A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stock, Calibration Standards and Quality Control Solution
2.3. Instrumentation and Chromatographic Conditions
2.4. HPLC Method Validation
2.4.1. Linearity, LOD, and LOQ
2.4.2. Accuracy and Precision
2.5. Stability Studies
2.6. Preparation of CUR-DPA Meshes for Wound-Healing Dressings Using a Robocasting 3D Printer
2.7. Application of the Method to In Vitro Release Study
2.8. Statistical Analysis
3. Results and Discussion
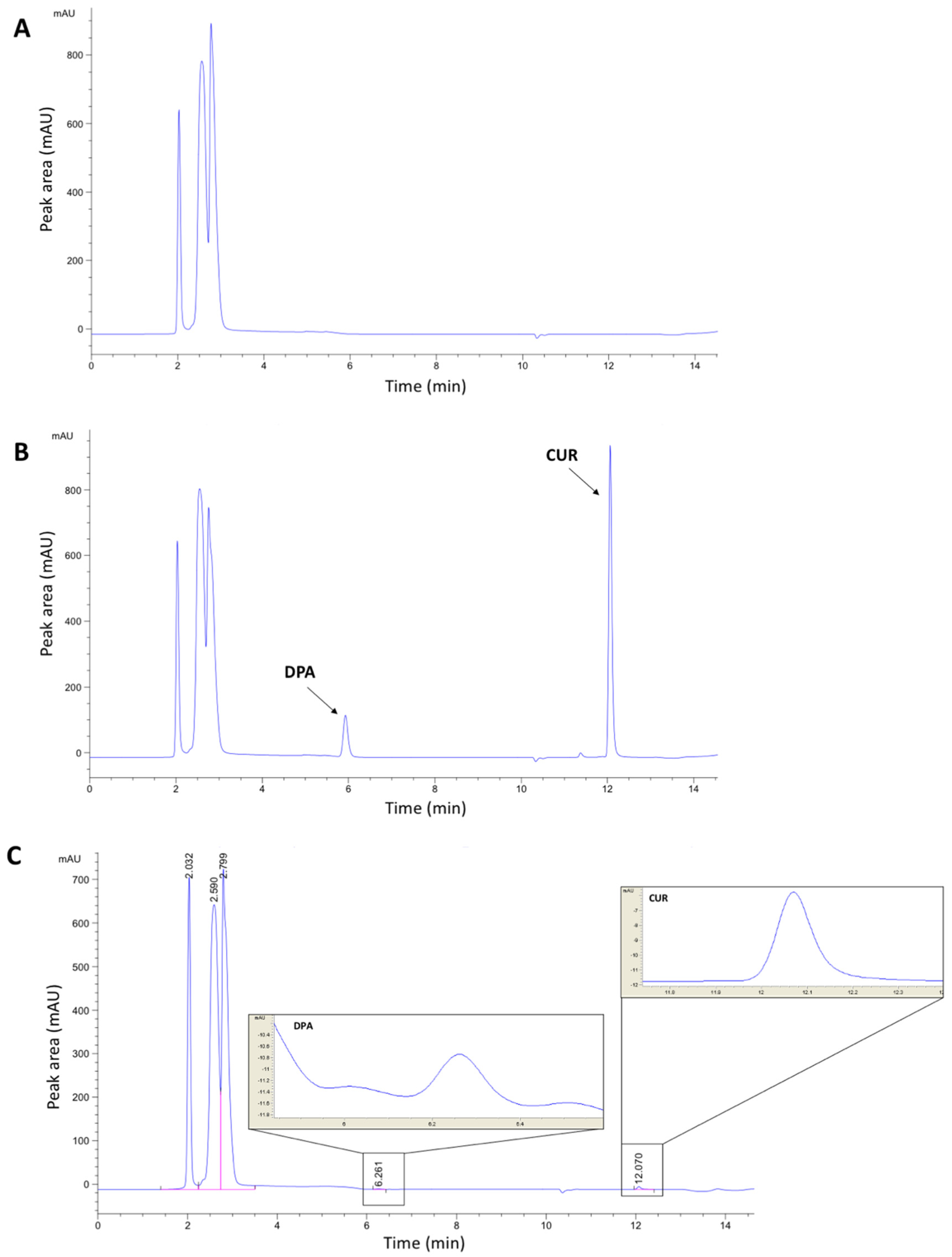
3.1. Chromatographic Method Development
3.2. HPLC Method Validation
3.2.1. Linearity, LOD, and LOQ
3.2.2. Accuracy and Precision
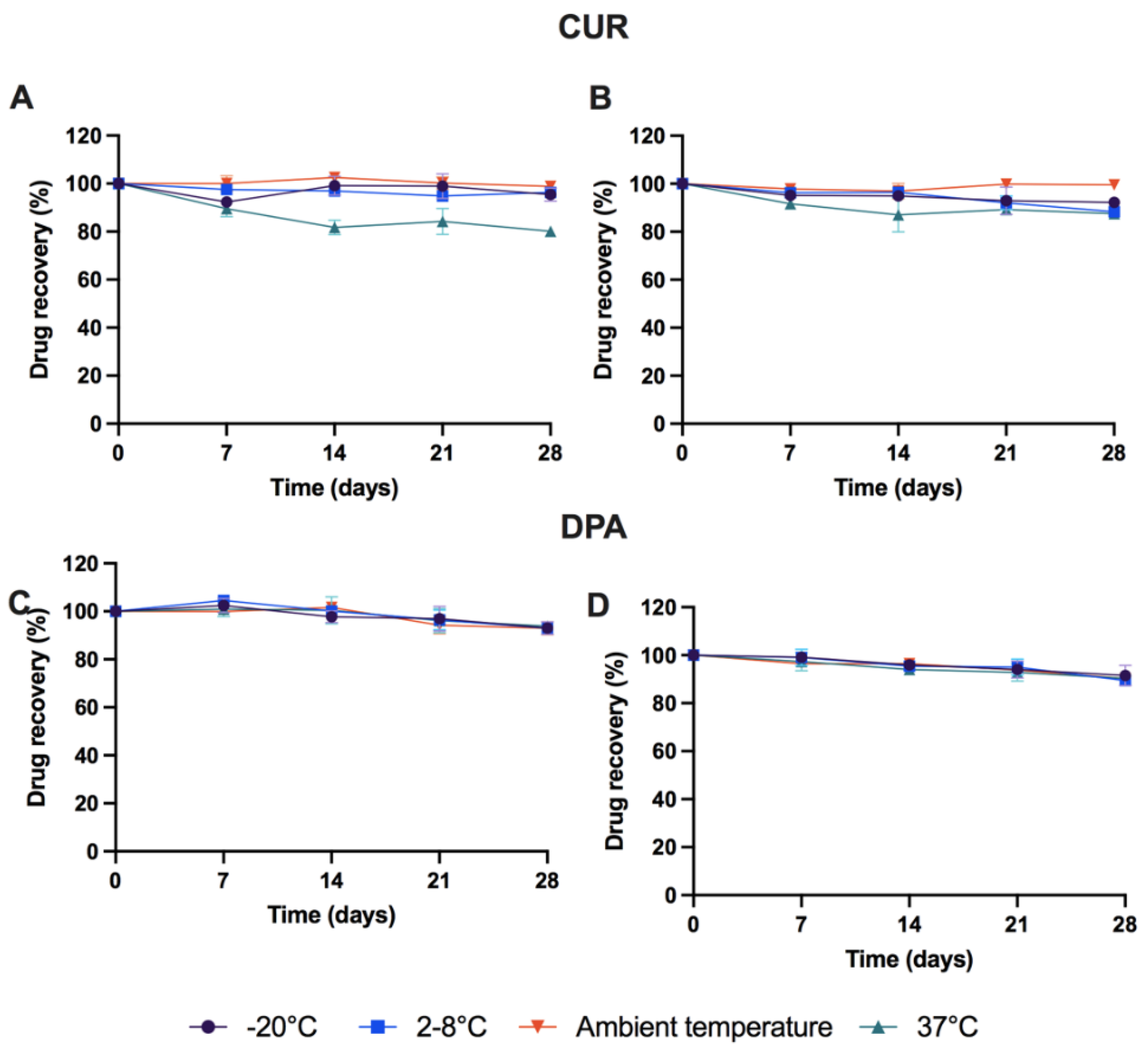
3.3. Stability Study
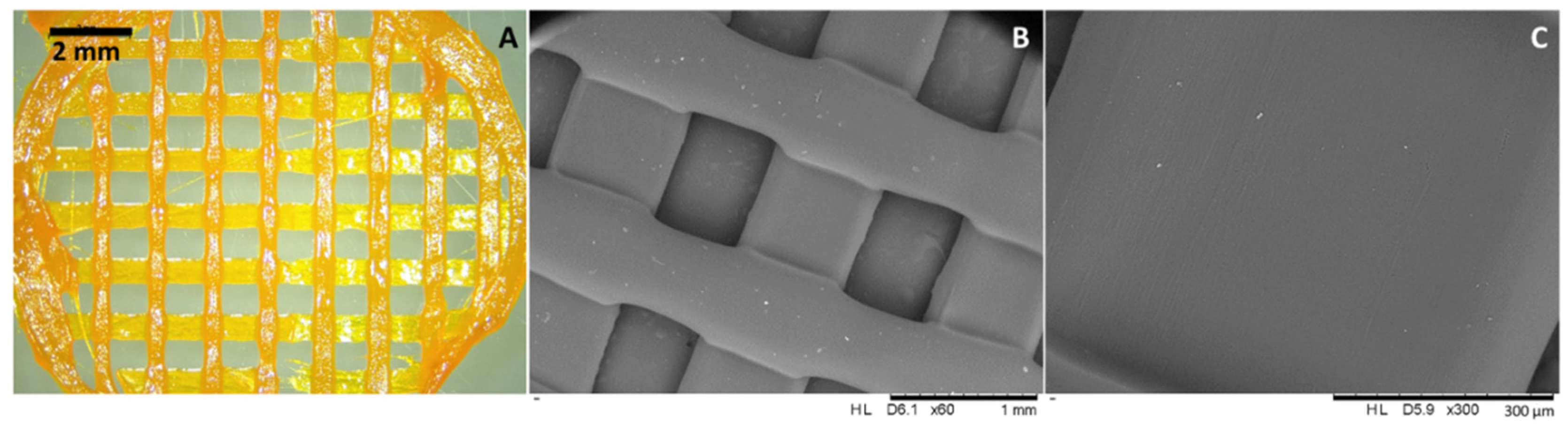
3.4. Preparation of CUR-DPA Topical Meshes Using a Robocasting 3D Printer
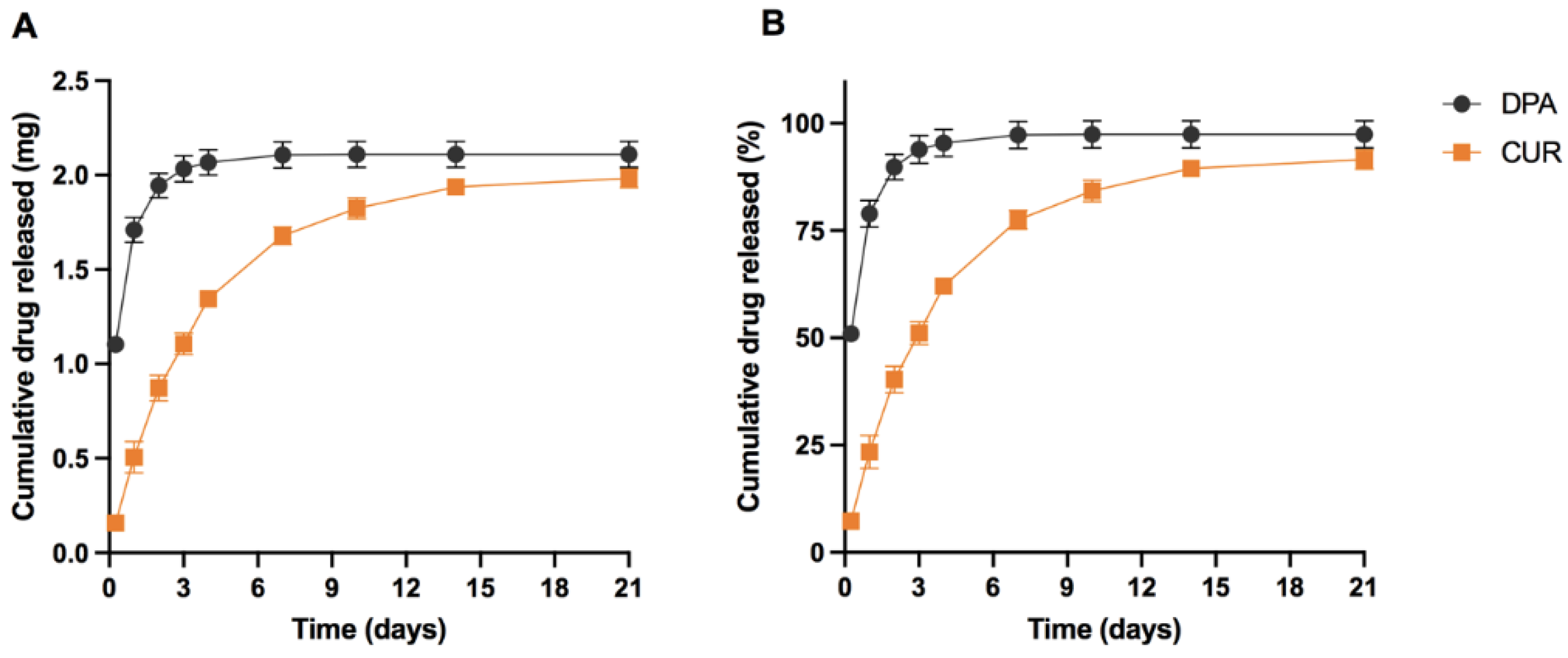
3.5. Application of the Method to In Vitro Release Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohanty, C.; Das, M.; Sahoo, S.K. Sustained Wound Healing Activity of Curcumin Loaded Oleic Acid Based Polymeric Bandage in a Rat Model. Mol. Pharm. 2012, 9, 2801–2811. [Google Scholar] [CrossRef] [PubMed]
