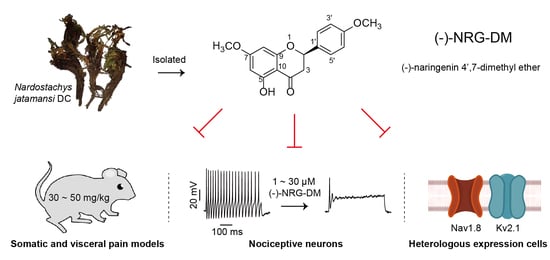
(−)-Naringenin 4′,7-dimethyl Ether Isolated from Nardostachys jatamansi Relieves Pain through Inhibition of Multiple Channels
Abstract
:1. Introduction
2. Results
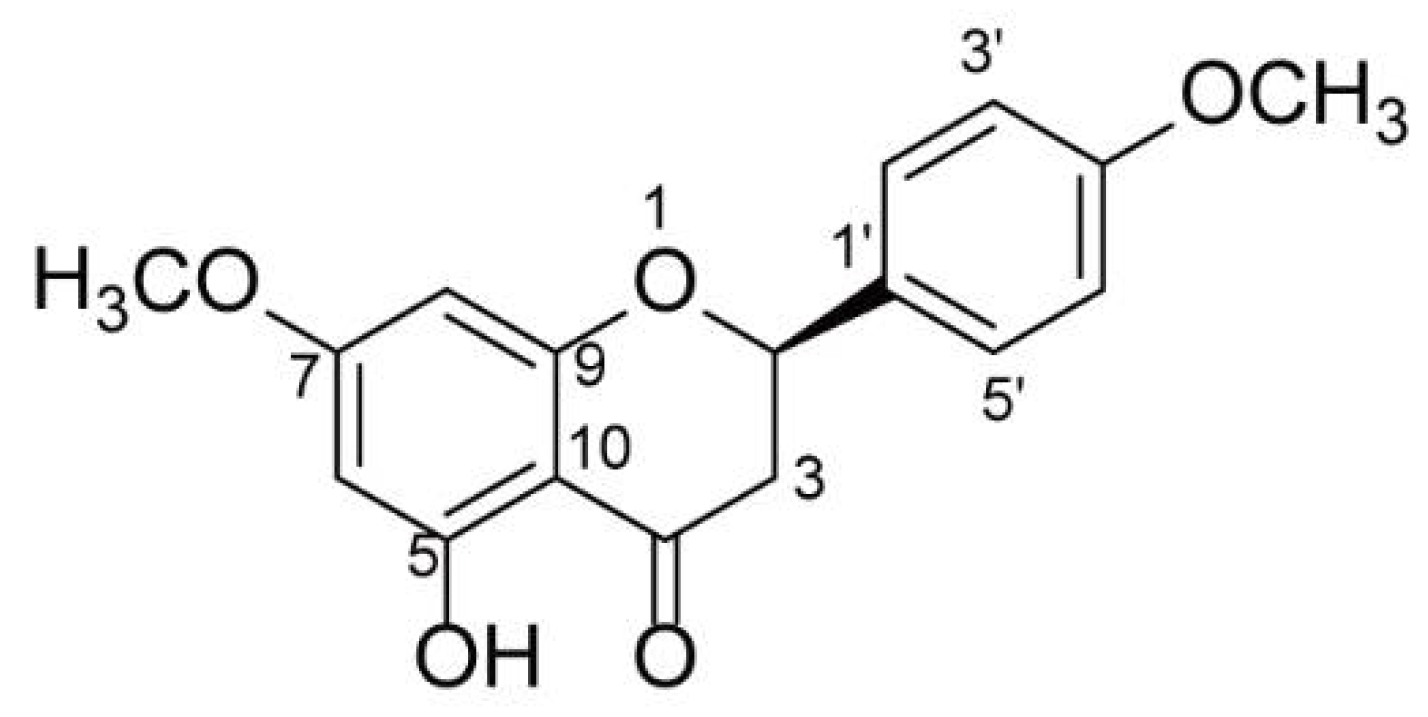
2.1. Structure Elucidation of (−)-NRG-DM
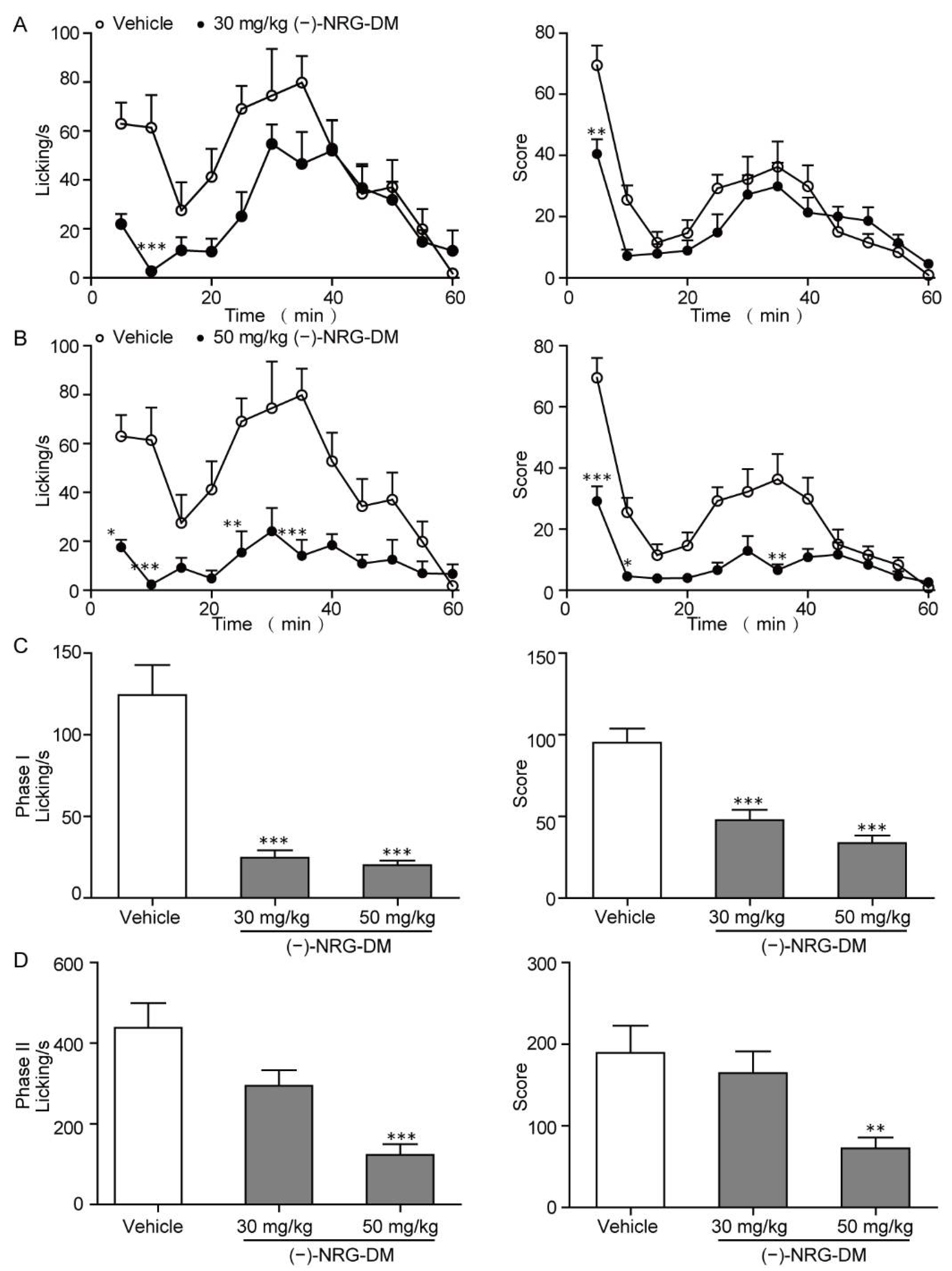
