Influence of Gamma Irradiation on Different Phytochemical Constituents of Dried Rose Hip (Rosa canina L.) Fruits
Abstract
:1. Introduction
2. Results
2.1. Influence of Gamma Irradiation on Sugar and Organic Acid Composition
2.2. Influence of Gamma Irradiation on the Lipid Composition of Seed Oil
2.3. Influence of Gamma Irradiation on the Phenolic Constituents and Antioxidant Activity
2.4. Influence of Gamma Irradiation on Polysaccharide Constituents
2.4.1. Influence of Gamma Irradiation on Monosaccharide Composition
2.4.2. Influence of Gamma Irradiation on Molecular Weight Distribution
2.4.3. Fourier Transformed Infrared (FT-IR) Spectroscopy
2.5. Scanning Electron Microscopy (SEM) of the Fruit Material
3. Discussion
3.1. Influence of Gamma Irradiation on Carbohydrate Constituents
3.2. Influence of Gamma Irradiation on Organic Acids
3.3. Influence of Gamma Irradiation on Lipid Constituents
3.4. Influence of Gamma Irradiation on Phenolic Constituents and In Vitro Antioxidant Activity
4. Materials and Methods
4.1. Fruit Material
4.2. Gamma Irradiation
4.3. Chemical Characterization of Non-irradiated and Irradiated Fruits
4.3.1. Preparation of Alcohol-Insoluble Solids
4.3.2. Crude Protein Content
4.3.3. Total Uronic Acid and Cellulose Content
4.3.4. Total Lipid Content
4.3.5. Total Carotenoids, β-Carotene, and Tocopherol Content
4.3.6. Conjugated Dienes and Trienes Contents
4.3.7. Acid Value, Peroxide Value, and Induction Period
4.3.8. Fatty Acid Composition
4.3.9. HPLC Determination of Free Sugars
4.3.10. HPLC Determination of Organic Acids
4.3.11. HPLC Determination of Phenolic Components
4.3.12. Total Polyphenolic Content and In Vitro Antioxidant Activity
4.3.13. Scanning Electron Microscopy
4.4. Extraction of the Polysaccharide Fraction
4.5. Endo-Polygalacturonase (endo-PG) Hydrolysis of the Polysaccharide Fraction
4.6. Physico-Chemical Characterization of Polysaccharides
4.6.1. General Analytical Methods
4.6.2. Monosaccharide Composition Analysis
4.6.3. Molecular Weight Distribution Analysis
4.7. FT-IR Spectroscopy
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Molins, R.A. Introduction. In Food Irradiation: Principles and Applications; Molins, R.A., Ed.; Wiley Interscience: New York, NY, USA, 2001; pp. 1–21. [Google Scholar]
- Golge, E.; Ova, G. The effects of food irradiation on quality of pine nut kernels. Radiat. Phys. Chem. 2008, 77, 365–369. [Google Scholar] [CrossRef]
- Diehl, J.F. Safety of Irradiated Foods, 2nd ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; ISBN 0-8247-9344-7. [Google Scholar]
- Farkas, J. Irradiation as a method for decontaminating food. A review. Int. J. Food Microbiol. 1998, 44, 189–204. [Google Scholar] [CrossRef]
- Farkas, J.; Mohácsi-Farkas, C. History and future of food irradiation. Trends. Food Sci. Technol. 2011, 22, 121–126. [Google Scholar] [CrossRef]
- Gecgel, U.; Gumus, T.; Tasan, M.; Daglioglu, O.; Arici, M. Determination of fatty acid composition of γ-irradiated hazelnuts, walnuts, almonds, and pistachios. Rad. Phys. Chem. 2011, 80, 578–581. [Google Scholar] [CrossRef]