- Akbik, D.; Ghadiri, M.; Chrzanowski, W.; Rohanizadeh, R. Curcumin as a Wound Healing Agent. Life Sci. 2014, 116, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, J.; Zheng, H.; Wichmann, J.; Hopfner, U.; Sudhop, S.; Prein, C.; Shen, Y.; Machens, H.-G.; Schilling, A.F. Nano-Formulated Curcumin Accelerates Acute Wound Healing through Dkk-1-Mediated Fibroblast Mobilization and MCP-1-Mediated Anti-Inflammation. NPG Asia Mater. 2017, 9, 368. [Google Scholar] [CrossRef] [Green Version]
- Proksch, E.; De Bony, R.; Trapp, S.; Boudon, S.; Ephanie Boudon, S. Topical Use of Dexpanthenol: A 70th Anniversary Article. J. Dermatol. Treat. 2017, 28, 766–773. [Google Scholar] [CrossRef]
- Proksch, E.; Nissen, H.P. Dexapanthenol Enhances Skin Barrier Repair and Reduces Inflammation after Sodium Lauryl Sulphate-Induced Irritation. J. Dermatol. Treat. 2010, 13, 173–178. [Google Scholar] [CrossRef]
- Gorski, J.; Proksch, E.; Baron, J.M.; Schmid, D.; Zhang, L. Dexpanthenol in Wound Healing after Medical and Cosmetic Interventions (Postprocedure Wound Healing). Pharmaceuticals 2020, 13, 138. [Google Scholar] [CrossRef]
