Evaluation and Prediction on the Effect of Ionic Properties of Solvent Extraction Performance of Oily Sludge Using Machine Learning
Abstract
:1. Introduction
2. Results and Discussion
2.1. Ionic Liquids and Deep Eutectic Solvents Physicochemical Properties
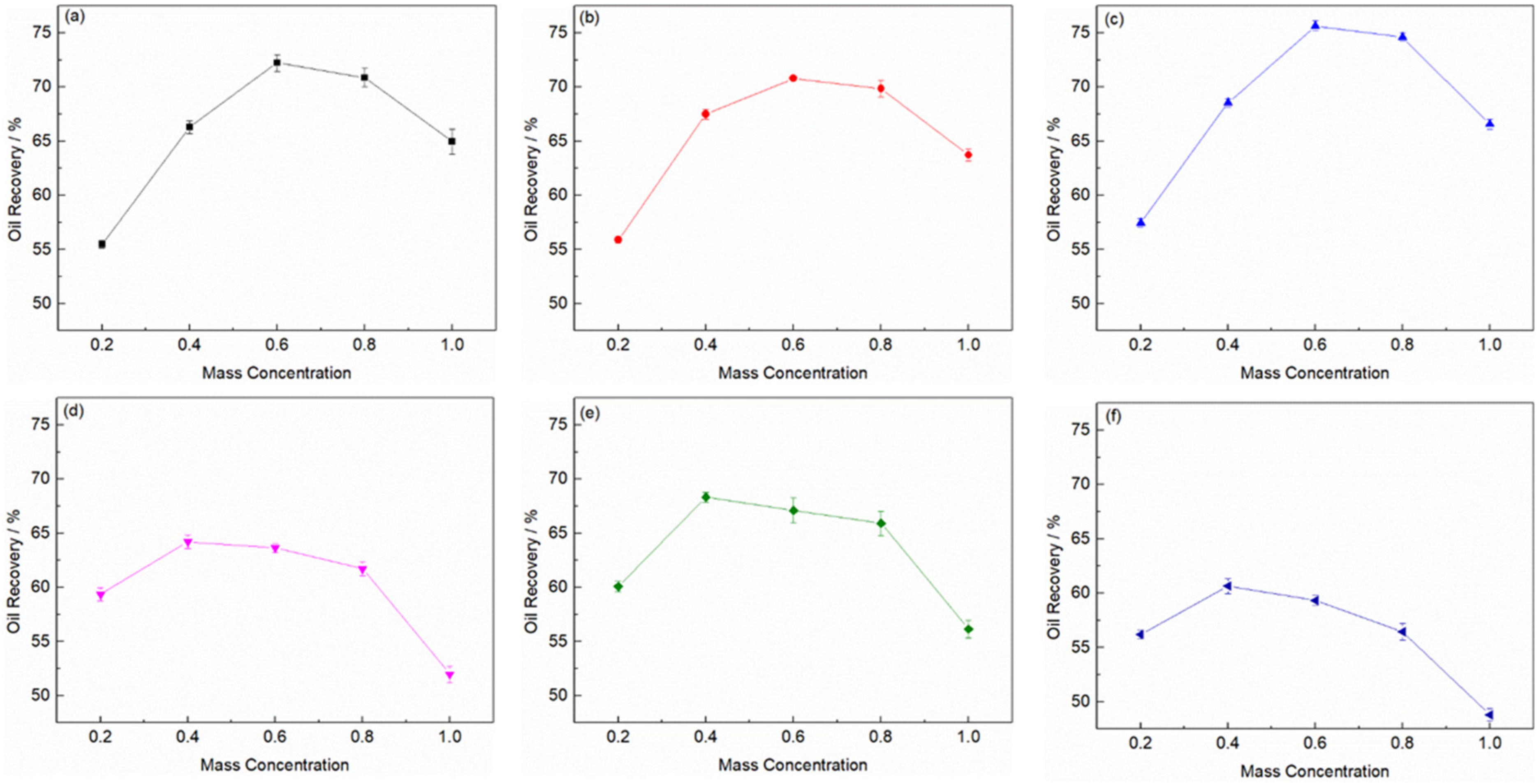
2.2. Effect of Dosage of Ionic Liquid on Oily Sludge Extraction Efficiency
2.3. Machine Learning Analysis and Prediction Results
3. Materials and Methods
3.1. Preparation of Ionic Liquids and Deep Eutectic Solvents
3.2. Determination of Oil Content in the Oily Sludge
3.3. Extraction Procedures
3.4. Physicochemical Analysis
3.5. Data Processing and Analysis
3.5.1. Ridge Regression
3.5.2. Support Vector Regression (SVR)
3.5.3. Multilayer Perceptron (MLP)
3.5.4. Model Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Mu, B.A.; Zhu, W.; Zhong, J.; Chen, L.; Lin, N.X.; Wang, C.Y.; Chen, S.P.; Li, Z. Mechanism of separation and removal of water from oily sludge using liquid dimethyl ether to dissolve hydrocarbons. Chemosphere 2021, 279, 134052–134059. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Yao, M.Z.; Liu, L.; Qin, C.R.; Qin, B.C.; Deng, N.K.; Liang, C.; Yao, S.Q. Mechanism and characteristics of oil recovery from oily sludge by sodium lignosulfonate treatment. ACS Omega 2021, 6, 25819–25827. [Google Scholar] [CrossRef]
- Hamidi, Y.; Ataei, S.A.; Sarrafi, A. Effect of dissolution of extracted hydrocarbons of oily sludge on petroleum products. Chem. Eng. Technol. 2021, 44, 1364–1370. [Google Scholar] [CrossRef]
- Tian, Y.; Mcgill, W.B.; Whitcombe, T.W. Ionic Liquid-enhanced solvent extraction for oil recovery from oily sludge. Energ Fuel 2019, 33, 3429–3438. [Google Scholar] [CrossRef]
- Painter, P.; Williams, P.; Lupinsky, A. Recovery of bitumen from Utah tar sands using ionic liquids. Energ Fuel 2010, 24, 5081–5088. [Google Scholar] [CrossRef]
- Hogshead, C.G.; Manias, E.; Williams, P.; Lupinsky, A.; Painter, P. Studies of bitumen−silica and oil−silica interactions in ionic liquids. Energ Fuel 2011, 25, 293–299. [Google Scholar] [CrossRef]
- Li, X.; Sun, W.J.; Wu, G.Z.; He, L.; Li, H.; Sui, H. Ionic liquid enhanced solvent extraction for bitumen recovery from oil sands. Energ Fuel 2011, 25, 5224–5231. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Yilmazi, Y.G. Prediction of the strength and elasticity modulus of gypsum using multiple regression, ANN, and ANFIS models. Int. J. Rock Mech. Min. 2009, 46, 803–810. [Google Scholar] [CrossRef]