- Shahbaz, H.; Ahn, J.; Akrama, K.; Kim, H.; Park, E.; Kwon, J. Chemical and sensory quality of fresh pomegranate fruits exposed to gamma radiation as quarantine treatment. Food Chem. 2014, 145, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Ognyanov, M.; Georgiev, Y.; Denev, P.; Yanakieva, I.; Kratchanova, M. Bioactive compounds and health effects of rose hip fruits. Nauka Diet. 2014, 3–4, 14–27. (In Bulgarian) [Google Scholar]
- Patel, S. Rose hip as an underutilized functional food: Evidence based review. Trends. Food Sci. Technol. 2017, 63, 29–38. [Google Scholar] [CrossRef]
- Denev, P.; Lojek, A.; Ciz, M.; Kratchanova, M. Antioxidant activity and polyphenol content of Bulgarian fruits. Bulg. J. Agric. Sci. 2013, 19, 22–27. [Google Scholar]
- Ognyanov, M.; Remoroza, C.; Schols, H.A.; Georgiev, Y.; Kratchanova, M.; Kratchanov, C. Isolation and structure elucidation of pectic polysaccharide from rose hip fruits (Rosa canina L.). Carbohydr. Polym. 2016, 151, 803–811. [Google Scholar] [CrossRef]
- Petkova, N.; Ognyanov, M.; Kirchev, M.; Stancheva, S. Bioactive compounds in water extracts prepared from rosehip-containing herbal blends. J. Food Process. Preserv. 2021, 45, e14645. [Google Scholar] [CrossRef]
- Burbidge, C.A.; Ford, C.M.; Melino, V.J.; Wong, D.C.J.; Jia, Y.; Jenkins, C.L.D.; Soole, K.L.; Castellarin, S.D.; Darriet, P.; Rienth, M.; et al. Biosynthesis and cellular functions of tartaric acid in grapevines. Front. Plant Sci. 2021, 12, 643024. [Google Scholar] [CrossRef] [PubMed]
- Katušin-Ražem, B.; Matić, S.; Ražem, D.; Mihoković, V. Radiation decontamination of tea herbs. J. Food Sci. 1988, 53, 1120–1126. [Google Scholar] [CrossRef]
- Harrison, K.; Were, L.M. Effect of gamma irradiation on total phenolic content yield and antioxidant capacity of almond skin extracts. Food Chem. 2007, 102, 932–937. [Google Scholar] [CrossRef]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Benen, J.A.E.; Kester, H.C.M.; Visser, J. Kinetic characterization of Aspergillus niger N400 endopolygalacturonases I., II and C. Eur. J. Biochem. 1999, 259, 577–585. [Google Scholar] [CrossRef] [Green Version]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Azelmat, K.; ElGarrouj, D.; Mouhib, M.; Sayah, F. Irradiation of ‘Boufeggous’ dates: Effects on chemical composition during storage. Postharvest Biol. Technol. 2006, 39, 217–222. [Google Scholar] [CrossRef]
- Jarvis, M.C. Plant cell walls: Supramolecular assemblies. Food Hydrocoll. 2011, 25, 257–262. [Google Scholar] [CrossRef]
- Takács, E.; Wojnárovits, L.; Borsa, J.; Földváry, C.; Hargittai, P.; Zöld, O. Effect of γ-irradiation on cotton-cellulose. Radiat. Phys. Chem. 1999, 55, 663–666. [Google Scholar] [CrossRef]
- Horio, M.; Imamura, R.; Mizukami, H. Effect of gamma irradiation upon cellulose. Bull. Inst. Chem. Res. Kyoto Univ. 1963, 41, 17–38. [Google Scholar]
- Wang, K.-Q.; Xiong, X.-Y.; Chen, J.-P.; Chen, L.; Liu, Y. Effect of gamma irradiation on microcrystalline structure of Phragmites cellulose. Wood Fiber Sci. 2011, 43, 225–231. [Google Scholar]
- Skinner, E.R.; Kertesz, Z.I. The effect of gamma radiation on the structure of pectin. An electrophoretic study. J. Polym. Sci. 1960, 47, 99–109. [Google Scholar] [CrossRef]
