Effect of Native Reservoir State and Oilfield Operations on Clay Mineral Surface Chemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Zeta Potential Sample Preparation
2.3. Zeta Potential () Measurement
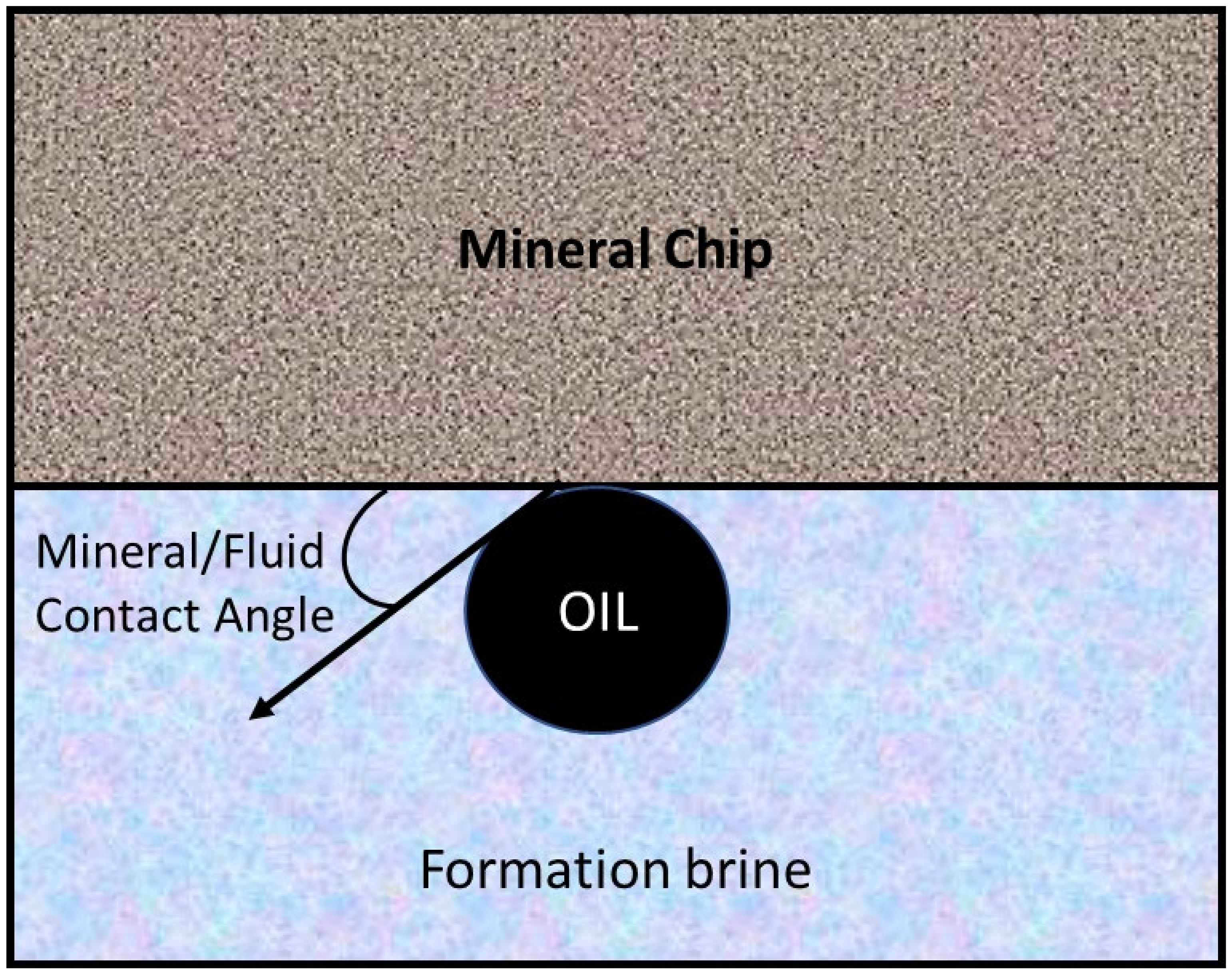
2.4. Contact Angle Measurement
3. Result and Discussions