- Yasen, Z.M.; El-shafie, A.; Jaafa, O.; Afan, H.A.; Sayl, K.N. Artificial intelligence based models for stream-flow forecasting: 2000–2015. J. Hydrol. 2015, 530, 829–844. [Google Scholar] [CrossRef]
- Maier, H.R.; Jain, A.; Dandy, G.C.; Sudheer, K.P. Methods used for the development of neural networks for the prediction of water resource variables in river systems: Current status and future directions. Environ. Model. Softw. 2010, 25, 891–909. [Google Scholar] [CrossRef]
- Pulati, N.; Lupinsky, A.; Miller, B.; Painter, P. Extraction of Bitumen from Oil Sands Using Deep Eutectic Ionic Liquid Analogues. Energ Fuel 2015, 29, 4927–4935. [Google Scholar] [CrossRef]
- Liu, W.; Cheng, L.; Zhang, Y. The physical properties of aqueous solution of room-temperature ionic liquids based on imidazolium: Database and evaluation. J. Mol. Liq. 2008, 140, 68–72. [Google Scholar] [CrossRef]
- Abbott, A.P.; Harris, R.C.; Ryder, K.S. Application of hole theory to define ionic liquids by their transport properties. J. Phy. Chem. B 2007, 111, 4910–4913. [Google Scholar] [CrossRef] [PubMed]
- Vila, J.; Gines, P.; Rilo, E.; Ginés, A.J.P.; Rilo, E. Great increase of the electrical conductivity of ionic liquids in aqueous solutions. Fluid Phase Equilibr. 2006, 247, 32–39. [Google Scholar] [CrossRef]
- Sirieix-plenet, J.; Gaillon, N.; Letellier, P. Behaviour of a binary solvent mixture constituted by an amphiphilic ionic liquid, 1-decyl-3-methylimidazolium bromide and water: Potentiometric and conductimetric studies. Talanta 2004, 63, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, K.; Mjalli, F.S.; Hashim, M.A. Prediction of the surface tension of deep eutectic solvents. Fluid Phase Equilibr. 2012, 319, 48–54. [Google Scholar] [CrossRef]
- Sung, J.; Jeon, Y.; Kim, D. Gibbs monolayer of ionic liquid + H2O mixtures studied by surface tension measurement and sum-frequency generation spectroscopy. Colloid Surface A 2006, 284–285, 84–88. [Google Scholar] [CrossRef]
- Guo, K.; Bi, Y.; Sun, L. Experiment and correlation of vapor-liquid equilibrium of aqueous solutions of hydrophilic ionic liquids: 1-Ethyl-3-methylimidazolium acetate and 1-hexyl-3-methylimidazolium chloride. J. Chem. Eng. Data 2012, 57, 2243–2251. [Google Scholar] [CrossRef]