- Kertesz, Z.I.; Morgan, B.H.; Tuttle, L.W.; Lavin, M. Effect of ionizing radiations on pectin. Radiat. Res. 1956, 5, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, A.-M. The effects of γ irradiation on the structure of apple pectin. Food Hydrocoll. 1987, 1, 271–276. [Google Scholar] [CrossRef]
- Zegota, H. Some quantitative aspects of hydroxyl radical induced reactions in γ-irradiated aqueous solutions of pectins. Food Hydrocoll. 2002, 16, 353–361. [Google Scholar] [CrossRef]
- Wen, H.-W.; Chung, H.-P.; Chou, F.-I.; Lin, I.-H.; Hsieh, P.-C. Effect of gamma irradiation on microbial decontamination, and chemical and sensory characteristic of lycium fruit. Radiat. Phys. Chem. 2006, 75, 596–603. [Google Scholar] [CrossRef]
- Calucci, L.; Pinzino, C.; Zandomeneghi, M.; Capocchi, A.; Ghiringhelli, S.; Saviozzi, F.; Tozzi, S.; Galleschi, L. Effects of γ-irradiation on the free radical and antioxidant contents in nine aromatic herbs and spices. J. Agric. Food Chem. 2003, 51, 927–934. [Google Scholar] [CrossRef]
- Golding, J.B.; Blades, B.L.; Satyan, S.; Jessup, A.J.; Spohr, L.J.; Harris, A.M.; Banos, C.; Davies, J.B. Low dose gamma irradiation does not affect the quality, proximate or nutritional profile of ‘Brigitta’ blueberry and ‘Maravilla’ raspberry fruit. Postharvest Biol. Technol. 2014, 96, 49–52. [Google Scholar] [CrossRef]
- Lisińska, G.; Aniołowski, K. Organic acids in potato tubers: Part 2—The effect of gamma irradiation on citric and malic acid contents of potato tubers. Food Chem. 1991, 40, 207–212. [Google Scholar] [CrossRef]
- Alothman, M.R.; Bhat, K.A. Effects of radiation processing on phytochemicals and antioxidants in plant produce. Trends. Food Sci. Technol. 2009, 20, 201–212. [Google Scholar] [CrossRef]
- McDonald, H.; Arpaia, M.L.; Caporaso, F.; Obenland, D.; Were, L.; Rakovski, C.; Prakash, A. Effect of gamma irradiation treatment at phytosanitary dose levels on the quality of ‘Lane Late’ navel oranges. Postharvest Biol. Technol. 2013, 86, 91–99. [Google Scholar] [CrossRef]
- Mali, A.; Khedkar, K.; Lele, S. Effect of gamma irradiation on total phenolic content and in vitro antioxidant activity of pomegranate (Punica granatum L.) peels. Food Nutr. Sci. 2012, 2, 428–433. [Google Scholar]
- Hussain, P.R.; Chatterjee, S.; Variyar, P.S.; Sharma, A.; Dar, M.A.; Wani, A.M. Bioactive compounds and antioxidant activity of gamma irradiated sun dried apricots (Prunus armeniaca L.). J. Food Compos. Anal. 2013, 30, 59–66. [Google Scholar] [CrossRef]
- GB 5009.5—400; Determination of Protein in Foods. National Food Safety Standard of the People’s Republic of China (NFSS). China National Center for Food Safety Risk Assessment: Beijing, China, 2016.
- Ahmed, A.E.R.; Labavitch, J.M. A simplified method for accurate determination of cell wall uronide content. J. Food Biochem. 1978, 1, 361–365. [Google Scholar] [CrossRef]
- Kürschner, K.; Hanak, A. Zur Bestimmung der sog. Rohfaser. Ein neues Verfahren der Bestimmung der Rohcellulose in Kakao. Z. Unters. Lebensm. 1930, 59, 484–494. [Google Scholar] [CrossRef]
- ISO 659:2009; Oilseeds—Determination of Oil Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2009.
