Influence of Different Microplastic Forms on pH and Mobility of Cu2+ and Pb2+ in Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microplastic Characterization
2.2. Soil Incubation Experiment
2.3. Metal Analysis in Soil
2.4. Statistical Analysis
3. Results
3.1. Effect on Soil pH
3.2. Effects on the Metal Species in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in Freshwater and Terrestrial Environments: Evaluating the Current Understanding to Identify the Knowledge Gaps and Future Research Priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [Green Version]
- Nizzetto, L.; Bussi, G.; Futter, M.N.; Butterfield, D.; Whitehead, P.G. A Theoretical Assessment of Microplastic Transport in River Catchments and Their Retention by Soils and River Sediments. Environ. Sci. Process. Impacts 2016, 18, 1050–1059. [Google Scholar] [CrossRef]
- Gao, F.; Li, J.; Sun, C.; Zhang, L.; Jiang, F.; Cao, W.; Zheng, L. Study on the Capability and Characteristics of Heavy Metals Enriched on Microplastics in Marine Environment. Mar. Pollut. Bull. 2019, 144, 61–67. [Google Scholar] [CrossRef] [PubMed]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an Emerging Threat to Terrestrial Ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yang, W.; Cheng, P.; Zhang, S.; Zhang, S.; Jiao, W.; Sun, Y. Adsorption Characteristics of Cadmium onto Microplastics from Aqueous Solutions. Chemosphere 2019, 235, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.; Zhou, X.; Kong, X.; Tao, S.; Xing, B. Sorption of Four Hydrophobic Organic Compounds by Three Chemically Distinct Polymers: Role of Chemical and Physical Composition. Environ. Sci. Technol. 2012, 46, 7252–7259. [Google Scholar] [CrossRef] [PubMed]
- Aghilinasrollahabadi, K.; Salehi, M.; Fujiwara, T. Investigate the Influence of Microplastics Weathering on Their Heavy Metals Uptake in Stormwater. J. Hazard. Mater. 2021, 408, 124439. [Google Scholar] [CrossRef]
- Besson, M.; Jacob, H.; Oberhaensli, F.; Taylor, A.; Swarzenski, P.W.; Metian, M. Preferential Adsorption of Cd, Cs and Zn onto Virgin Polyethylene Microplastic versus Sediment Particles. Mar. Pollut. Bull. 2020, 156, 111223. [Google Scholar] [CrossRef]
- Rochman, C.M.; Hoh, E.; Kurobe, T.; Teh, S.J. Ingested Plastic Transfers Hazardous Chemicals to Fish and Induces Hepatic Stress. Sci. Rep. 2013, 3, 3263. [Google Scholar] [CrossRef]
- Ashton, K.; Holmes, L.; Turner, A. Association of Metals with Plastic Production Pellets in the Marine Environment. Mar. Pollut. Bull. 2010, 60, 2050–2055. [Google Scholar] [CrossRef]