- Cammarata, L.; Kazarian, S.G.; Salter, P.A. Molecular states of water in room temperature ionic liquids. Phy. Chem. Chem. Phys. 2001, 3, 5192–5200. [Google Scholar] [CrossRef] [Green Version]
- Sui, H.; Ma, G.; Yuan, Y. Bitumen-silica interactions in the presence of hydrophilic ionic liquids. Fuel 2018, 233, 860–866. [Google Scholar] [CrossRef]
- Hayyan, M.; Hashim, M.A.; Hayyan, A.; Al-Saadi, M.A.; AlNashef, I.M.; Mirghani, M.E.S.; Saheed, O.K. Are deep eutectic solvents benign or toxic? Chemosphere 2013, 90, 2193–2195. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, X.; Liu, L. Effect of ultrasonic reactor and auxiliary stirring on oil removal from oily sludge. Environ. Technol. 2017, 38, 3109–3114. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Yilamaz, I.; Kaynar, O. Multiple regression, ANN (RBF, MLP) and ANFIS models for prediction of swell potential of clayey soils. Expert Syst. Appl. 2011, 38, 5958–5966. [Google Scholar] [CrossRef]
- De Vlaming, R.; Groenen, P.J.F. The current and future use of ridge regression for prediction in quantitative genetics. BioMed Res. Int. 2015, 2015, 143712–143729. [Google Scholar] [CrossRef] [Green Version]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Smola, A.J.; Scholkopf, B. A tutorial on support vector regression. Stat. Comput. 2004, 14, 199–222. [Google Scholar] [CrossRef] [Green Version]
- Granata, F.; Papirio, S.; Esranata, F.; Papirio, S.; Esposito, G. Machine learning algorithms for the forecasting of wastewater quality indicators. Water 2017, 9, 105. [Google Scholar] [CrossRef] [Green Version]
- Zaghloul, M.S.; Hamza, R.A.; Iorhemen, O.T. Comparison of adaptive neuro-fuzzy inference systems (ANFIS) and support vector regression (SVR) for data-driven modelling of aerobic granular sludge reactors. J. Environ. Chem. Eng. 2020, 8, 103742. [Google Scholar] [CrossRef]
- Wu, C.H.; Ho, J.M.; Lee, D.T. Travel-time prediction with support vector regression. IEEE Transact. Intell. Transp. 2004, 5, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Hand, D.; Chan, L.; Zhu, U. Food forecasting using support vector machines. J. Hydroinform. 2007, 9, 267–276. [Google Scholar]
- Seshan, H.; Goyal, M.K.; Falk, M.W. Support vector regression model of wastewater bioreactor performance using microbial community diversity indices: Effect of stress and bioaugmentation. Water Res. 2014, 53, 282–296. [Google Scholar] [CrossRef]