- British Standard Methods of Analysis—B.S.684, Section 2.20:1997; Determination of Carotene in Vegetable Oils. BSI Group: London, UK, 1997.
- ISO 9936:2012; Animal and Vegetable Fats and Oils—Determination of Tocopherol and Tocotrienol Content by High-Performance Liquid Chromatography. International Organization for Standardization: Geneva, Switzerland, 2012.
- AOCS Official method Ch 5-91. Specific Extinction of Oils and Fats, Ultraviolet Absorption. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Urbana, IL, USA, 1991.
- AOCS Official method Cd 3d-63. Acid Value of Fats and Oils. In Official Methods and Recommended Practices of the AOCS; AOCS Press: Champaign, IL, USA, 1999.
- Yanishlieva, N.; Popov, A.; Marinova, E. Eine Modifizierte Jodometrische Methode zur Bestimmung der Peroxidzahl in kleinen Lipidproben. C. R. Acad. Bulg. Sci. 1978, 31, 869. [Google Scholar]
- Le Tutour, B.; Guedon, D. Antioxidative activities of Olea europaea leaves and related phenolic compounds. Phytochemistry 1992, 31, 1173–1178. [Google Scholar] [CrossRef]
- Christie, W.W. Lipid Analysis: Isolation, Separation, Identification, and Structural Analysis of Lipids, 3rd ed.; Oily Press: Bridgwater, UK, 2003; pp. 205–224. [Google Scholar]
- Singleton, V.; Rossi, J. Colorimetry of total phenolic with phosphomolibdiphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Denev, P.; Ciz, M.; Ambrozova, G.; Lojek, A.; Yanakieva, I.; Kratchanova, M. Solid-phase extraction of berries’ anthocyanins and evaluation of their antioxidative properties. Food Chem. 2010, 123, 1055–1061. [Google Scholar] [CrossRef]