2.2. Analgesic Effects of (−)-NRG-DM in Formalin-Induced Mouse Inflammatory Pain Model
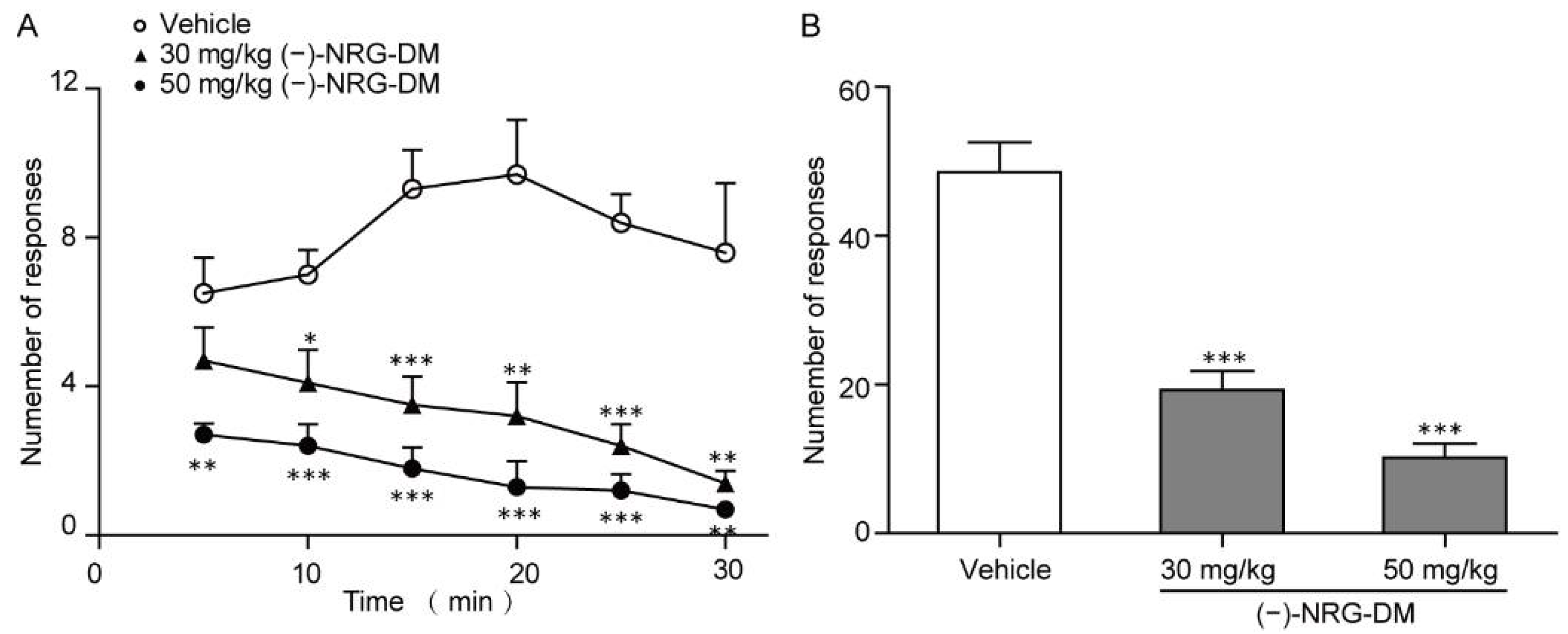