- Cao, Y.; Zhao, M.; Ma, X.; Song, Y.; Zuo, S.; Li, H.; Deng, W. A Critical Review on the Interactions of Microplastics with Heavy Metals: Mechanism and Their Combined Effect on Organisms and Humans. Sci. Total Environ. 2021, 788, 147620. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Santos, T.; Duarte, A.C. A Critical Overview of the Analytical Approaches to the Occurrence, the Fate and the Behavior of Microplastics in the Environment. TrAC Trends Anal. Chem. 2015, 65, 47–53. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwanga, E.; Geissen, V. Evidence of Microplastic Accumulation in Agricultural Soils from Sewage Sludge Disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Chen, L.; Chao, J.; Teng, J.; Wang, Q. Microplastics in Soils: A Review of Possible Sources, Analytical Methods and Ecological Impacts. J. Chem. Technol. Biotechnol. 2020, 95, 2052–2068. [Google Scholar] [CrossRef]
- Qi, R.; Jones, D.L.; Li, Z.; Liu, Q.; Yan, C. Behavior of Microplastics and Plastic Film Residues in the Soil Environment: A Critical Review. Sci. Total Environ. 2020, 703, 134722. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Phil. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liu, X.; Li, Y.; Powell, T.; Wang, X.; Wang, G.; Zhang, P. Microplastics as Contaminants in the Soil Environment: A Mini-Review. Sci. Total Environ. 2019, 691, 848–857. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X.; Liu, G.; Liang, C.; Xue, S.; Chen, H.; Ritsema, C.J.; Geissen, V. Response of Soil Dissolved Organic Matter to Microplastic Addition in Chinese Loess Soil. Chemosphere 2017, 185, 907–917. [Google Scholar] [CrossRef]
- Yu, H.; Hou, J.; Dang, Q.; Cui, D.; Xi, B.; Tan, W. Decrease in Bioavailability of Soil Heavy Metals Caused by the Presence of Microplastics Varies across Aggregate Levels. J. Hazard. Mater. 2020, 395, 122690. [Google Scholar] [CrossRef]
- Hodson, M.E.; Duffus-Hodson, C.A.; Clark, A.; Prendergast-Miller, M.T.; Thorpe, K.L. Plastic Bag Derived-Microplastics as a Vector for Metal Exposure in Terrestrial Invertebrates. Environ. Sci. Technol. 2017, 51, 4714–4721. [Google Scholar] [CrossRef] [Green Version]
- Selonen, S.; Dolar, A.; Kokalj, A.J.; Skalar, T.; Dolcet, L.P.; Hurley, R.; van Gestel, C.A.M. Exploring the Impacts of Plastics in Soil—The Effects of Polyester Textile Fibers on Soil Invertebrates. Sci. Total Environ. 2020, 700, 134451. [Google Scholar] [CrossRef]
- Lehmann, A.; Leifheit, E.F.; Feng, L.; Bergmann, J.; Wulf, A.; Rillig, M.C. Microplastic Fiber and Drought Effects on Plants and Soil Are Only Slightly Modified by Arbuscular Mycorrhizal Fungi. Soil Ecol. Lett. 2020, 4, 32–44. [Google Scholar] [CrossRef]
- Schwertfeger, D.M.; Hendershot, W.H. Spike/Leach Procedure to Prepare Soil Samples for Trace Metal Ecotoxicity Testing: Method Development Using Copper. Commun. Soil Sci. Plant Anal. 2013, 44, 1570–1587. [Google Scholar] [CrossRef]