- Klavons, J.A.; Bennett, R.D. Determination of methanol using alcohol oxidase and its application to methyl ester content of pectins. J. Agric. Food Chem. 1986, 34, 597–599. [Google Scholar] [CrossRef]
- Anthon, G.E.; Barrett, D.M. Combined enzymatic and colorimetric method for determining the uronic acid and methylester content of pectin: Application to tomato products. Food Chem. 2008, 110, 239–247. [Google Scholar] [CrossRef] [PubMed]
- McComb, E.A.; McCready, R.M. Determination of acetyl in pectin and in acetylated carbohydrate polymers. Anal. Chem. 1957, 29, 819–821. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Y.; Wang, Q.; Wang, H.; Mei, Q. Analysis of the monosaccharide components in Angelica polysaccharides by high performance liquid chromatography. Anal. Sci. 2005, 21, 1177–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]



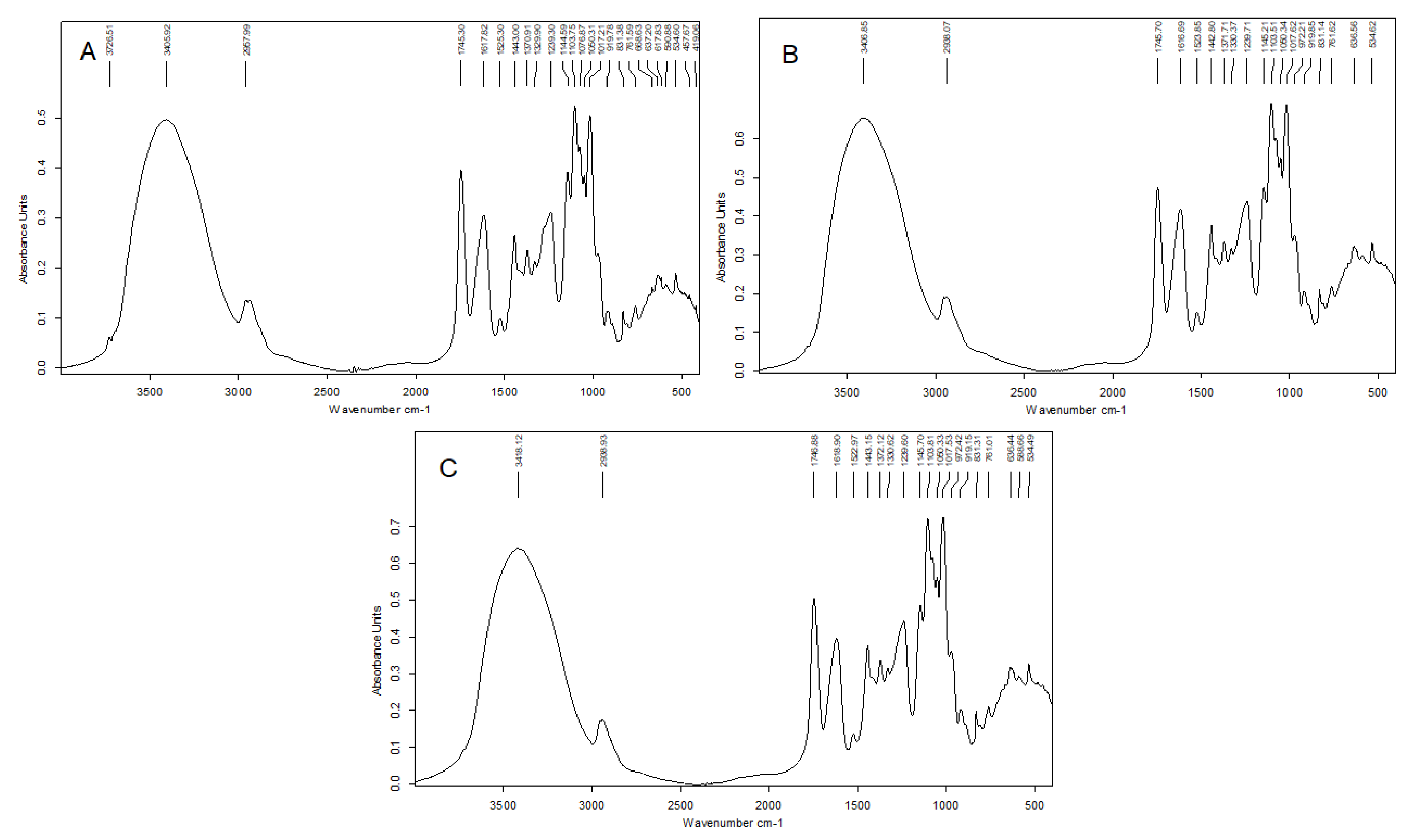
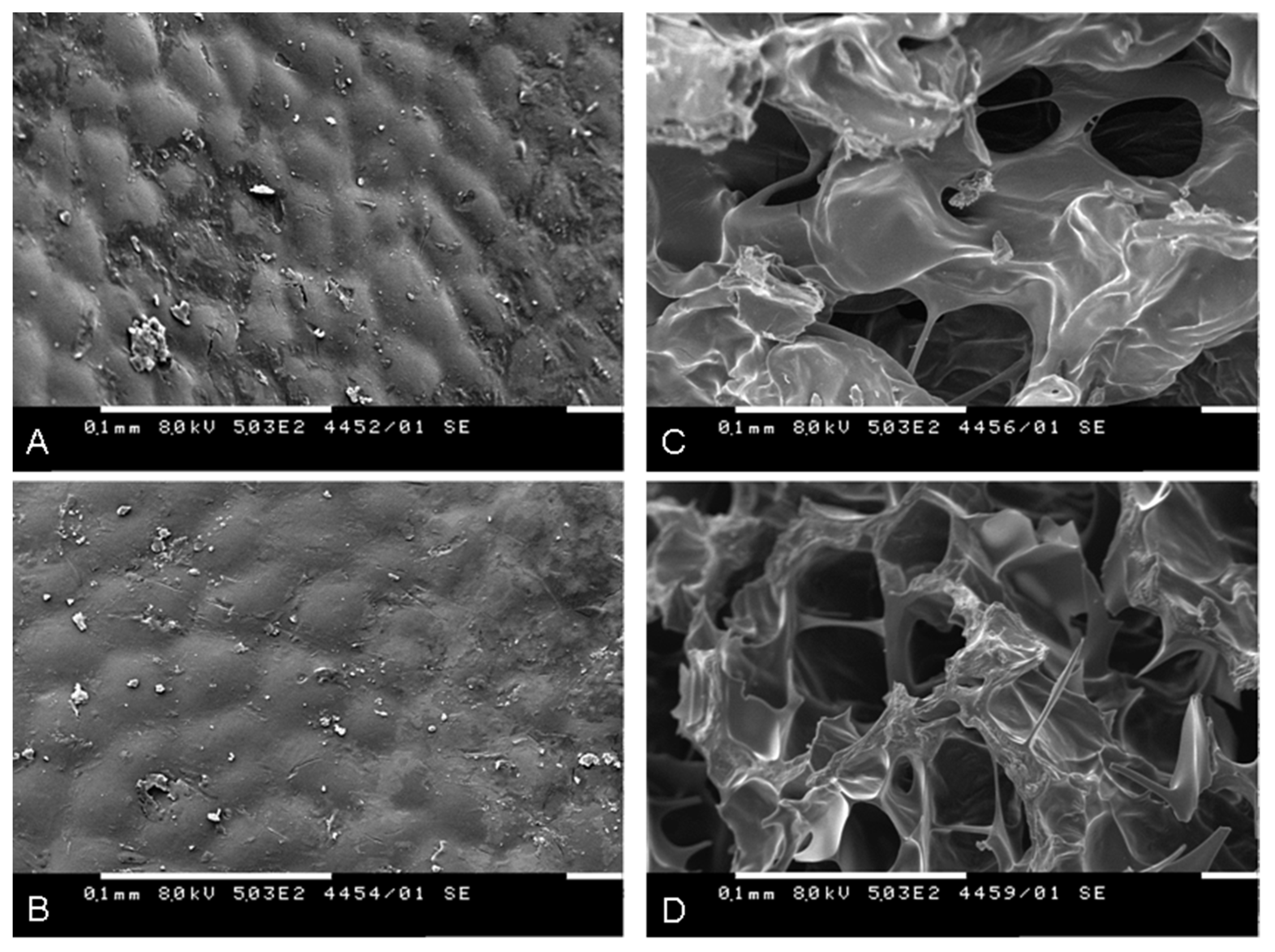
| Organic Acid | Non-Irradiated | 10 kGy | 25 kGy |
|---|---|---|---|
| Quinic acid | 1344.7 ± 187.5 ab * | 1568.0 ± 48.4 a | 1376.8 ± 81.0 b |
| Malic acid | 111.8 ± 6.4 b | 133.0 ± 3.1 a | 118.7 ± 9.2 b |
| Ascorbic acid | 623.5 ± 13.6 a | 646.0 ± 12.2 a | 654.1 ± 19.0 a |
| Citric acid | 2768.7 ± 196.6 b | 3299.9 ± 55.8 a | 3536.7 ± 303.5 a |
| α-Ketoglutaric acid | <50 b | 75.8 ± 5.1 a | 71.5 ± 5.2 a |
| Succinic acid | 70.8 ± 1.6 b | 79.9 ± 10.4 b | 107.6 ± 7.3 a |
| Tartaric acid | 49.2 ± 4.9 a | 50.7 ± 1.2 a | 34.2 ± 1.8 b |
| Non-Irradiated | 10 kGy | 25 kGy | |