2.3. Analgesic Effects of (−)-NRG-DM in Mustard Oil-Induced Mouse Colorectal Pain Model
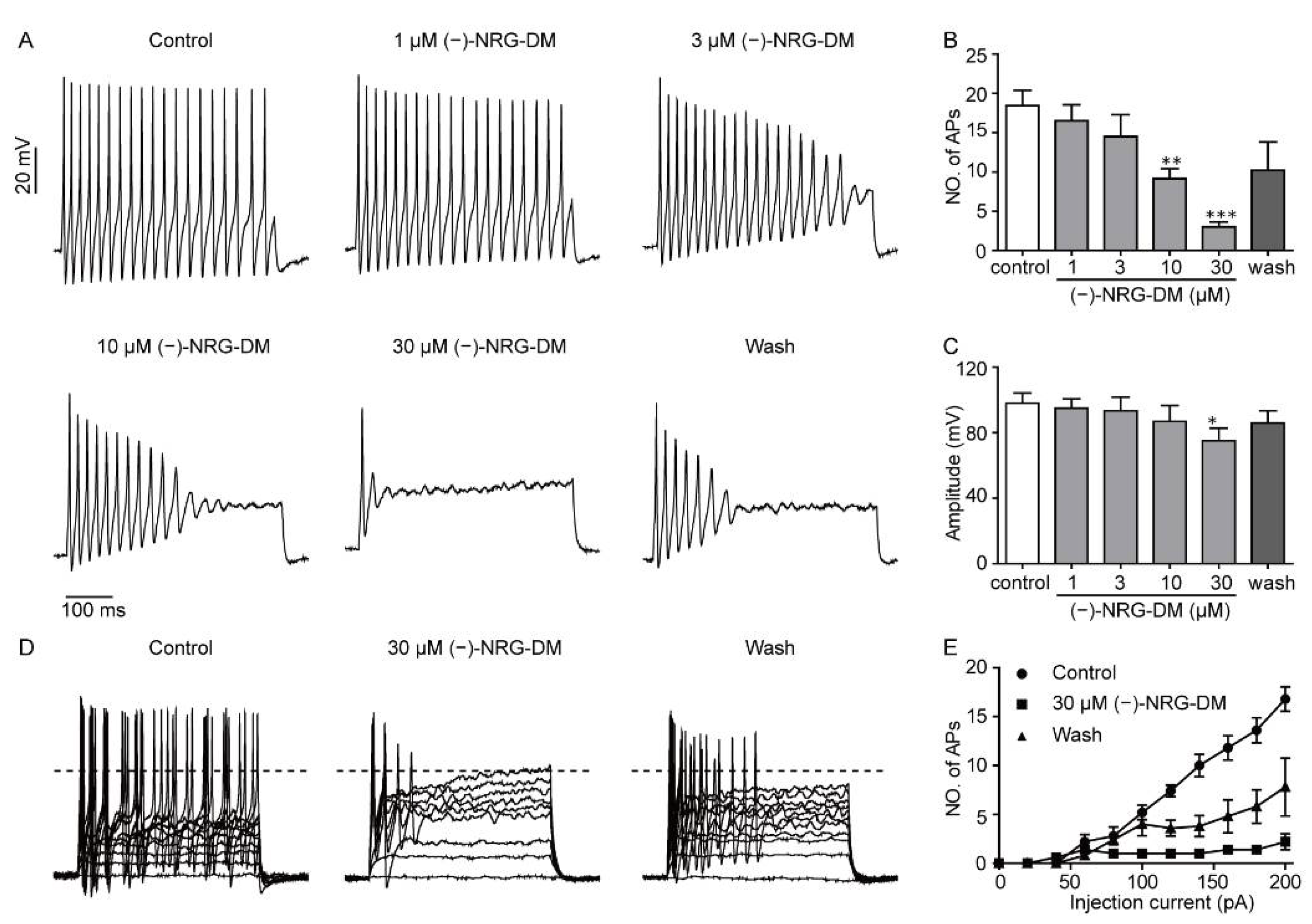
2.4. Inhibitory Effects of (−)-NRG-DM on