- Olsavszky, R.; Nanu, E.A.; Macura-biegun, A.; Kurka, P. Skin Barrier Restoration upon Topical Use of Two 5% Dexpanthenol Water-in-Oil Formulations on Freshly Tattooed Skin: Results from a Single-Blind Prospective Study Formal. Wounds Int. 2019, 10, 33–39. [Google Scholar]
- Heise, R.; Skazik, C.; Marquardt, Y.; Czaja, K.; Sebastian, K.; Kurschat, P.; Gan, L.; Denecke, B.; Ekanayake-Bohlig, S.; Wilhelm, K.-P.; et al. Dexpanthenol Modulates Gene Expression in Skin Wound Healing in Vivo. Ski. Pharm. Physiol. 2012, 25, 241–248. [Google Scholar] [CrossRef]
- Wichitnithad, W.; Jongaroonngamsang, N.; Pummangura, S.; Rojsitthisak, P. A Simple Isocratic HPLC Method for the Simultaneous Determination of Curcuminoids in Commercial Turmeric Extracts. Phytochem. Anal. 2009, 20, 314–319. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. A Simple Reversed Phase High-Performance Liquid Chromatography (HPLC) Method for Determination of in Situ Gelling Curcumin-Loaded Liquid Crystals in in Vitro Performance Tests. Arab. J. Chem. 2017, 10, 1029–1037. [Google Scholar] [CrossRef] [Green Version]