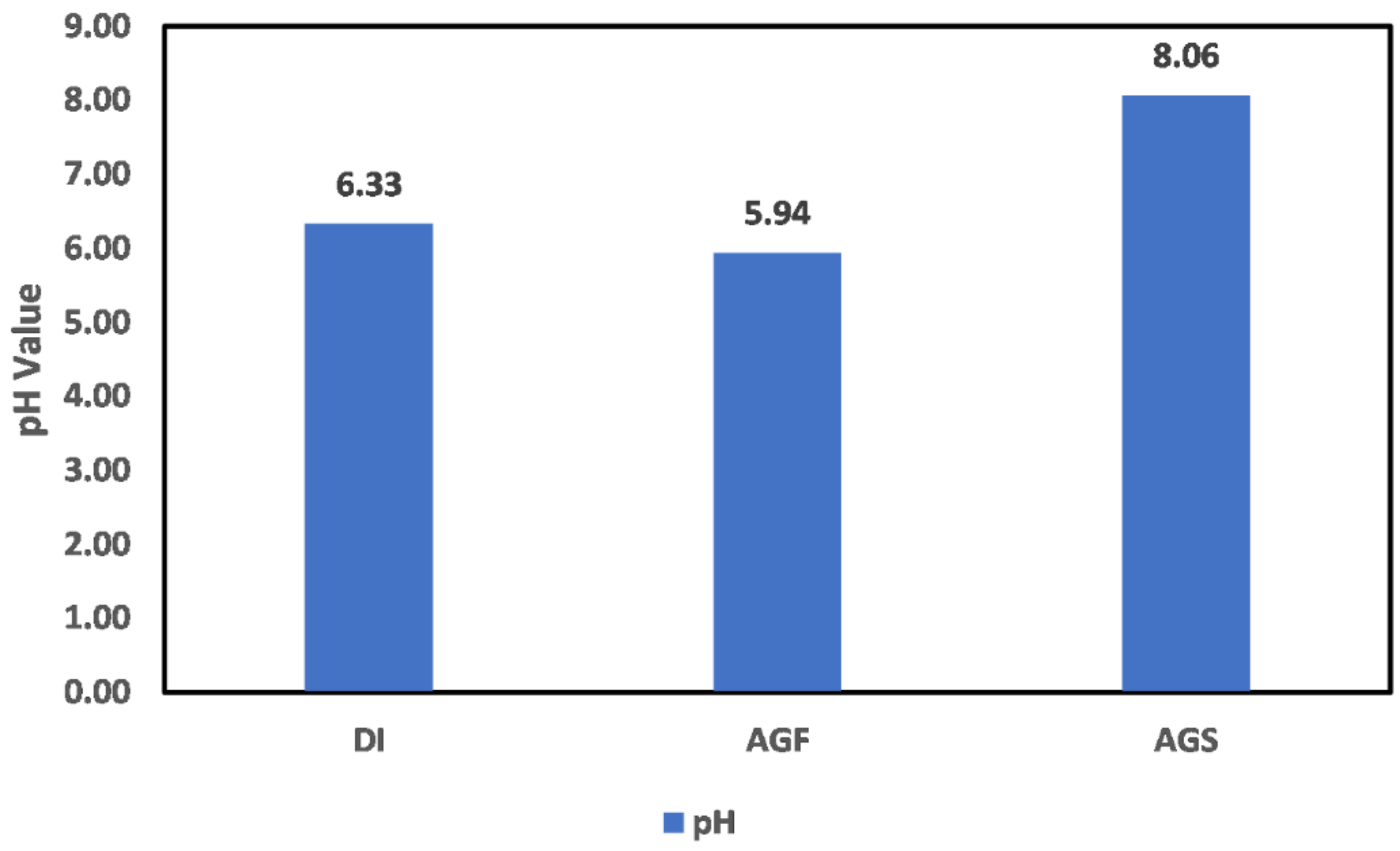
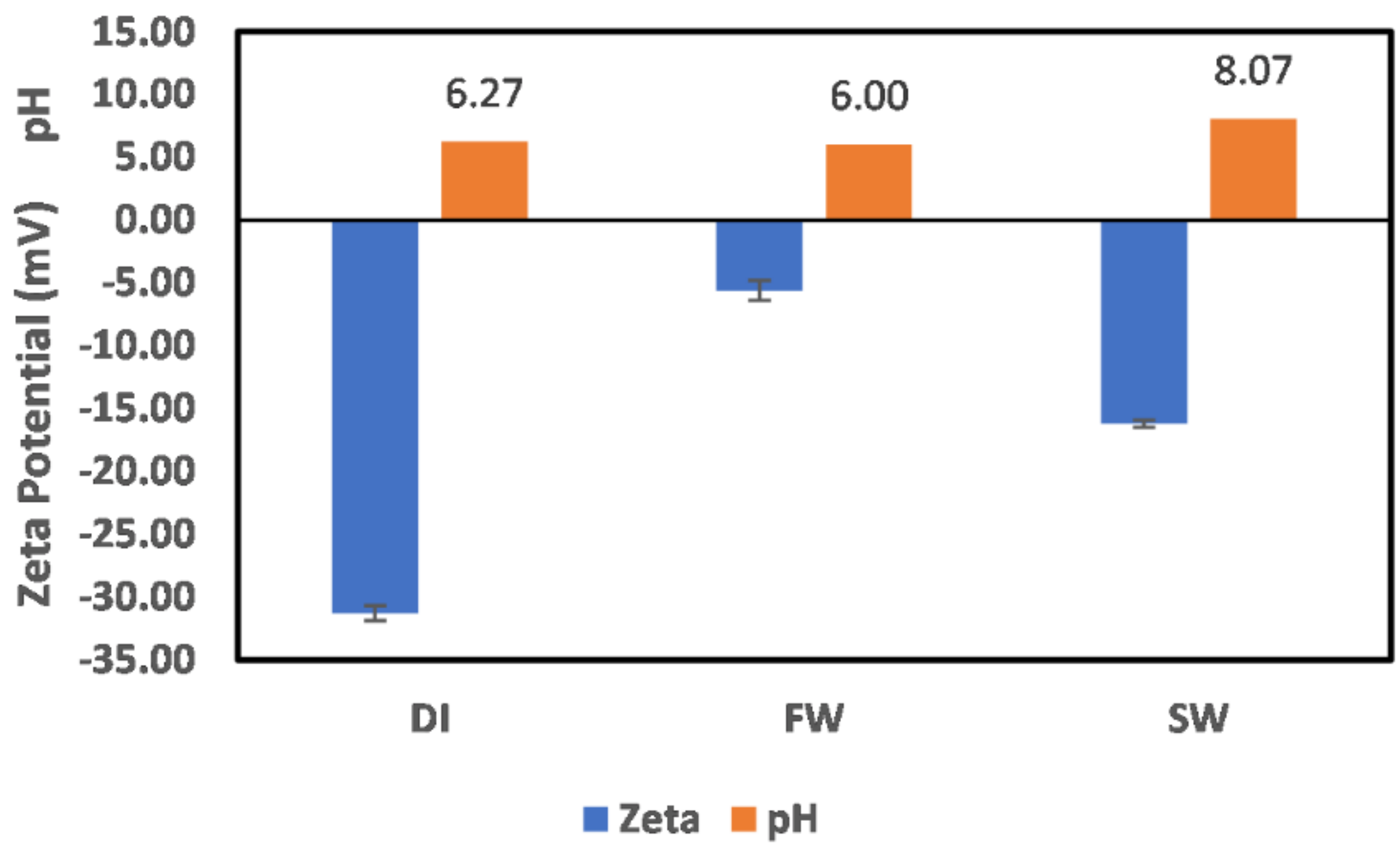
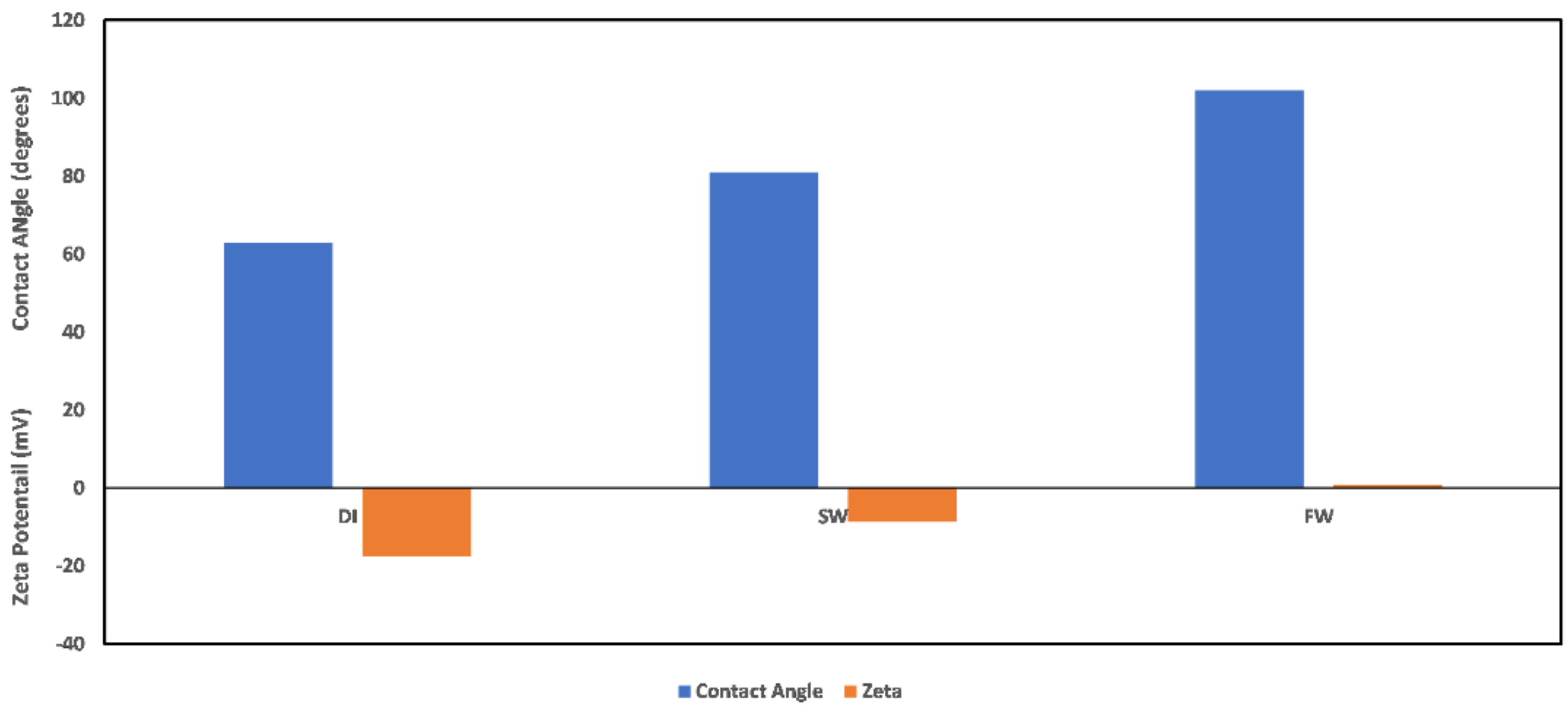
3.1. Reservoir Environment
3.1.1. Chlorite
3.1.2. Illite
3.1.3. Kaolinite
3.1.4. Montmorillonite
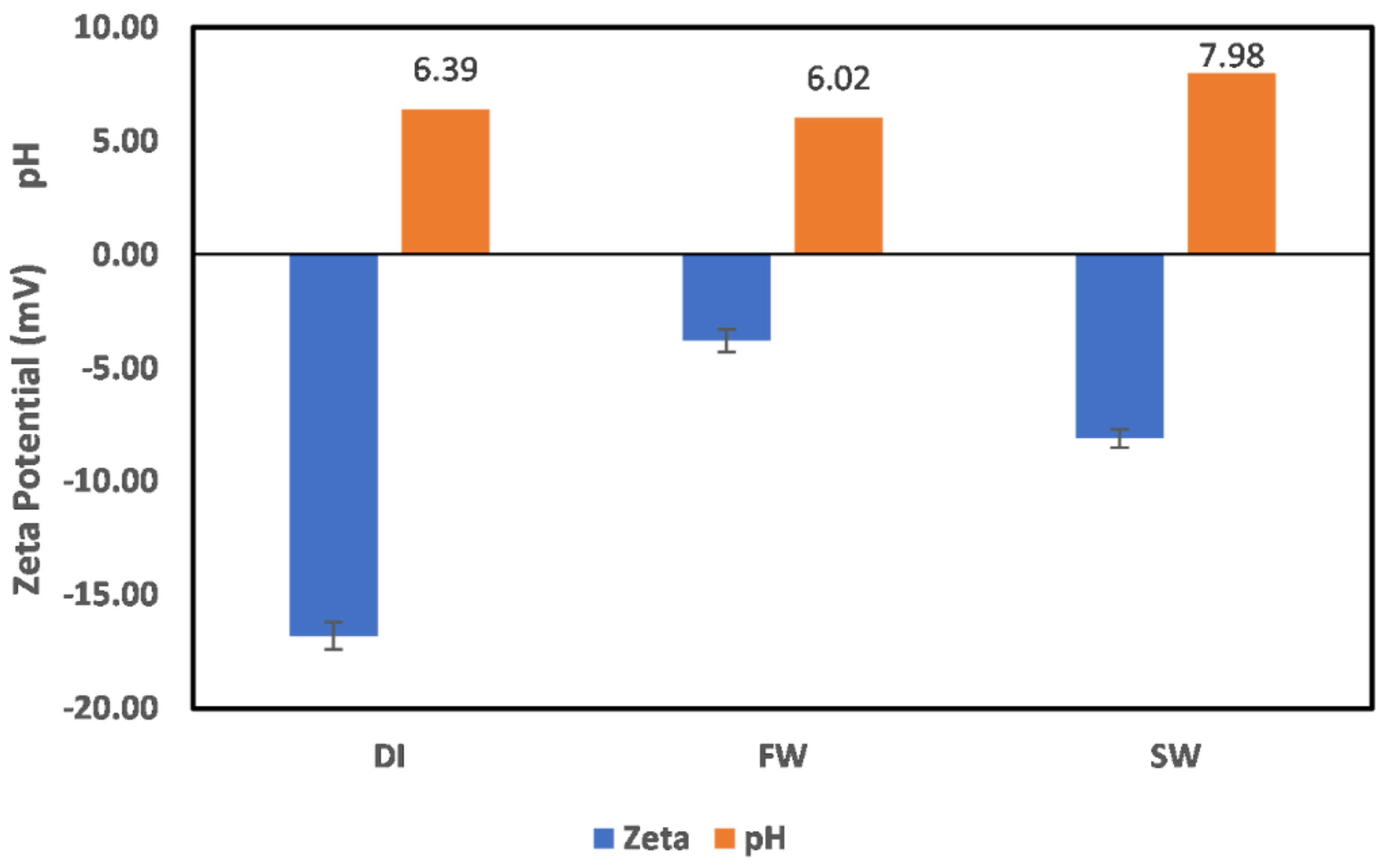
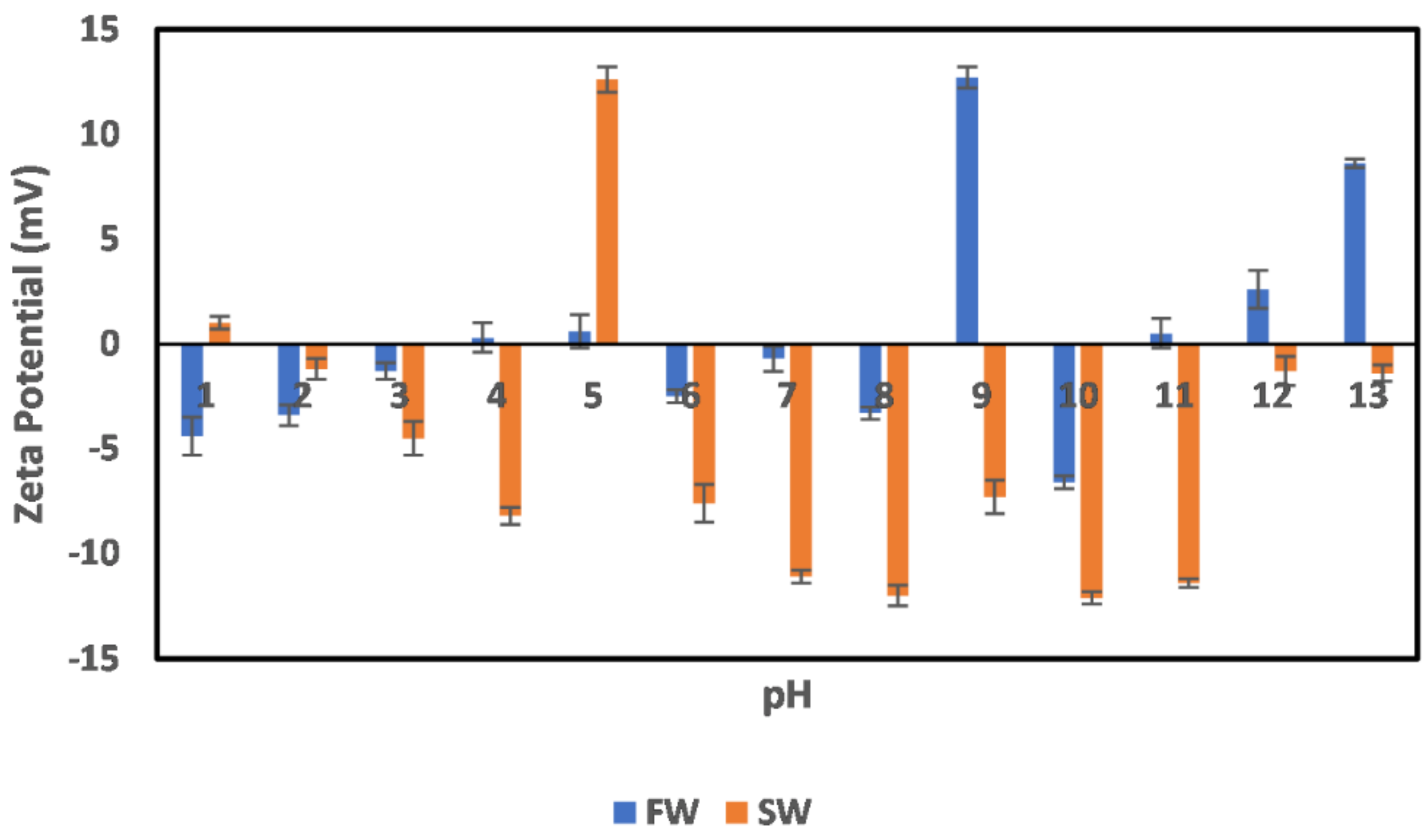
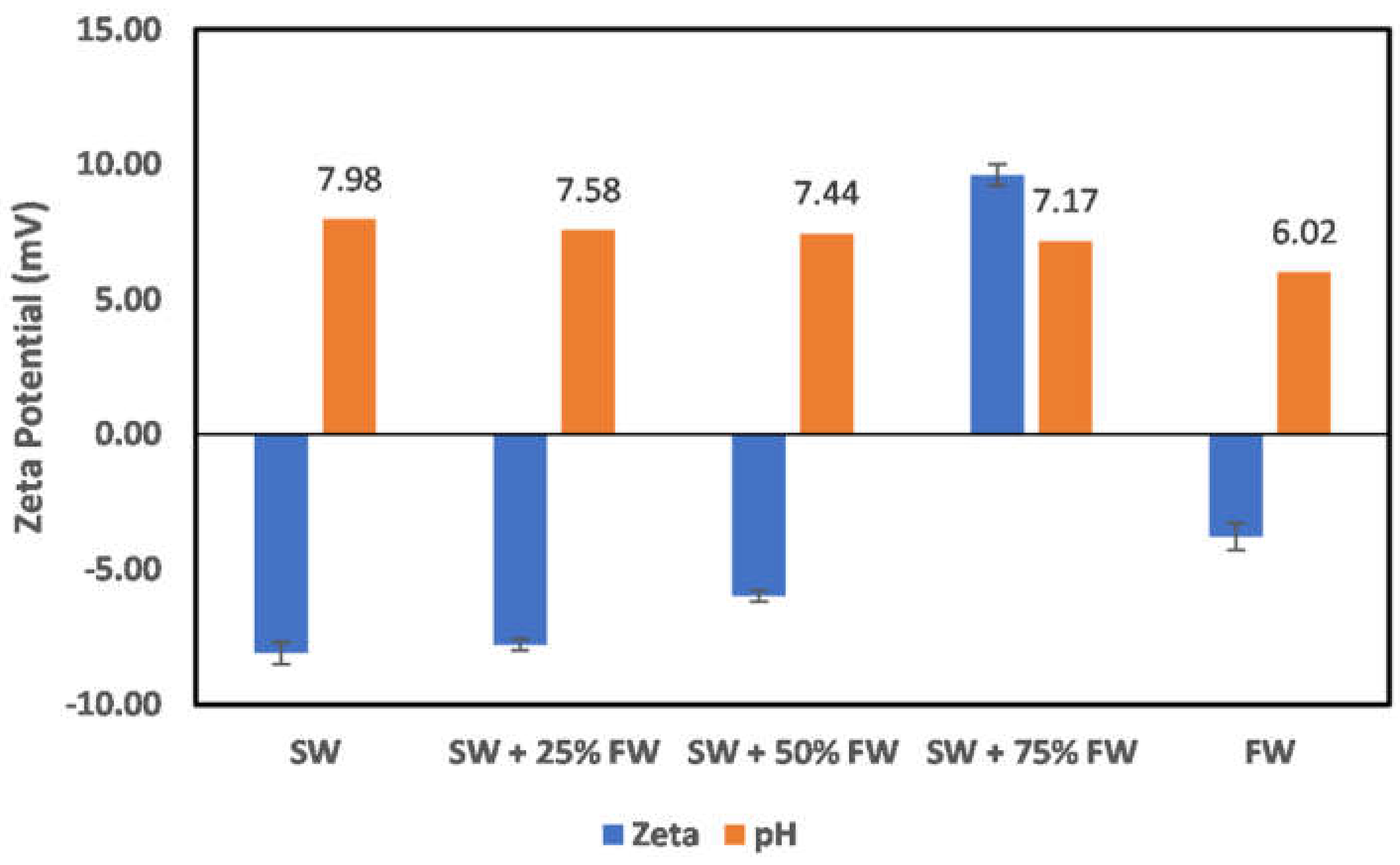
