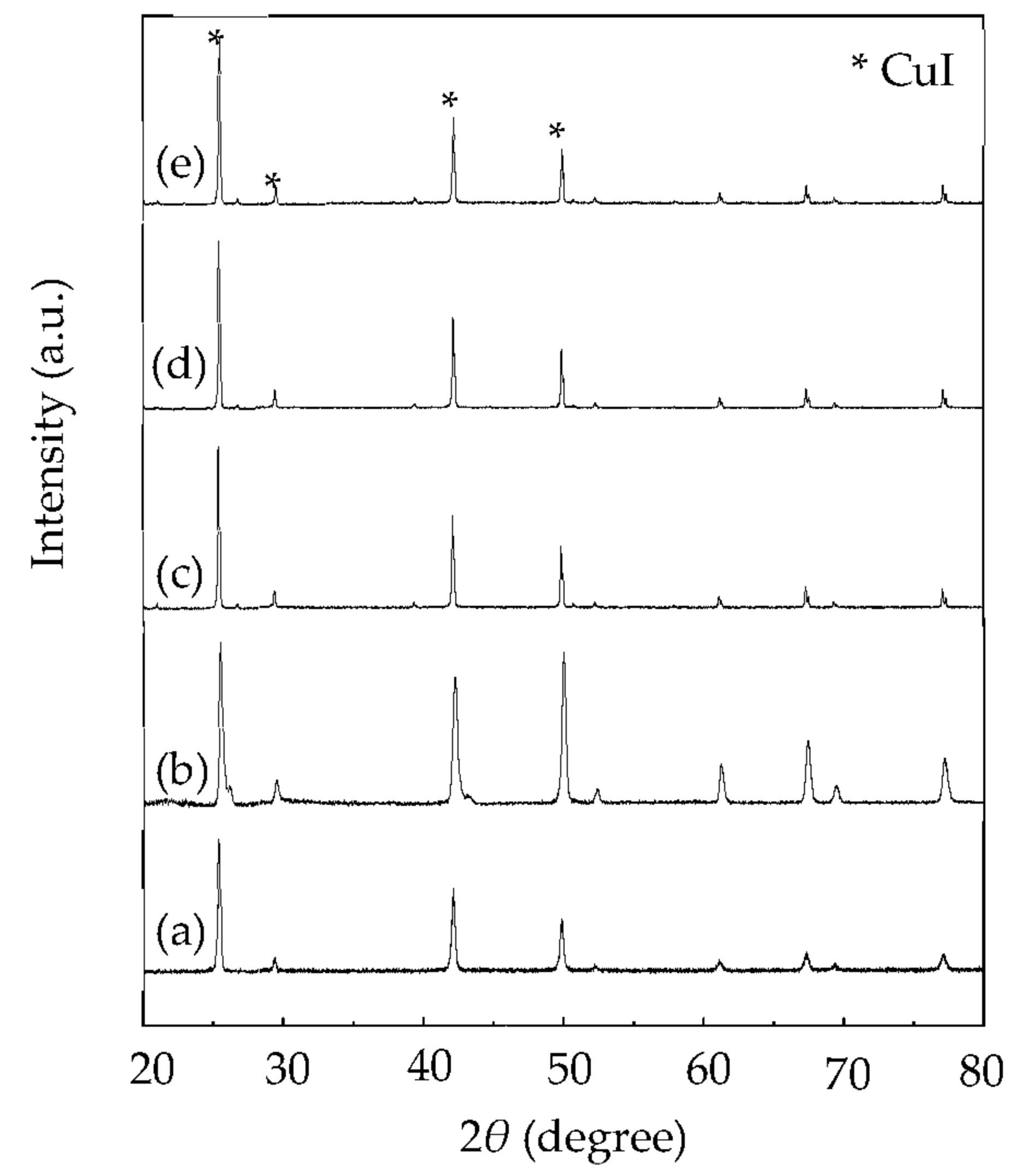
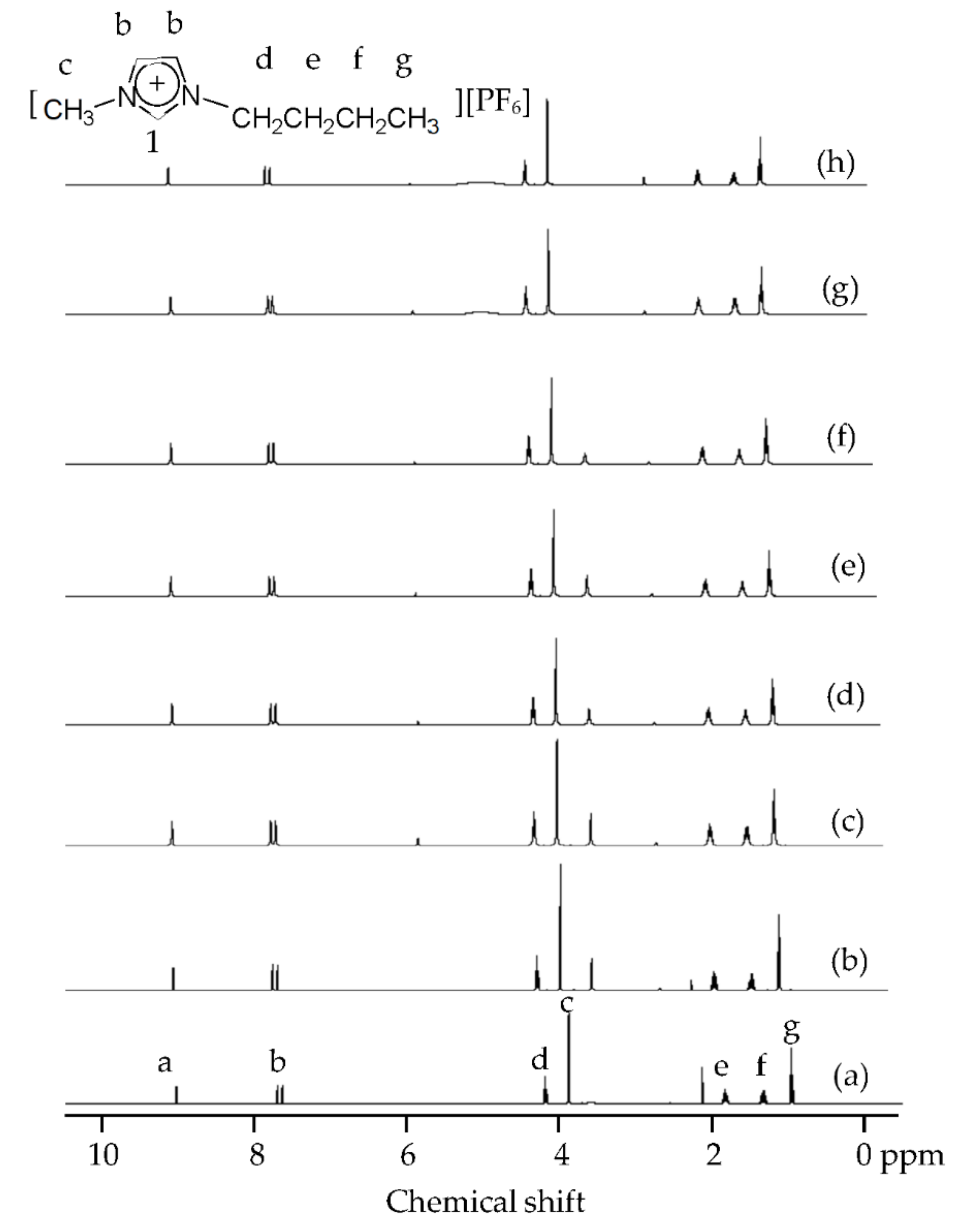
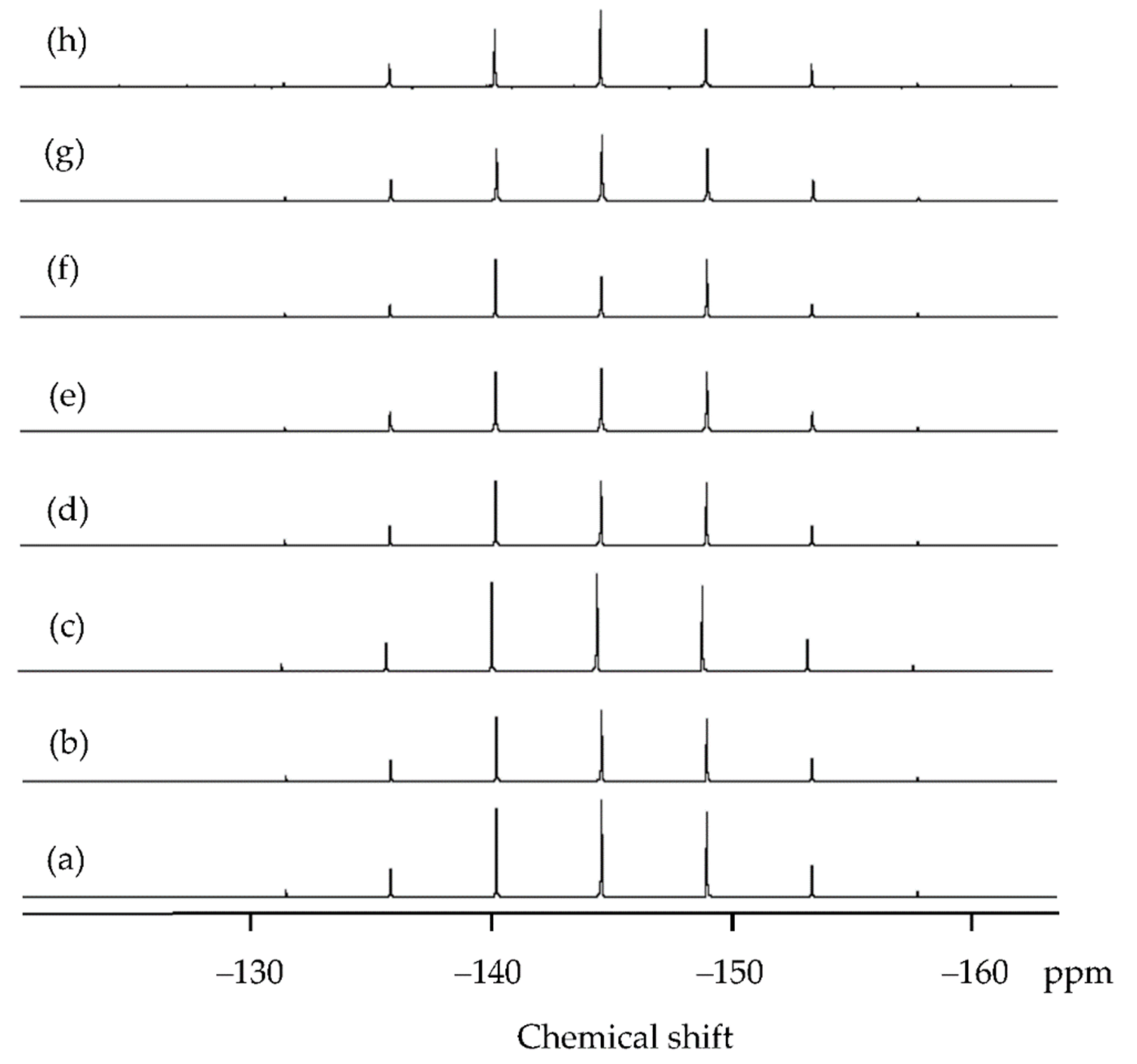

| Shell | Bond Distance (Å) | Coordination Number | σ2 (Å2) | |
|---|---|---|---|---|
| Extracted copper in the IL-I | ||||
| at 303 K | Cu-I | 2.55 | 1.9 | 0.007 |
| at 343 K | Cu-I | 2.55 | 1.7 | 0.008 |
| at 413 K | Cu-I | 2.55 | 1.8 | 0.008 |
| at 473 K | Cu-I | 2.55 | 1.9 | 0.008 |
| at 543 K | Cu-I | 2.55 | 1.8 | 0.008 |
| Shell | Bond Distance (Å) | Coordination Number | σ2 (Å2) | |
|---|---|---|---|---|
| Residual copper on the Cu/ITO thin film | ||||