- Kulikov, A.U.; Zinchenko, A.A. Development and Validation of Reversed Phase High Performance Liquid Chromatography Method for Determination of Dexpanthenol in Pharmaceutical Formulations. J. Pharm. Biomed. Anal. 2007, 43, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Doganay, A.; Koksel, B.; Gundogdu, S.O.; Capan, Y. Simultaneous Determination of Dexpanthenol, Lidocaine Hydrochloride, Mepyramine Maleate and Their Related Substances by a RP-HPLC Method in Topical Dosage Forms. J. Chromatogr. Sci. 2018, 56, 903–911. [Google Scholar] [CrossRef] [PubMed]
- González, O.; Blanco, M.E.; Iriarte, G.; Bartolomé, L.; Maguregui, M.I.; Alonso, R.M. Bioanalytical Chromatographic Method Validation According to Current Regulations, with a Special Focus on the Non-Well Defined Parameters Limit of Quantification, Robustness and Matrix Effect. J. Chromatogr. 2014, 1353, 10–27. [Google Scholar] [CrossRef] [PubMed]
- Van Nong, H.; Hung, L.X.; Thang, P.N.; Chinh, V.D.; Van Vu, L.; Dung, P.T.; Van Trung, T.; Nga, P.T. Fabrication and Vibration Characterization of Curcumin Extracted from Turmeric (Curcuma longa) Rhizomes