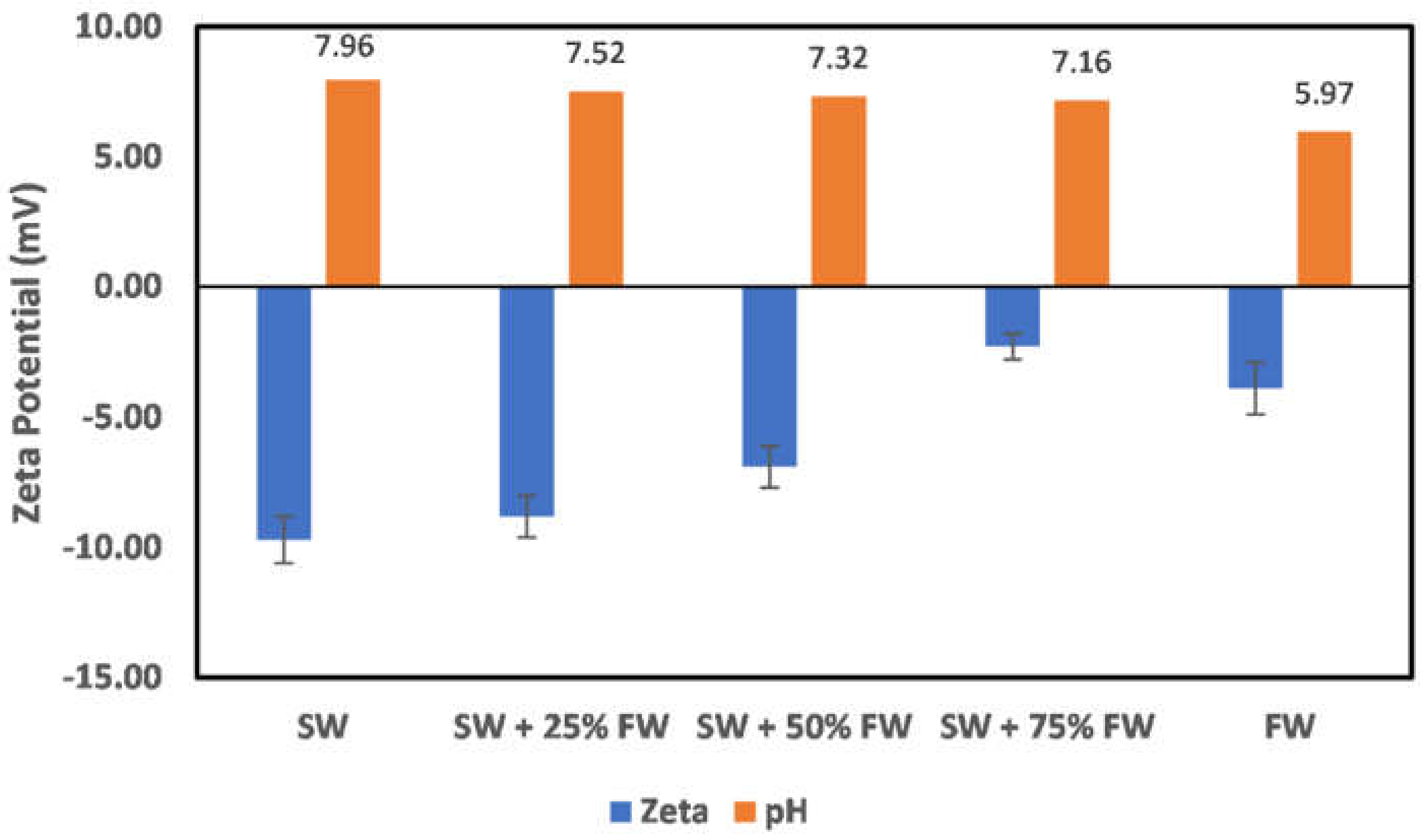
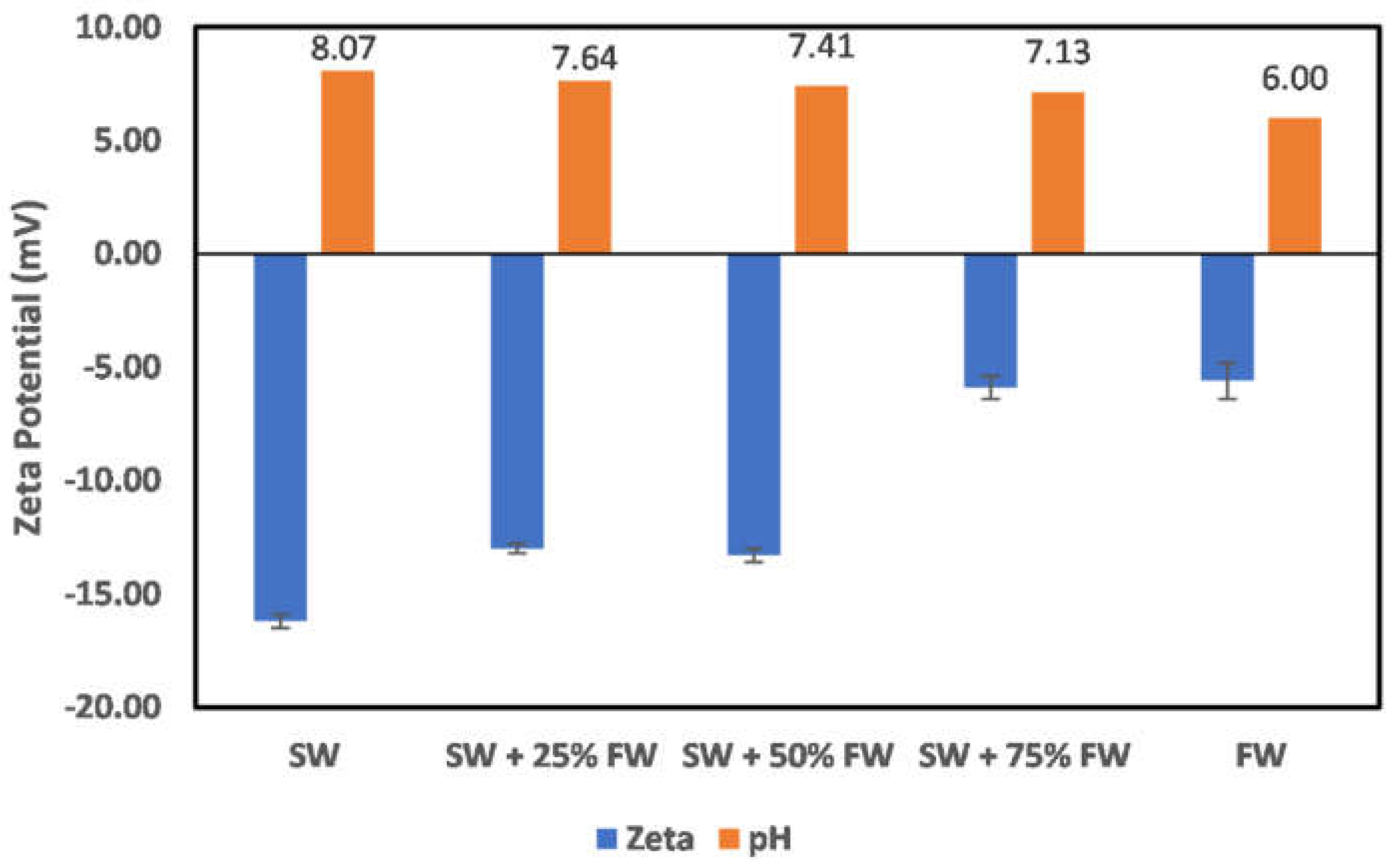
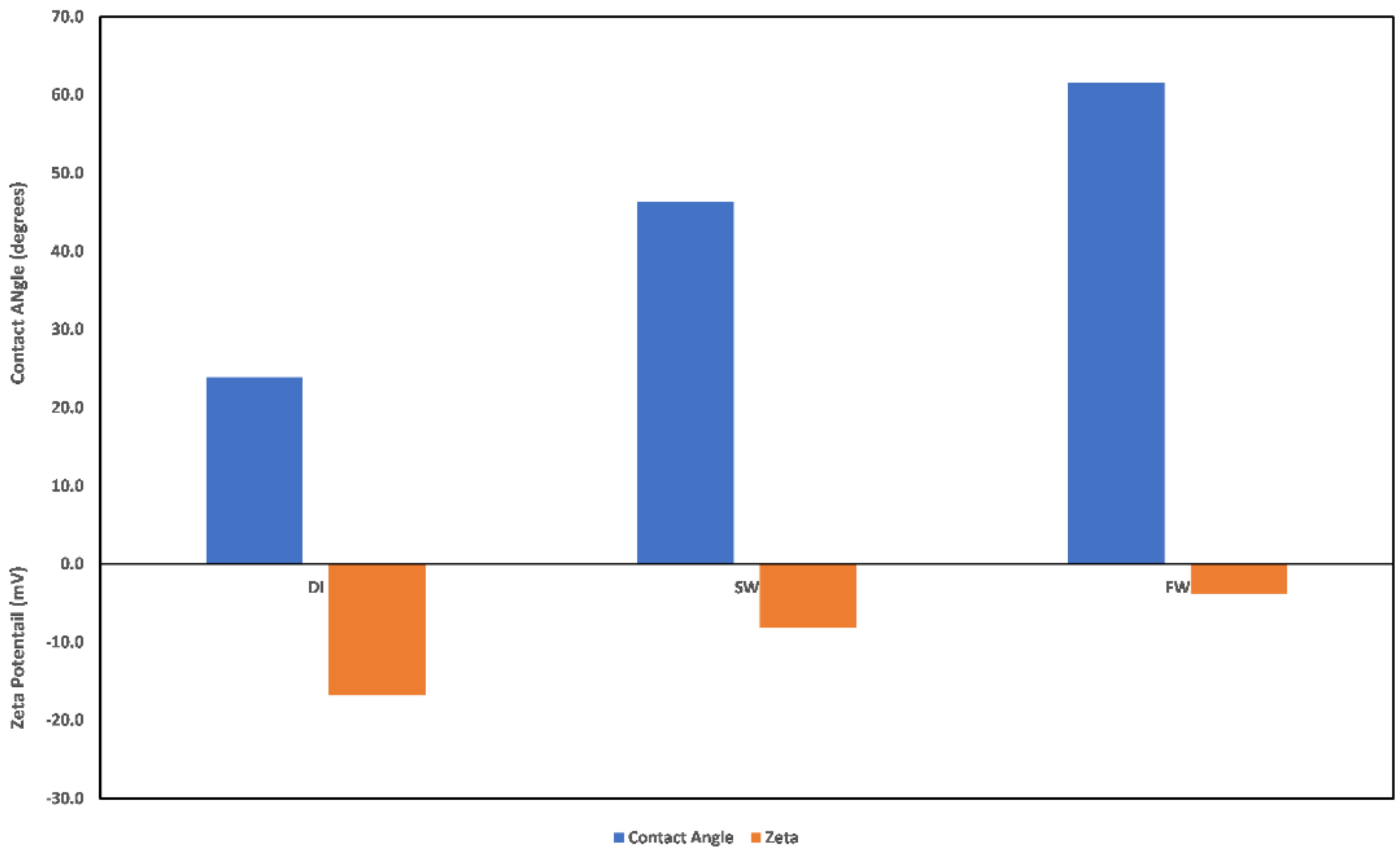
3.2. Effect of Oilfield Operations
3.2.1. Chlorite
3.2.2. Illite
3.2.3. Kaolinite
3.2.4. Montmorillonite
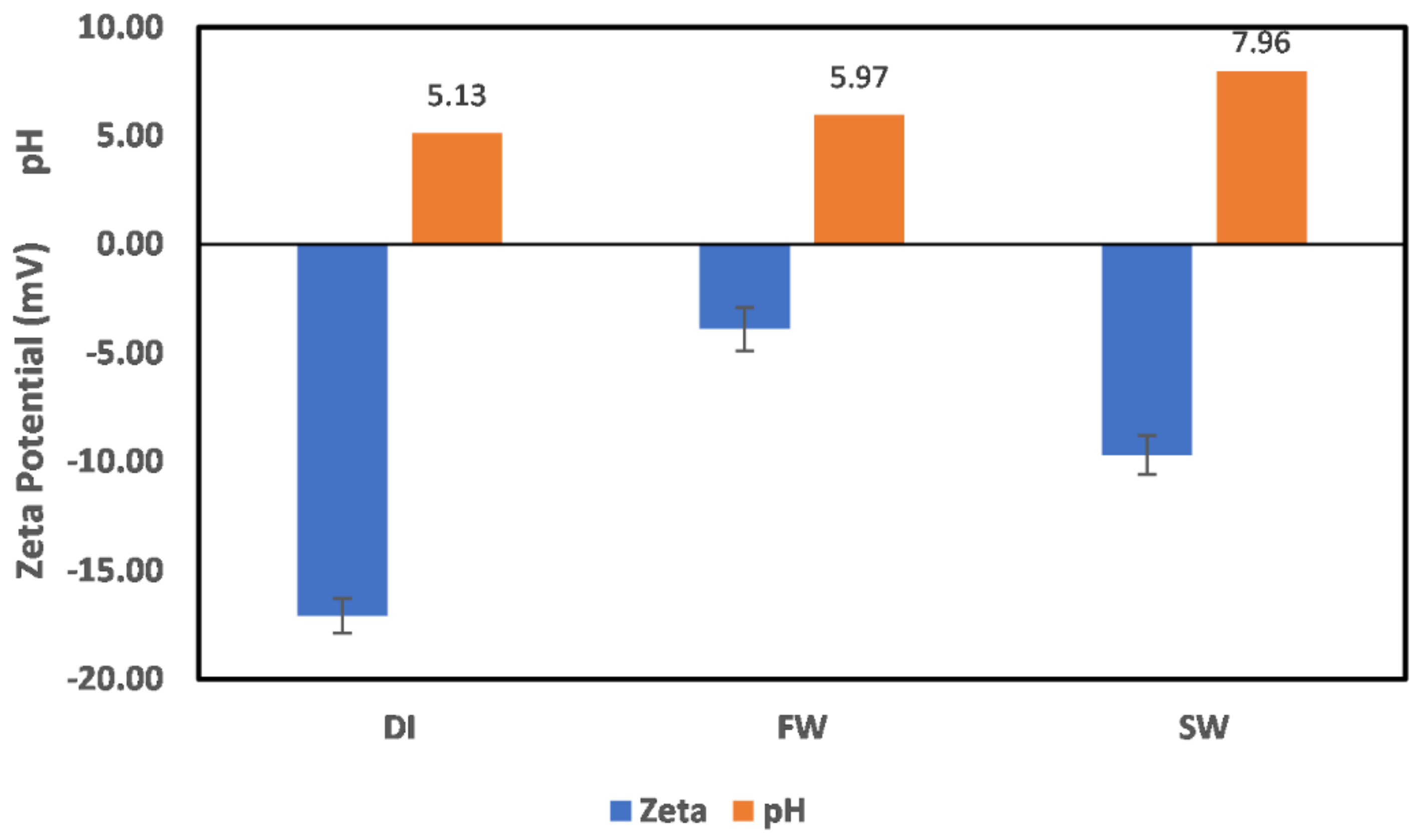
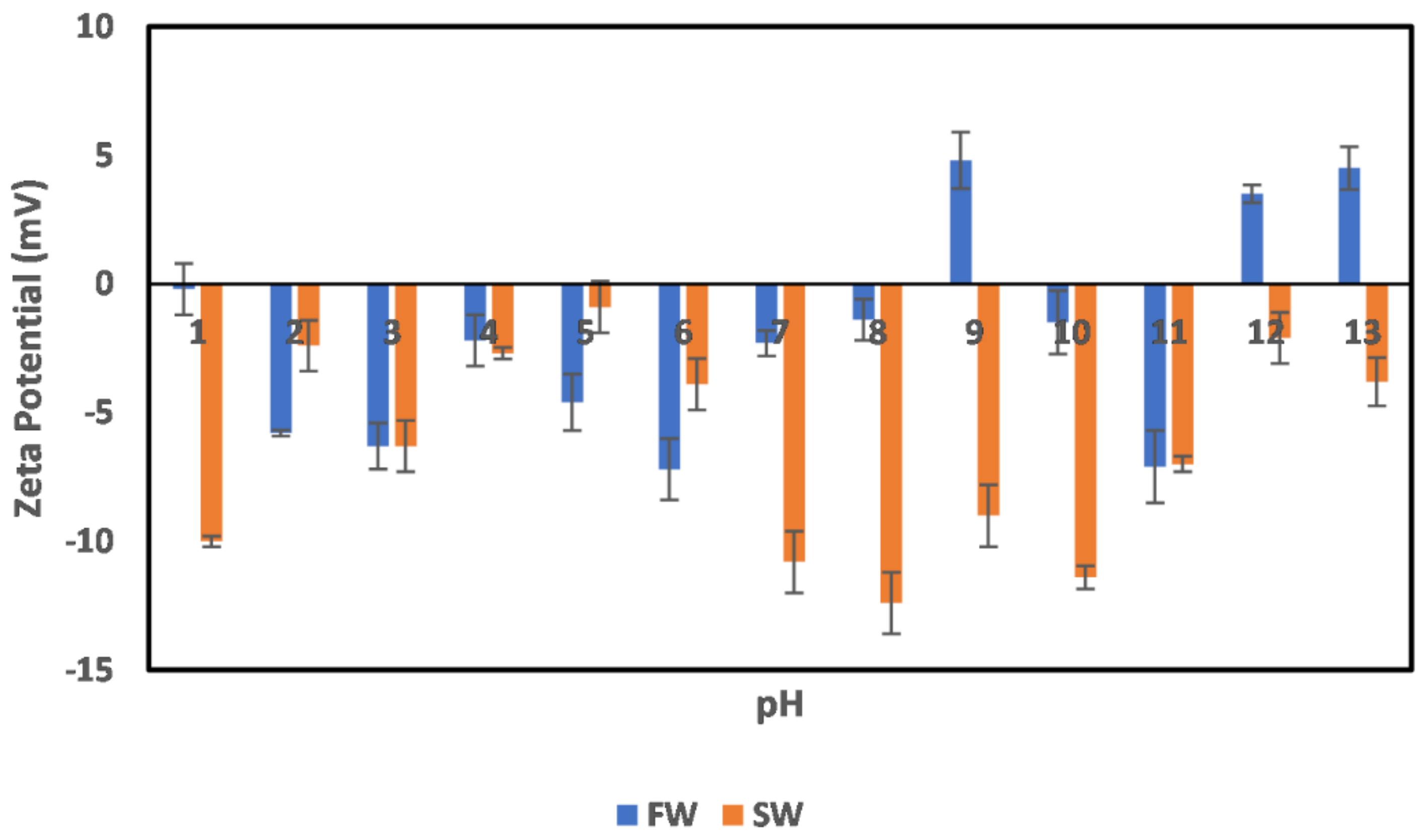