- Schuwirth, N.; Hofmann, T. Comparability of and Alternatives to Leaching Tests for the Assessment of the Emission of Inorganic Soil Contamination (11 Pp). J. Soils Sediments 2006, 6, 102–112. [Google Scholar] [CrossRef]
- Medyńska-Juraszek, A.; Bednik, M.; Chohura, P. Assessing the Influence of Compost and Biochar Amendments on the Mobility and Uptake of Heavy Metals by Green Leafy Vegetables. Int. J. Environ. Res. Public Health 2020, 17, 7861. [Google Scholar] [CrossRef]
- Gao, X.; Hassan, I.; Peng, Y.; Huo, S.; Ling, L. Behaviors and Influencing Factors of the Heavy Metals Adsorption onto Microplastics: A Review. J. Clean. Prod. 2021, 319, 128777. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The Potential of Microplastics as Carriers of Metals. Environ. Pollut. 2019, 255, 113363. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Y.; Liu, G.; He, G.; Liu, W. Adsorption Mechanism of Cadmium on Microplastics and Their Desorption Behavior in Sediment and Gut Environments: The Roles of Water PH, Lead Ions, Natural Organic Matter and Phenanthrene. Water Res. 2020, 184, 116209. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of Microplastics and the Association of Heavy Metals with Microplastics in Suburban Soil of Central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Maaß, S.; Daphi, D.; Lehmann, A.; Rillig, M.C. Transport of Microplastics by Two Collembolan Species. Environ. Pollut. 2017, 225, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Adsorption of Trace Metals to Plastic Resin Pellets in the Marine Environment. Environ. Pollut. 2012, 160, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Tang, S.; Wang, X.; Sun, X.; Yu, A. Hexabromocyclododecane Alters Malachite Green and Lead(II) Adsorption Behaviors onto Polystyrene Microplastics: Interaction Mechanism and Competitive Effect. Chemosphere 2021, 265, 129079. [Google Scholar] [CrossRef]
- Zou, J.; Liu, X.; Zhang, D.; Yuan, X. Adsorption of Three Bivalent Metals by Four Chemical Distinct Microplastics. Chemosphere 2020, 248, 126064. [Google Scholar] [CrossRef]
- Wu, X.; Lyu, X.; Li, Z.; Gao, B.; Zeng, X.; Wu, J.; Sun, Y. Transport of Polystyrene Nanoplastics in Natural Soils: Effect of Soil Properties, Ionic Strength and Cation Type. Sci. Total Environ. 2020, 707, 136065. [Google Scholar] [CrossRef]
- Zhao, T.; Lozano, Y.M.; Rillig, M.C. Microplastics Increase Soil PH and Decrease Microbial Activities as a Function of Microplastic Shape, Polymer Type, and Exposure Time. Front. Environ. Sci. 2021, 9, 675803. [Google Scholar] [CrossRef]
- Lozano, Y.M.; Rillig, M.C. Effects of Microplastic Fibers and Drought on Plant Communities. Environ. Sci. Technol. 2020, 54, 6166–6173. [Google Scholar] [CrossRef]