of the Northern Vietnam. SpringerPlus 2016, 5, 1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hudson, T.S.; Subramanian, S.; Allen, R.J. Determination of Pantothenic Acid, Biotin, and Vitamin B12 in Nutritional Products. J. Assoc. Off. Anal. Chem. 1984, 67, 994–998. [Google Scholar] [CrossRef]
- Permana, A.D.; Wahyudin, E.; Amir, M.N.; Raihan, M.; Anjani, Q.K.; Utomo, E.; Layadi, P.; Donnelly, R.F. New and Sensitive HPLC-UV Method for Concomitant Quantification of a Combination of Antifilariasis Drugs in Rat Plasma and Organs after Simultaneous Oral Administration. Anal. Methods 2021, 13, 933–945. [Google Scholar] [CrossRef]
- ChemAxon Calculator Plugins Were Used for Structure Property Prediction and Calculation. Available online: https://www.chemaxon.com (accessed on 10 January 2022).
- ICH Q2 (R1) Proceedings of the International Conference on Harmonization. In Harmonised Tripartite Guideline: Validation of Analytical Procedures: Text and Methodology. Geneva. 1994. Available online: https://www.ema.europa.eu/en/ich-q2-r1-validation-analytical-procedures-text-methodology (accessed on 10 January 2022).