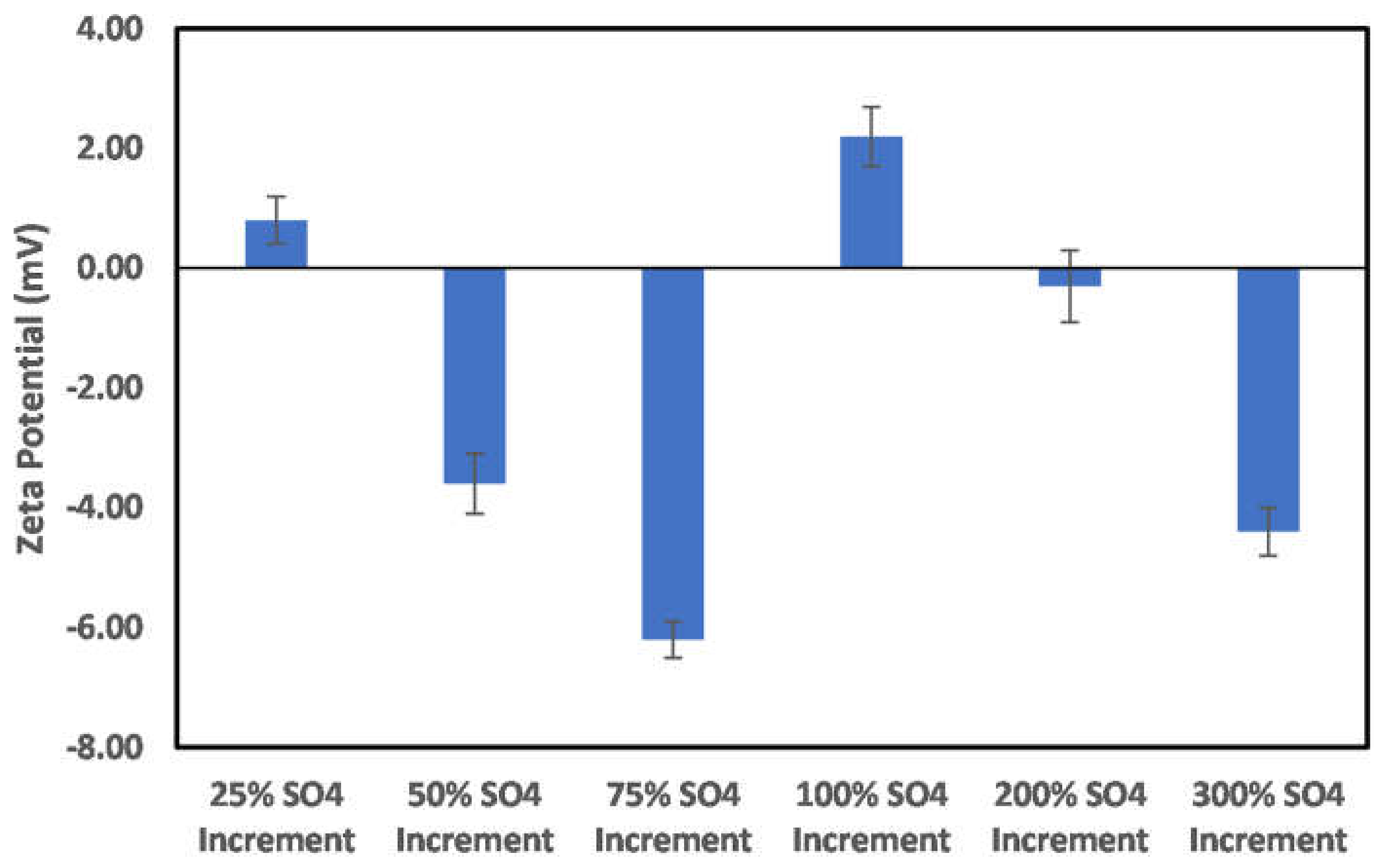
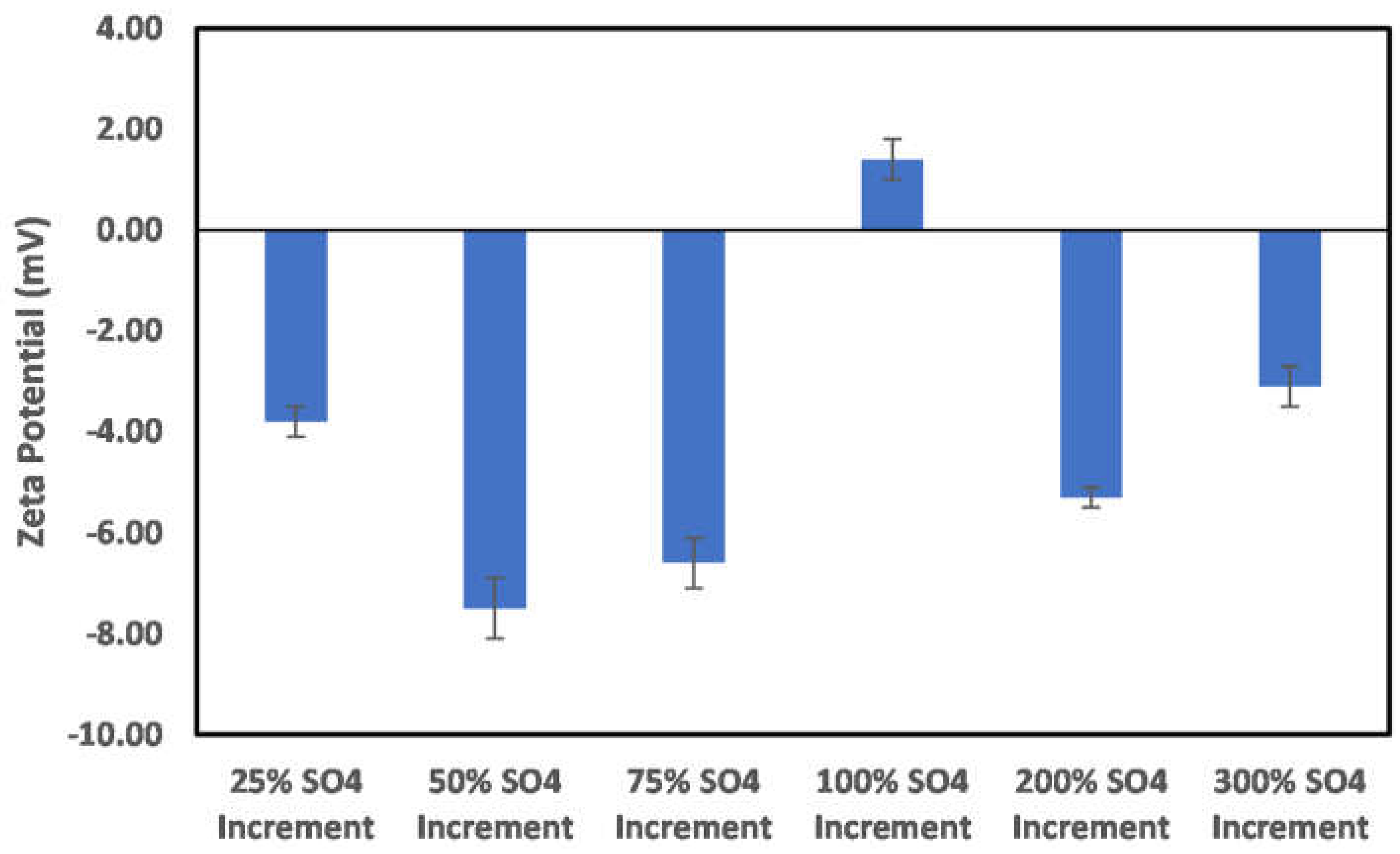
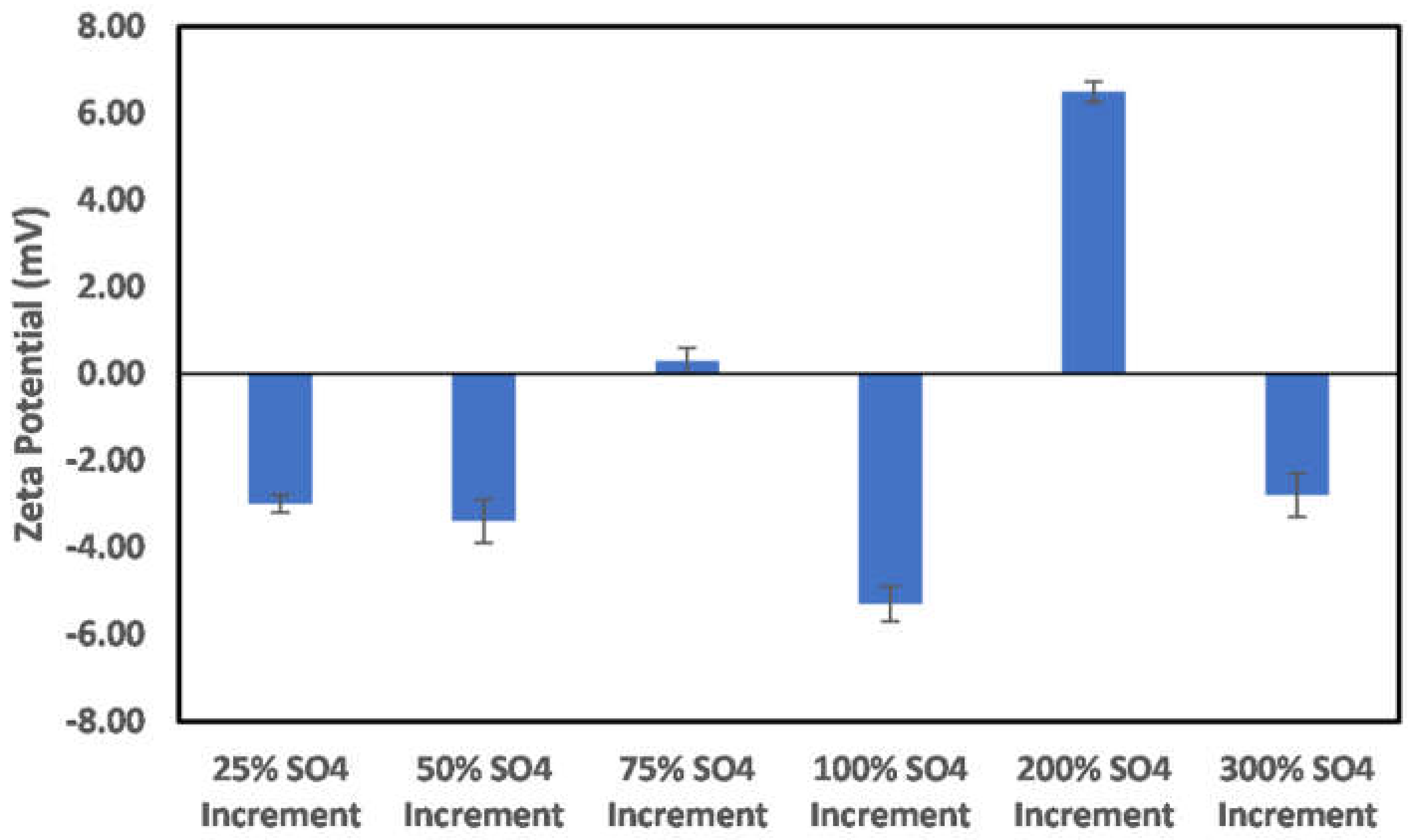
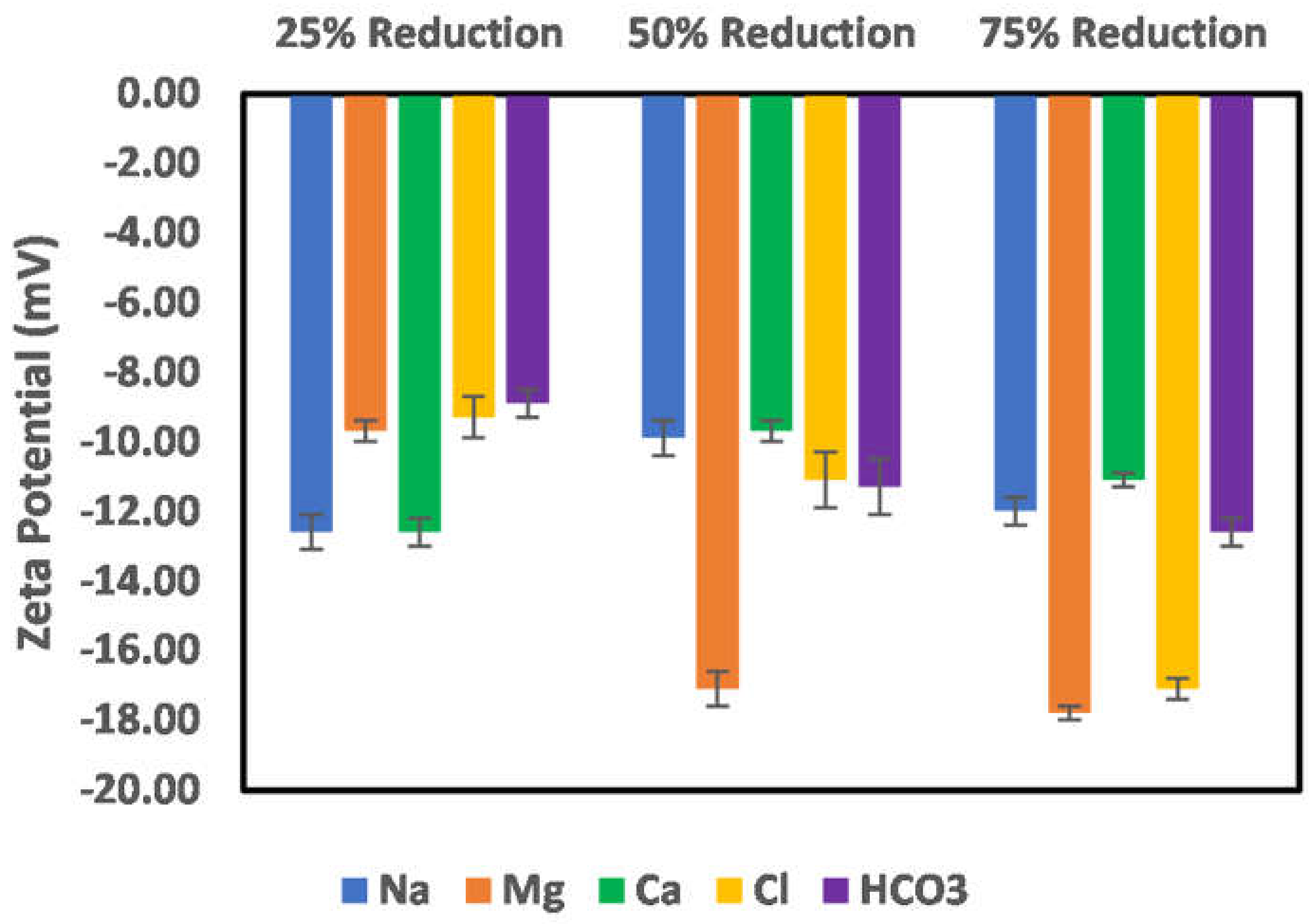
3.3. Effects of Ion Engineering
3.3.1. Chlorite
3.3.2. Illite
3.3.3. Kaolinite
3.3.4. Montmorillonite
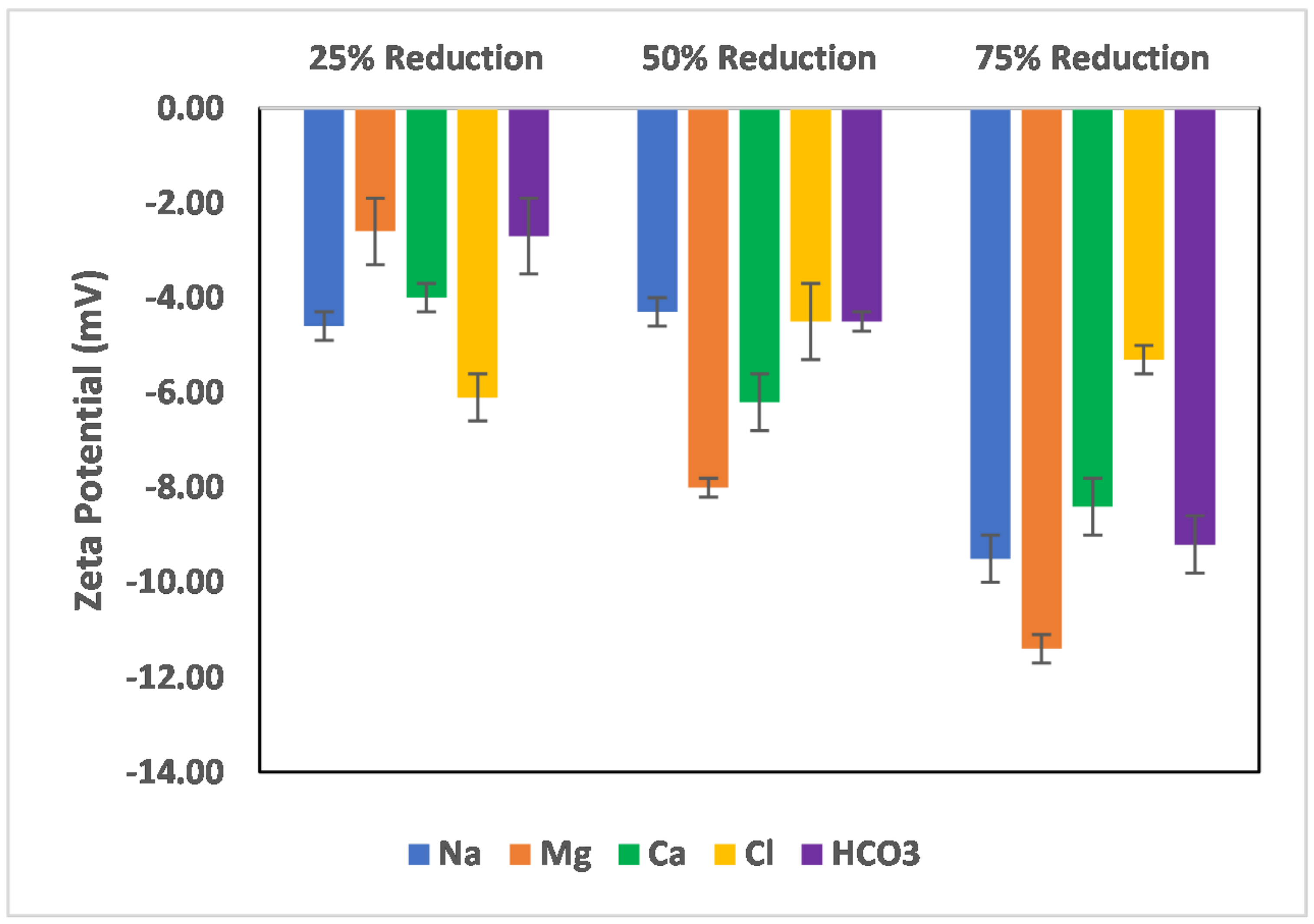