- Tourinho, P.S.; Kočí, V.; Loureiro, S.; van Gestel, C.A.M. Partitioning of Chemical Contaminants to Microplastics: Sorption Mechanisms, Environmental Distribution and Effects on Toxicity and Bioaccumulation. Environ. Pollut. 2019, 252, 1246–1256. [Google Scholar] [CrossRef]
- Yasir, A.M.; Ma, J.; Ouyang, X.; Zhao, J.; Zhao, Y.; Weng, L.; Islam, M.S.; Chen, Y.; Li, Y. Effects of Selected Functional Groups on Nanoplastics Transport in Saturated Media under Diethylhexyl Phthalate Co-Contamination Conditions. Chemosphere 2022, 286, 131965. [Google Scholar] [CrossRef]
- Brennecke, D.; Duarte, B.; Paiva, F.; Caçador, I.; Canning-Clode, J. Microplastics as Vector for Heavy Metal Contamination from the Marine Environment. Estuar. Coast. Shelf Sci. 2016, 178, 189–195. [Google Scholar] [CrossRef]
- Holmes, L.A.; Turner, A.; Thompson, R.C. Interactions between Trace Metals and Plastic Production Pellets under Estuarine Conditions. Mar. Chem. 2014, 167, 25–32. [Google Scholar] [CrossRef]
- Chen, W.; Ouyang, Z.-Y.; Qian, C.; Yu, H.-Q. Induced Structural Changes of Humic Acid by Exposure of Polystyrene Microplastics: A Spectroscopic Insight. Environ. Pollut. 2018, 233, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Shahbaz, M.; Liang, Y.; Yang, J.; Wang, S.; Chadwicka, D.R.; Jones, D.; Chen, J.; Ge, T. Effect of Microplastics on Organic Matter Decomposition in Paddy Soil Amended with Crop Residues and Labile C: A Three-Source-Partitioning Study. J. Hazard. Mater. 2021, 416, 126221. [Google Scholar] [CrossRef]
- Han, X.; Wang, S.; Yu, X.; Vogt, R.D.; Feng, J.; Zhai, L.; Ma, W.; Zhu, L.; Lu, X. Kinetics and Size Effects on Adsorption of Cu(II), Cr(III), and Pb(II) Onto Polyethylene, Polypropylene, and Polyethylene Terephthalate Microplastic Particles. Front. Mar. Sci. 2021, 8, 785146. [Google Scholar] [CrossRef]
- Guo, X.; Hu, G.; Fan, X.; Jia, H. Sorption Properties of Cadmium on Microplastics: The Common Practice Experiment and A Two-Dimensional Correlation Spectroscopic Study. Ecotoxicol. Environ. Saf. 2020, 190, 110118. [Google Scholar] [CrossRef]
- Vo, T.S.; Hossain, M.M.; Jeong, H.M.; Kim, K. Heavy Metal Removal Applications Using Adsorptive Membranes. Nano Converg. 2020, 7, 36. [Google Scholar] [CrossRef]
- Racho, P.; Waiwong, W. Modified Textile Waste for Heavy Metals Removal. Energy Rep. 2020, 6, 927–932. [Google Scholar] [CrossRef]
- Asaad, J.N.; Ikladious, N.E.; Awad, F.; Müller, T. Evaluation of Some New Hyperbranched Polyesters as Binding Agents for Heavy Metals. Can. J. Chem. Eng. 2013, 91, 257–263. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, M.; Wang, L.; Ke, R.; Lou, Y.; Zhang, X.; Dong, X.; Zhang, Y. Sorption Behaviors of Tris-(2,3-Dibromopropyl) Isocyanurate and Hexabromocyclododecanes on Polypropylene Microplastics. Mar. Pollut. Bull. 2018, 135, 581–586. [Google Scholar] [CrossRef]