- Anjani, Q.K.; Sabri, A.H.; Donnelly, R. Development and Validation of Simple and Sensitive HPLC-UV Method for Ethambutol Hydrochloride Detection Following Transdermal Application. Anal. Methods 2021, 14, 125–134. [Google Scholar] [CrossRef]
- De, A.K.; Chowdhury, P.P.; Chattapadhyay, S. Simultaneous Quantification of Dexpanthenol and Resorcinol from Hair Care Formulation Using Liquid Chromatography: Method Development and Validation. Scientifica 2016, 2016, 1537952. [Google Scholar] [CrossRef] [Green Version]
- Yu, W.; Wen, D.; Cai, D.; Zheng, J.; Gan, H.; Jiang, F.; Liu, X.; Lao, B.; Yu, W.; Guan, Y.; et al. Simultaneous Determination of Curcumin, Tetrahydrocurcumin, Quercetin, and Paeoniflorin by UHPLC-MS/MS in Rat Plasma and Its Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal. 2019, 172, 58–66. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Kapoor, B.; Gulati, M.; Wadhwa, S.; Gupta, S.; Prasher, P.; Kumar, D.; Dua, K.; Kumar, L.M.S.; et al. Development and Validation of RP-HPLC Method for Simultaneous Determination of Curcumin and Quercetin in Extracts, Marketed Formulations, and Self-Nanoemulsifying Drug Delivery System. Re GEN Open 2021, 1, 43–52. [Google Scholar] [CrossRef]
- Long, Y.; Zhang, W.; Wang, F.; Chen, Z. Simultaneous Determination of Three Curcuminoids in Curcuma longa L. by High Performance Liquid Chromatography Coupled with Electrochemical Detection. J. Pharm. Anal. 2014, 4, 325–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of PH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Skrzynski, M.; Decker, E.A.; McClements, D.J. Enhancement of Chemical Stability of Curcumin-Enriched Oil-in-Water Emulsions: Impact of Antioxidant Type and Concentration. Food Chem. 2020, 320, 126653. [Google Scholar] [CrossRef]
- Larrañeta, E.; Barturen, L.; Ervine, M.; Donnelly, R.F. Hydrogels Based on Poly(Methyl Vinyl Ether-Co-Maleic Acid) and Tween 85 for Sustained Delivery of Hydrophobic Drugs. Int. J. Pharm. 2018, 538, 147–158. [Google Scholar] [CrossRef] [Green Version]
- Indriani, R.; Hatta, A.M.; Irawati, N. Effect of the Storage Temperature on Curcumin Content in Food Supplement by Spectrophotometry Method. Third Int. Semin. Photonics Opt. Appl. 2019, 11044, 123–125. [Google Scholar] [CrossRef]
- Nimiya, Y.; Wang, W.; Du, Z.; Sukamtoh, E.; Zhu, J.; Decker, E.; Zhang, G. Redox Modulation of Curcumin Stability: Redox Active Antioxidants Increase Chemical Stability of Curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Economidou, S.N.; Lamprou, D.A.; Douroumis, D. 3D Printing Applications for Transdermal Drug Delivery. Int. J. Pharm. 2018, 544, 415–424. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.S.; Fantaus, S.S.; Guillot, A.J.; Melero, A.; Beck, R.C.R. 3D-Printed Products for Topical Skin Applications: From Personalized Dressings to Drug Delivery. Pharmaceutics 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Alizadehgiashi, M.; Nemr, C.R.; Chekini, M.; Pinto Ramos, D.; Mittal, N.; Ahmed, S.U.; Khuu, N.; Kelley, S.O.; Kumacheva, E. Multifunctional 3D-Printed Wound Dressings. ACS Nano 2021, 15, 12375–12387. [Google Scholar] [CrossRef]
- Trenfield, S.J.; Awad, A.; Madla, C.M.; Hatton, G.B.; Firth, J.; Goyanes, A.; Gaisford, S.; Basit, A.W. Shaping the Future: Recent Advances of 3D Printing in Drug Delivery and Healthcare. Expert Opin. Drug Deliv. 2019, 16, 1081–1094. [Google Scholar] [CrossRef]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D Scanning and 3D Printing as Innovative Technologies for Fabricating Personalized Topical Drug Delivery Systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Development of a Biodegradable Subcutaneous Implant for Prolonged Drug Delivery Using 3D Printing. Pharmaceutics 2020, 12, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, N.K.; Domínguez-Robles, J.; Stewart, S.A.; Cornelius, V.A.; Anjani, Q.K.; Utomo, E.; García-Romero, I.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; et al. Fused Deposition Modelling for the Development of Drug Loaded Cardiovascular Prosthesis. Int. J. Pharm. 2021, 595, 120243. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.A.; Domínguez-Robles, J.; McIlorum, V.J.; Gonzalez, Z.; Utomo, E.; Mancuso, E.; Lamprou, D.A.; Donnelly, R.F.; Larrañeta, E. Poly(Caprolactone)-Based Coatings on 3D-Printed Biodegradable Implants: A Novel Strategy to Prolong Delivery of Hydrophilic Drugs. Mol. Pharm. 2020, 17, 3487–3500. [Google Scholar] [CrossRef]
- Domínguez-Robles, J.; Shen, T.; Cornelius, V.A.; Corduas, F.; Mancuso, E.; Donnelly, R.F.; Margariti, A.; Lamprou, D.A.; Larrañeta, E. Development of Drug Loaded Cardiovascular Prosthesis for Thrombosis Prevention Using 3D Printing. Mater. Sci. Eng. C 2021, 129, 112375. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Diaz-Gomez, L.; Utomo, E.; Shen, T.; Picco, C.J.; Alvarez-Lorenzo, C.; Concheiro, A.; Donnelly, R.F.; Larrañeta, E. Use of 3D Printing for the Development of Biodegradable Antiplatelet Materials for Cardiovascular Applications. Pharmaceuticals 2021, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Dexpanthenol|C9H19NO4—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dexpanthenol (accessed on 10 January 2022).