- Schmidhuber, J. Deep Learning in neural networks: An overview. Neural Netw. 2015, 61, 85–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altun, H.; Gelen, G. Enhancing performance of MLP/RBF neural classifiers via an multivariate data distribution scheme. In Proceedings of the International Conference on Computational Intelligence (ICCI2004), Nicosia, North Cyprus, 24 May 2014; pp. 1–6. [Google Scholar]
- Gokceoglu, C. A fuzzy triangular chart to predict the uniaxial compressive strength of the Ankara agglomerates from their petrographic composition. Eng. Geol. 2002, 66, 39–51. [Google Scholar] [CrossRef]
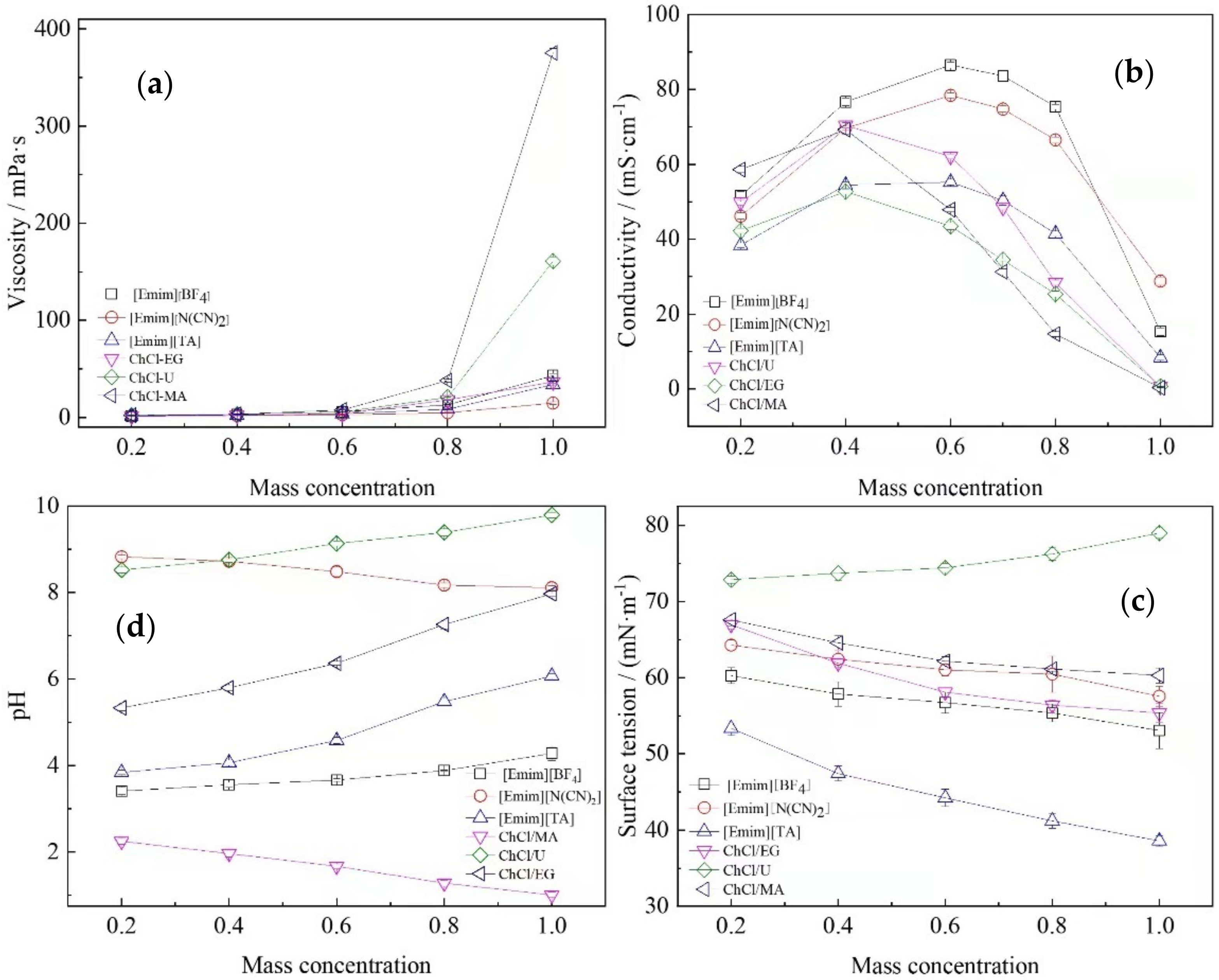


| Variable | Extraction Method | Unit | Data Range |
|---|---|---|---|
| Viscosity () | determined in Section 4 with the mass concentration of the extraction solvent 0.2 to 1.0 | [mPa·s] | 1–400 |
| Conductivity () | [mS·cm−1] | 0–100 | |
| pH | - | 1–10 | |
| Surface Tension () | [mN·m−1] | 30–80 |
| VAF | RMSE | MAPE | R2 | |
|---|---|---|---|---|
| RR | 95.72 | 0.95 | 1.37 | 0.96 |
| MLP | 95.14 | 1.01 | 1.44 | 0.95 |
| SVR | 95.50 | 1.03 | 1.38 | 0.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, C.; Fu, S.; Zhu, L.; Dang, W.; Zhang, T. Evaluation and Prediction on the Effect of Ionic Properties of Solvent Extraction Performance of Oily Sludge Using Machine Learning. Molecules 2021, 26, 7551. https://doi.org/10.3390/molecules26247551
Hu C, Fu S, Zhu L, Dang W, Zhang T. Evaluation and Prediction on the Effect of Ionic Properties of Solvent Extraction Performance of Oily Sludge Using Machine Learning. Molecules. 2021; 26(24):7551. https://doi.org/10.3390/molecules26247551
Chicago/Turabian StyleHu, Changchao, Shuhan Fu, Lingfu Zhu, Wei Dang, and Tingting Zhang. 2021. "Evaluation and Prediction on the Effect of Ionic Properties of Solvent Extraction Performance of Oily Sludge Using Machine Learning" Molecules 26, no. 24: 7551. https://doi.org/10.3390/molecules26247551
APA StyleHu, C., Fu, S., Zhu, L., Dang, W., & Zhang, T. (2021). Evaluation and Prediction on the Effect of Ionic Properties of Solvent Extraction Performance of Oily Sludge Using Machine Learning. Molecules, 26(24), 7551. https://doi.org/10.3390/molecules26247551