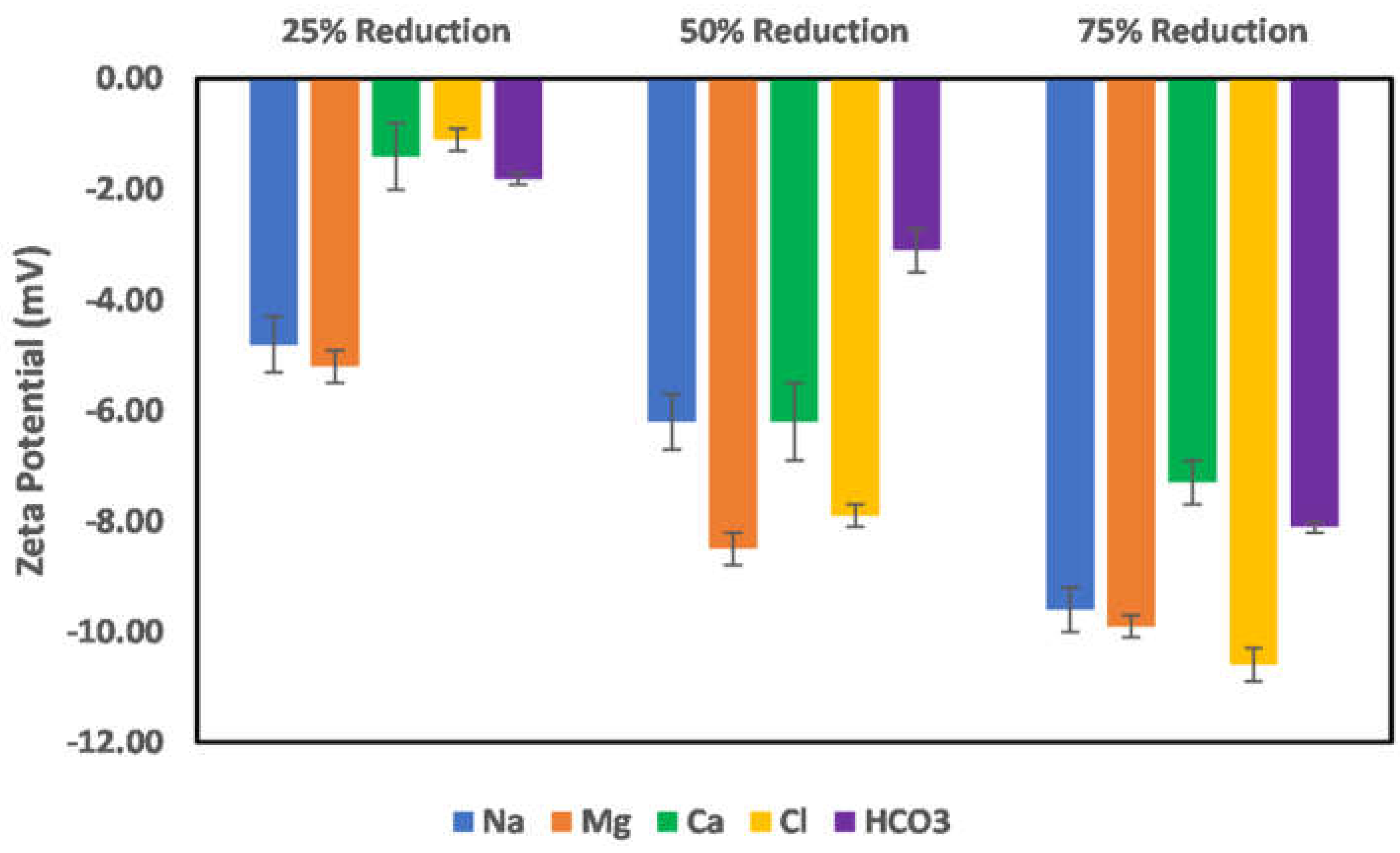
3.4. Effect of Salinity Gradient
3.5. Contact Angle Correlation
4. Conclusions
- 1.
- Clay minerals, except for in the case of chlorite in the native reservoir environment, do not provide an adsorption-prone surface to polar crude oil components due to their surface charge.
- 2.
- Oilfield operations do not impact the nature of the clay mineral surface charge; however, they affect the magnitude of the surface charge. Moreover, significant effects of the oilfield operations are dominant in the alkaline pH regions.
- 3.
- The dominant mechanisms responsible for clay mineral surface charge development are the electrical double-layer effect and ion adsorption.
- 4.
- Ion-engineered water has a significant effect on the clay mineral surface charge, especially in the case of sulfate ions.
- 5.
- The salinity gradient across the reservoir results in different clay mineral surface charges across the reservoir.
- 6.