| at 303 K | Cu-I | 2.57 | 3.4 | 0.01 |
| at 343 K | Cu-I | 2.57 | 3.1 | 0.01 |
| at 413 K | Cu-I | 2.56 | 3.4 | 0.01 |
| at 473 K | Cu-I | 2.57 | 3.2 | 0.01 |
| at 543 K | Cu-I | 2.57 | 3.4 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-L.; Wei, Y.J. In Situ XANES Studies on Extracted Copper from Scrap Cu/ITO Thin Film in an Ionic Liquid Containing Iodine/Iodide. Molecules 2022, 27, 1771. https://doi.org/10.3390/molecules27061771
Huang H-L, Wei YJ. In Situ XANES Studies on Extracted Copper from Scrap Cu/ITO Thin Film in an Ionic Liquid Containing Iodine/Iodide. Molecules. 2022; 27(6):1771. https://doi.org/10.3390/molecules27061771
Chicago/Turabian StyleHuang, Hsin-Liang, and Yu Jhe Wei. 2022. "In Situ XANES Studies on Extracted Copper from Scrap Cu/ITO Thin Film in an Ionic Liquid Containing Iodine/Iodide" Molecules 27, no. 6: 1771. https://doi.org/10.3390/molecules27061771
APA StyleHuang, H.-L., & Wei, Y. J. (2022). In Situ XANES Studies on Extracted Copper from Scrap Cu/ITO Thin Film in an Ionic Liquid Containing Iodine/Iodide. Molecules, 27(6), 1771. https://doi.org/10.3390/molecules27061771