- Curcumin|C21H20O6—PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Curcumin (accessed on 10 January 2022).
- Anjana, D.; Anitha Nair, K.; Somashekara, N.; Venkata, M.; Sripathy, R.; Yelucheri, R.; Parmar, H.; Upadhyay, R.; Rama Verma, S.; Ramchand, C.N. Development of Curcumin Based Ophthalmic Formulation. Am. J. Infect. Dis. 2012, 8, 41–49. [Google Scholar] [CrossRef] [Green Version]
| Time (min) | A (%) | B (%) | Detector Wavelength (nm) |
|---|---|---|---|
| 0–7 | 94 | 6 | 200 |
| 7–12 | 25 | 75 | 425 |
| 12–15 | 94 | 6 | 200 |
| Analyte | Concentration Range (µg mL−1) | Slope | y-Intercept | R2 | LOD (µg mL−1) | LOQ (µg mL−1) | RSS | |
|---|---|---|---|---|---|---|---|---|
| Intra-day | CUR | 0.39–12.5 | 226.63 | −20.81 | 0.9999 | 0.09 | 0.29 | 673.35 |
| DPA | 0.39–25 | 40.69 | −0.19 | 1 | 0.08 | 0.24 | 28.08 | |
| Inter-day | CUR | 0.39–12.5 | 209.98 | −24.258 | 1 | 0.12 | 0.36 | 809.11 |
| DPA | 0.39–25 | 40.93 | −1.32 | 1 | 0.10 | 0.31 | 14.72 |
| Analyte | Concentration Added (µg mL−1) | Intraday | Interday | ||||
|---|---|---|---|---|---|---|---|
| Concentration Found (µg mL−1) | Precision (%RSD) | Accuracy (%RE) | Concentration Found (µg mL−1) | Precision (%RSD) | Accuracy (%RE) | ||
| CUR | 10 | 10.09 | 2.79 | 0.91 | 10.16 | 1.01 | 1.58 |
| 2 | 1.83 | 2.20 | −8.74 | 1.89 | 5.54 | −5.75 | |
| 0.75 | 0.77 | 2.30 | 3.32 | 0.75 | 1.16 | 0.56 | |
| DPA | 20 | 19.44 | 1.00 | −2.82 | 21.39 | 2.54 | 6.93 |
| 5 | 4.88 | 1.77 | −2.36 | 5.11 | 2.71 | 2.15 | |
| 0.74 | 0.75 | 2.90 | 0.96 | 0.73 | 5.27 | −1.91 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anjani, Q.K.; Utomo, E.; Domínguez-Robles, J.; Detamornrat, U.; Donnelly, R.F.; Larrañeta, E. A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings. Molecules 2022, 27, 1759. https://doi.org/10.3390/molecules27061759
Anjani QK, Utomo E, Domínguez-Robles J, Detamornrat U, Donnelly RF, Larrañeta E. A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings. Molecules. 2022; 27(6):1759. https://doi.org/10.3390/molecules27061759
Chicago/Turabian StyleAnjani, Qonita Kurnia, Emilia Utomo, Juan Domínguez-Robles, Usanee Detamornrat, Ryan F. Donnelly, and Eneko Larrañeta. 2022. "A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings" Molecules 27, no. 6: 1759. https://doi.org/10.3390/molecules27061759
APA StyleAnjani, Q. K., Utomo, E., Domínguez-Robles, J., Detamornrat, U., Donnelly, R. F., & Larrañeta, E. (2022). A New and Sensitive HPLC-UV Method for Rapid and Simultaneous Quantification of Curcumin and D-Panthenol: Application to In Vitro Release Studies of Wound Dressings. Molecules, 27(6), 1759. https://doi.org/10.3390/molecules27061759