|---|---|---|---|
| Yield, w/w% | 6.3 ± 0.1 a * | 6.5 ± 0.6 a | 6.4 ± 0.8 a |
| Saturated fatty acids (rel. %) | |||
| Palmitic acid (16:0) | 3.7 ± 0.1 a | 3.6 ± 0.1 a | 3.7 ± 0.1 a |
| Stearic acid (18:0) | 2.2 ± 0.0 a | 2.2 ± 0.0 a | 2.2 ± 0.0 a |
| Arachidic acid (20:0) | 0.9 ± 0.1 a | 0.9 ± 0.1 a | 0.9 ± 0.1 a |
| Behenic acid (22:0) | 0.1 ± 0.0 a | 0.1 ± 0.0 a | 0.1 ± 0.0 a |
| Unsaturated fatty acids (rel. %) | |||
| Palmitoleic acid (16:1) | 0.2 ± 0.0 a | 0.2 ± 0.0 a | 0.2 ± 0.0 a |
| Oleic acid (18:1) | 16.1 ± 0.5 a | 16.5 ± 0.6 a | 16.4 ± 0.5 a |
| cis-Vaccenic acid (18:1) | 0.5 ± 0.0 a | 0.5 ± 0.0 a | 0.5 ± 0.0 a |
| Linoleic acid (18:2) | 54.8 ± 1.2 a | 54.4 ± 1.3 a | 54.6 ± 1.5 a |
| α-Linolenic acid (18:3) | 21.1 ± 0.8 a | 21.3 ± 0.9 a | 21.0 ± 1.0 a |
| Gondoic acid (20:1) | 0.4 ± 0.0 a | 0.4 ± 0.0 a | 0.4 ± 0.0 a |
| Total carotenoids (mg/kg) | 365 ± 12 (816 ± 30) a ** | 336 ± 35 (818 ± 26) a | 350 ± 10 (825 ± 30) a |
| β-Carotene (mg/kg) | 105 ± 7 a | 110 ± 13 a | 115 ± 11 a |
| α-Tocopherol (mg/kg) | 364 ± 28 a | 295 ± 5 b | 272 ± 1 c |
| γ-Tocopherol (mg/kg) | 1042 ± 16 a | 937 ± 17 ab | 914 ± 9 b |
| Acid value (mg KOH/g) | 1.3 ± 0.0 b | 1.3 ± 0.0 b | 1.7 ± 0.0 a |
| conj. Dienes (A232, 1%) | 2.4 ± 0.1 c | 3.0 ± 0.1 b | 4.3 ± 0.8 a |
| conj. Trienes (A268, 1%) | 0.6 ± 0.0 b | 0.6 ± 0.0 b | 0.8 ± 0.0 a |
| Non-Irradiated | 10 kGy | 25 kGy | ||
|---|---|---|---|---|
| Total phenolics, mg GAE/100 g dw | 13,148 ± 775 a * | 13,840 ± 625 a | 13,677 ± 646 a | |
| Phenolic constituents, mg/100 g dw | Chlorogenic acid | 139.7 ± 2.9 a | 129.9 ± 2.7 a | 128.8 ± 1.9 b |