- Bhagwat, G.; Tran, T.K.A.; Lamb, D.; Senathirajah, K.; Grainge, I.; O’Connor, W.; Juhasz, A.; Palanisami, T. Biofilms Enhance the Adsorption of Toxic Contaminants on Plastic Microfibers under Environmentally Relevant Conditions. Environ. Sci. Technol. 2021, 55, 8877–8887. [Google Scholar] [CrossRef] [PubMed]

| Microplastics Form | Polymer | Shape | Size Range |
|---|---|---|---|
| Fibers (FB) | Polyester (PEs) | Lines | 0.3 mm–3.0 mm |
| Plastic bag (PB) | Biodegradable polyethylene | Fragments with sharped edges | 0.5–4.5 mm |
| Plastic bottle (PET) | Polyethylene terephthalate PET | Squares | 1.0–5.0 mm |
| Glitter (G) | Polyethylene | Microbeads | 0.5–1.0 mm |
| Characteristics | Value |
|---|---|
| Texture | loose sand (92% sand, 5% silt, 2% clay) |
| pH in distilled water | 4.49 |
| EC * | 1.23 µS cm−1 |
| CEC | 1.04 cmol(+) kg−1 |
| TOC | 0.43% |
| TN | 0.12% |
| Total Cu | 0.82 mg kg−1 |
| Total Pb | 0.43 mg kg−1 |
| CuH20ex PbH2Oex | 0.05 mg kg−1 <0.02 mg kg−1 |
| Treatment | Cu-Spiked Soils | Pb-Spiked Soils |
|---|---|---|
| CS * | 4.49 ** ± 0.07 ***a | 4.49 ± 0.14 a |
| FBS | 5.13 ± 0.06 b | 5.09 ± 0.01 b |
| PBS | 4.89 ± 0.12 b | 4.91 ± 0.17 b |
| PETS | 4.75 ± 0.02 a | 4.79 ± 0.14 b |
| GLS | 4.82 ± 0.16 b | 4.62 ± 0.17 b |
| Treatment | Cu mg kg−1 | Pb mg kg−1 |
|---|---|---|
| CS * | 0.82 ** ± 0.10 *** a | 0.9 ± 0.06 a |
| FBS | 1.70 ± 0.06 b | 1.21 ± 0.08 b |
| PBS | 1.30 ± 0.18 b | 1.57 ± 0.12 b |
| PETS | 1.02 ± 0.22 b | 0.96 ± 0.12 b |
| GS | 1.74 ± 0.26 b | 1.68 ± 0.24 b |
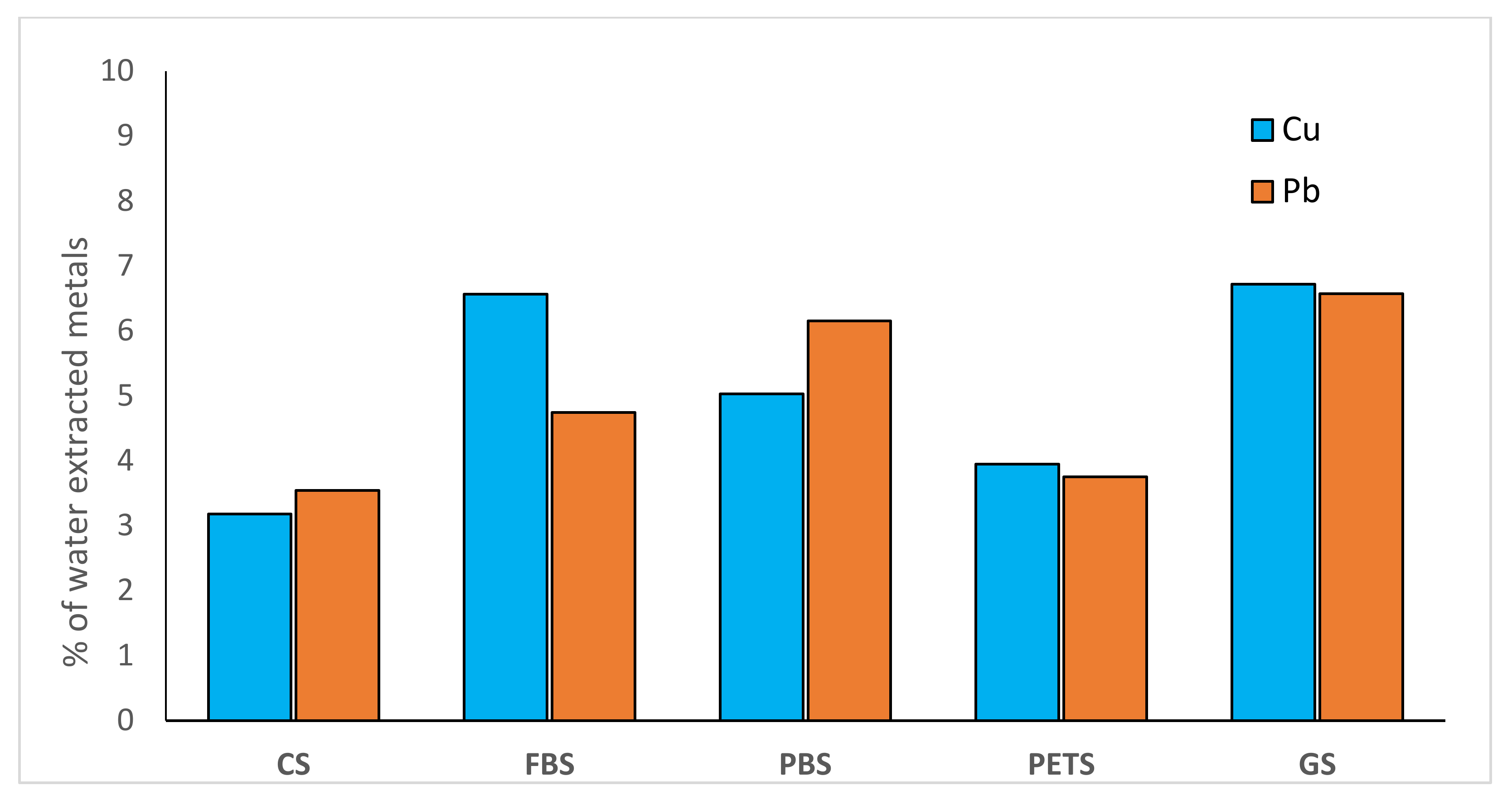
| Treatment | Cu mg kg−1 | Pb mg kg−1 |
|---|---|---|
| CS * | 1.57 ** ± 0.11 *** a | 2.62 ± 0.08 a |
| FBS | 3.63 ± 0.09 b | 3.78 ± 0.08 b |
| PBS | 2.46 ± 0.08 b | 6.92 ± 0.08 b |
| PETS | 2.74 ± 0.12 b | 3.45 ± 0.09 b |
| GS | 4.36 ± 0.06 b | 8.23 ± 0.14 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medyńska-Juraszek, A.; Jadhav, B. Influence of Different Microplastic Forms on pH and Mobility of Cu2+ and Pb2+ in Soil. Molecules 2022, 27, 1744. https://doi.org/10.3390/molecules27051744
Medyńska-Juraszek A, Jadhav B. Influence of Different Microplastic Forms on pH and Mobility of Cu2+ and Pb2+ in Soil. Molecules. 2022; 27(5):1744. https://doi.org/10.3390/molecules27051744
Chicago/Turabian StyleMedyńska-Juraszek, Agnieszka, and Bhakti Jadhav. 2022. "Influence of Different Microplastic Forms on pH and Mobility of Cu2+ and Pb2+ in Soil" Molecules 27, no. 5: 1744. https://doi.org/10.3390/molecules27051744
APA StyleMedyńska-Juraszek, A., & Jadhav, B. (2022). Influence of Different Microplastic Forms on pH and Mobility of Cu2+ and Pb2+ in Soil. Molecules, 27(5), 1744. https://doi.org/10.3390/molecules27051744