- Increased fluid salinity results in an increase in the contact angle, which consequently leads to a less water-wet surface condition.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Isah, M.; Mukhtar, A.; Bilyaminu, S. Energy Integration of Kero Hydrotreating Unit, A Case Study of Nigerian Refinery. J. Chem. Eng. Process Technol. 2018, 9, 2. [Google Scholar] [CrossRef]
- Muhammed, N.S.; Olayiwola, T.; Elkatatny, S. A review on clay chemistry, characterization and shale inhibitors for water-based drilling fluids. J. Pet. Sci. Eng. 2021, 206, 109043. [Google Scholar] [CrossRef]
- Mohammed, I.; Al Shehri, D.A.; Mahmoud, M.; Kamal, M.S.; Alade, O. Surface Charge Investigation of Reservoir Rock Minerals. Energy Fuels 2021, 35, 6003–6021. [Google Scholar] [CrossRef]
- Mohammed, I.; Al Shehri, D.; Mahmoud, M.; Kamal, M.S.; Alade, O.S. A Surface Charge Approach to Investigating the Influence of Oil Contacting Clay Minerals on Wettability Alteration. ACS Omega 2021, 6, 12841–12852. [Google Scholar] [CrossRef]
- Mohammed, I.; Mahmoud, M.; El-Husseiny, A.; Al Shehri, D.; Al-Garadi, K.; Kamal, M.S.; Alade, O.S. Impact of Asphaltene Precipitation and Deposition on Wettability and Permeability. ACS Omega 2021, 6, 20091–20102. [Google Scholar] [CrossRef]
- Mohammed, I.; Mahmoud, M.; Al Shehri, D.; El-Husseiny, A.; Alade, O. Asphaltene precipitation and deposition: A critical review. J. Pet. Sci. Eng. 2021, 197, 107956. [Google Scholar] [CrossRef]
- Ebaga-Ololo, J.; Chon, B.H. Experimental investigation of the influence of salinity gradient on low-concentration surfactant flooding in Berea sandstone. J. Ind. Eng. Chem. 2018, 68, 355–363. [Google Scholar] [CrossRef]
- Fauziah, C.A.; Al-Yaseri, A.Z.; Beloborodov, R.; Siddiqui, M.A.Q.; Lebedev, M.; Parsons, D.; Roshan, H.; Barifcani, A.; Iglauer, S. Carbon Dioxide/Brine, Nitrogen/Brine, and Oil/Brine Wettability of Montmorillonite, Illite, and Kaolinite at Elevated Pressure and Temperature. Energy Fuels 2019, 33, 441–448. [Google Scholar] [CrossRef]
- Zeitler, T.R.; Greathouse, J.A.; Cygan, R.T.; Fredrich, J.T.; Jerauld, G.R. Molecular Dynamics Simulation of Resin Adsorption at Kaolinite Edge Sites: Effect of Surface Deprotonation on Interfacial Structure. J. Phys. Chem. C 2017, 121, 22787–22796. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Q.; Niasar, V.J. Insights into the nano-structure of oil-brine-kaolinite interfaces: Molecular dynamics and implications for enhanced oil recovery. Appl. Clay Sci. 2021, 211, 106203. [Google Scholar] [CrossRef]
- Chen, G.; Lu, S.; Zhang, J.; Xue, Q.; Han, T.; Xue, H.; Tian, S.; Li, J.; Xu, C.; Pervukhina, M.; et al. Research of CO2 and N2 Adsorption Behavior in K-Illite Slit Pores by GCMC Method. Sci. Rep. 2016, 6, 37579. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.; Myshakin, E.M. Molecular simulations of competitive adsorption of carbon dioxide—Methane mixture on illitic clay surfaces. Fluid Phase Equilib. 2018, 472, 185–195. [Google Scholar] [CrossRef]
- Loganathan, N.; Yazaydin, A.O.; Bowers, G.M.; Ngouana-Wakou, B.F.; Kalinichev, A.G.; Kirkpatrick, R.J. Role of Cations in the Methane/Carbon Dioxide Partitioning in Nano- and Mesopores of Illite Using Constant Reservoir Composition Molecular Dynamics Simulation. J. Phys. Chem. C 2020, 124, 2490–2500. [Google Scholar] [CrossRef]
- Da Silva Alvim, R.; Miranda, C.R. Noncontact AFM First-Principles Simulations of Functionalized Silicon Tips on the Montmorillonite (001) Surface. J. Phys. Chem. C 2016, 120, 13503–13513. [Google Scholar] [CrossRef]
- Peng, C.; Zhong, Y.; Min, F. Adsorption of alkylamine cations on montmorillonite (001) surface: A density functional theory study. Appl. Clay Sci. 2018, 152, 249–258. [Google Scholar] [CrossRef]
- Li, H.; Bian, L.; Dong, F.; Li, W.; Song, M.; Nie, J.; Liu, X.; Huo, T.; Zhang, H.; Xu, B.; et al. DFT and 2D-CA methods unravelling the mechanism of interfacial interaction between amino acids and Ca-montmorillonite. Appl. Clay Sci. 2019, 183, 105356. [Google Scholar] [CrossRef]
- Ulian, G.; Moro, D.; Valdrè, G. DFT Simulation of the Water Molecule Interaction with the (00l) Surface of Montmorillonite. Minerals 2021, 11, 501. [Google Scholar] [CrossRef]
- Abdel-Azeim, S.; Sakthivel, S.; Kandiel, T.A.; Kanj, M.Y. Specificity and Synergy at the Oil–Brine Interface: New Insights from Experiments and Molecular Dynamics Simulations. Energy Fuels 2021, 35, 14647–14657. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S. Demulsification of water-in-oil emulsions using ionic liquids: Effects of counterion and water type. J. Mol. Liq. 2019, 279, 411–419. [Google Scholar] [CrossRef]
- Bourdelle, F.; Dubois, M.; Lloret, E.; Durand, C.; Addad, A.; Bounoua, S.; Ventalon, S.; Recourt, P. Kaolinite-to-Chlorite Conversion from Si,Al-Rich Fluid-Origin Veins/Fe-Rich Carboniferous Shale Interaction. Minerals 2021, 11, 804. [Google Scholar] [CrossRef]
- Lee, S.Y.; Webb, K.J.; Collins, I.R.; Lager, A.; Clarke, S.M.; O’Sullivan, M.; Routh, A.F.; Wang, X. Low salinity oil recovery-increasing understanding of the underlying mechanisms. In Proceedings of the SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 24–28 April 2010; pp. 534–544. [Google Scholar]
- Alfazazi, U.; AlAmeri, W.; Hashmet, M.R. Experimental investigation of polymer flooding with low-salinity preconditioning of high temperature–high-salinity carbonate reservoir. J. Pet. Explor. Prod. Technol. 2019, 9, 1517–1530. [Google Scholar] [CrossRef] [Green Version]
- Mokhtari, R.; Anabaraonye, B.U.; Afrough, A.; Mohammadkhani, S.; Feilberg, K.L. Experimental investigation of low salinity water-flooding in tight chalk oil reservoirs. J. Pet. Sci. Eng. 2022, 208, 109282. [Google Scholar] [CrossRef]
- Derkani, M.; Fletcher, A.; Abdallah, W.; Sauerer, B.; Anderson, J.; Zhang, Z. Low Salinity Waterflooding in Carbonate Reservoirs: Review of Interfacial Mechanisms. Colloids Interfaces 2018, 2, 20. [Google Scholar] [CrossRef] [Green Version]
- Darvish Sarvestani, A.; Rostami, B.; Mahani, H. Polymer-Enhanced Low-Salinity Brine to Control In Situ Mixing and Salt Dispersion in Low-Salinity Waterflooding. Energy Fuels 2021, 35, 10540–10550. [Google Scholar] [CrossRef]
- Shiran, B.S.; Skauge, A. Wettability and oil recovery by low salinity injection. In Proceedings of the SPE EOR Conference at Oil and Gas West Asia, Muscat, Oman, 16–18 April 2012; Volume 2, pp. 887–901. [Google Scholar] [CrossRef] [Green Version]
- Myint, P.C.; Firoozabadi, A. Thin liquid films in improved oil recovery from low-salinity brine. Curr. Opin. Colloid Interface Sci. 2015, 20, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Kashiri, R.; Kalantariasl, A.; Parsaei, R.; Zeinijahromi, A. Experimental Study of the Effect of Clay and Oil Polarity on Oil Recovery by Low Salinity Water Flooding Using Glass Micromodel. Nat. Resour. Res. 2021, 30, 3695–3716. [Google Scholar] [CrossRef]
- El-Din Mahmoud, M.A. Effect of Chlorite Clay-Mineral Dissolution on the Improved Oil Recovery From Sandstone Rocks During Diethylenetriaminepentaacetic Acid Chelating-Agent Flooding. SPE J. 2018, 23, 1880–1898. [Google Scholar] [CrossRef]






| Ions | Formation Water Ions (ppm) | Seawater Ions (ppm) |
|---|---|---|
| Na+ | 59,491 | 18,300 |
| Ca2+ | 19,040 | 650 |
| Mg2+ | 2439 | 2110 |
| SO42− | 350 | 4290 |
| Cl− | 132,060 | 32,200 |
| HCO3− | 354 | 120 |
| TDS | 213,734 | 57,670 |
| Physical Characterization/Mass Percentage | Value |
|---|---|
| API gravity at 15 °C | 32.49 |
| API specific gravity at 15 °C | 0.863 |
| Density (g/cm3) at 15 °C | 0.8620 |
| Viscosity (mPa·s) at 15 °C | 10.9 |
| SARA fractions: | |
| Saturates (wt.%) | 36.2 |
| Aromatic (wt.%) | 50 |
| Resins (wt.%) | 11 |
| Asphaltenes (wt.%) | 2.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, I.; Al Shehri, D.; Mahmoud, M.; Kamal, M.S.; Alade, O.; Arif, M.; Patil, S. Effect of Native Reservoir State and Oilfield Operations on Clay Mineral Surface Chemistry. Molecules 2022, 27, 1739. https://doi.org/10.3390/molecules27051739
Mohammed I, Al Shehri D, Mahmoud M, Kamal MS, Alade O, Arif M, Patil S. Effect of Native Reservoir State and Oilfield Operations on Clay Mineral Surface Chemistry. Molecules. 2022; 27(5):1739. https://doi.org/10.3390/molecules27051739
Chicago/Turabian StyleMohammed, Isah, Dhafer Al Shehri, Mohamed Mahmoud, Muhammad Shahzad Kamal, Olalekan Alade, Muhammad Arif, and Shirish Patil. 2022. "Effect of Native Reservoir State and Oilfield Operations on Clay Mineral Surface Chemistry" Molecules 27, no. 5: 1739. https://doi.org/10.3390/molecules27051739
APA StyleMohammed, I., Al Shehri, D., Mahmoud, M., Kamal, M. S., Alade, O., Arif, M., & Patil, S. (2022). Effect of Native Reservoir State and Oilfield Operations on Clay Mineral Surface Chemistry. Molecules, 27(5), 1739. https://doi.org/10.3390/molecules27051739