| Quercetin | 13.9 ± 0.6 b | 18.9 ± 0.8 a | 18.3 ± 0.3 a | |
| Quercetin 3-O-β-D-glucopyranoside | 68.2 ± 0.6 a | 67.0 ± 2.6 ab | 63.9 ± 0.5 b | |
| Rutin | 355.7 ± 2.8 a | 350.7 ± 4.9 ab | 347.1 ± 2.7 b | |
| Catechin | 645.4 ± 21.7 a | 633.3 ± 23.1 a | 661.6 ± 9.2 a | |
| Epicatechin | 425.2 ± 5.8 a | 426.1 ± 15.1 a | 429.1 ± 1.5 a | |
| Antioxidant activity | ORAC (µmol TE/g) | 3890 ± 282 a | 3862 ± 198 a | 3899 ± 208 a |
| HORAC (µmol GAE/g) | 593 ± 68 a | 573 ± 55 a | 599 ± 50 a |
| Characteristic Parameter | Non-Irradiated | 10 kGy | 25 kGy | |
|---|---|---|---|---|
| Alcohol-insoluble solids | Yield | 44.1 ± 0.2 a * | 42.5 ± 0.3 b | 40.6 ± 0.2 c |
| Initial RH fruits | Uronic acids | 10.5 ± 0.5 a | 9.5 ± 0.5 a | 10.1 ± 0.2 a |
| % of total uronic acids extracted | 51.0 | 63.4 | 70.5 | |
| Cellulose | 7.0 ± 0.1 a | 6.0 ± 0.2 b | 5.7 ± 0.0 c | |
| Crude protein (N×6.25) | 1.7 | - | - | |
| Polysaccharides | Yield | 10.8 ± 0.2 c | 12.8 ± 0.1 b | 13.4 ± 0.3 a |
| Uronic acids | 49.5 ± 1.5 b | 47.5 ± 1.0 b | 53.1 ± 0.6 a | |
| Degree of methyl-esterification ** | 69.6 ± 0.5 a | 70.5 ± 1.0 a | 70.1 ± 0.7 a | |
| Degree of acetylation ** (Acetyl content) | 10.5 ± 0.5 b (1.3) | 13.0 ± 0.2 a (1.5) | 13.3 ± 0.3 a (1.7) | |
| Protein | 1.7 ± 0.2 a | 1.6 ± 0.0 a | 1.7 ± 0.1 a |
| Monosaccharide | Non-Irradiated | 25 kGy |
|---|---|---|
| Neutral sugars | ||
| Rhamnose | 7.1 ± 1.0 a * | 8.8 ± 0.8 a |
| Arabinose | 9.6 ± 1.0 a | 11.0 ± 1.2 a |
| Galactose | 6.5 ± 0.7 a | 5.7 ± 1.0 a |
| Glucose | 11.2 ± 1.3 a | 7.5 ± 0.4 b |
| Mannose | 0.1 ± 0.0 a | 0.0 ± 0.0 a |
| Fucose | 1.5 ± 0.6 b | 4.2 ± 1.0 a |
| Uronic acids | ||
| Galacturonic acid | 56.5 ± 1.5 a | 58.8 ± 0.5 a |
| Glucuronic acid | 7.4 ± 0.4 a | 3.9 ± 1.2 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ognyanov, M.; Denev, P.; Teneva, D.; Georgiev, Y.; Taneva, S.; Totseva, I.; Kamenova-Nacheva, M.; Nikolova, Y.; Momchilova, S. Influence of Gamma Irradiation on Different Phytochemical Constituents of Dried Rose Hip (Rosa canina L.) Fruits. Molecules 2022, 27, 1765. https://doi.org/10.3390/molecules27061765
Ognyanov M, Denev P, Teneva D, Georgiev Y, Taneva S, Totseva I, Kamenova-Nacheva M, Nikolova Y, Momchilova S. Influence of Gamma Irradiation on Different Phytochemical Constituents of Dried Rose Hip (Rosa canina L.) Fruits. Molecules. 2022; 27(6):1765. https://doi.org/10.3390/molecules27061765
Chicago/Turabian StyleOgnyanov, Manol, Petko Denev, Desislava Teneva, Yordan Georgiev, Sabina Taneva, Iskra Totseva, Mariana Kamenova-Nacheva, Yana Nikolova, and Svetlana Momchilova. 2022. "Influence of Gamma Irradiation on Different Phytochemical Constituents of Dried Rose Hip (Rosa canina L.) Fruits" Molecules 27, no. 6: 1765. https://doi.org/10.3390/molecules27061765
APA StyleOgnyanov, M., Denev, P., Teneva, D., Georgiev, Y., Taneva, S., Totseva, I., Kamenova-Nacheva, M., Nikolova, Y., & Momchilova, S. (2022). Influence of Gamma Irradiation on Different Phytochemical Constituents of Dried Rose Hip (Rosa canina L.) Fruits. Molecules, 27(6), 1765. https://doi.org/10.3390/molecules27061765