Action Potential Firing in Mouse DRG Neurons
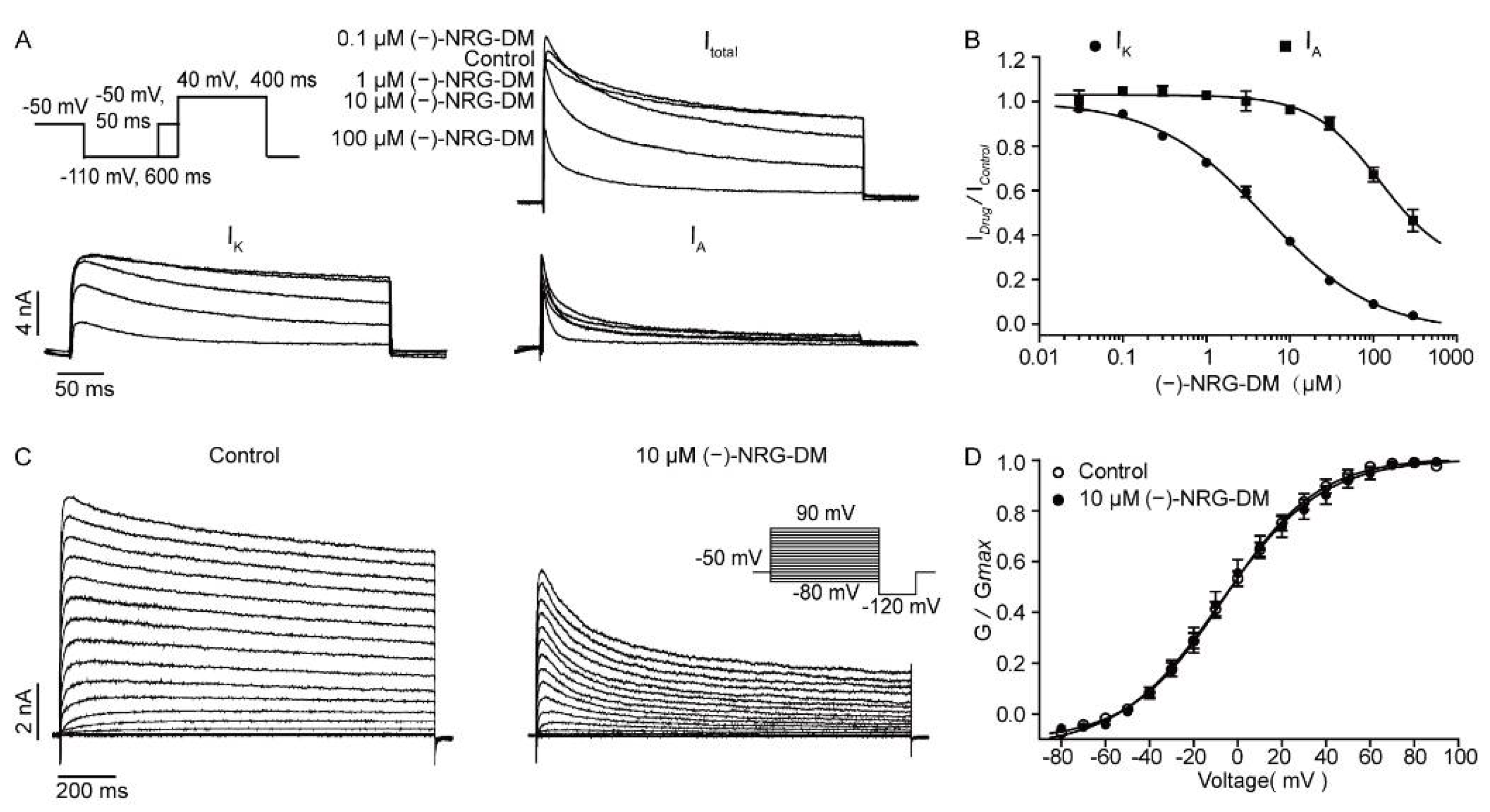
2.5. Inhibitory Effects of (−)-NRG-DM on Neuronal Potassium Currents
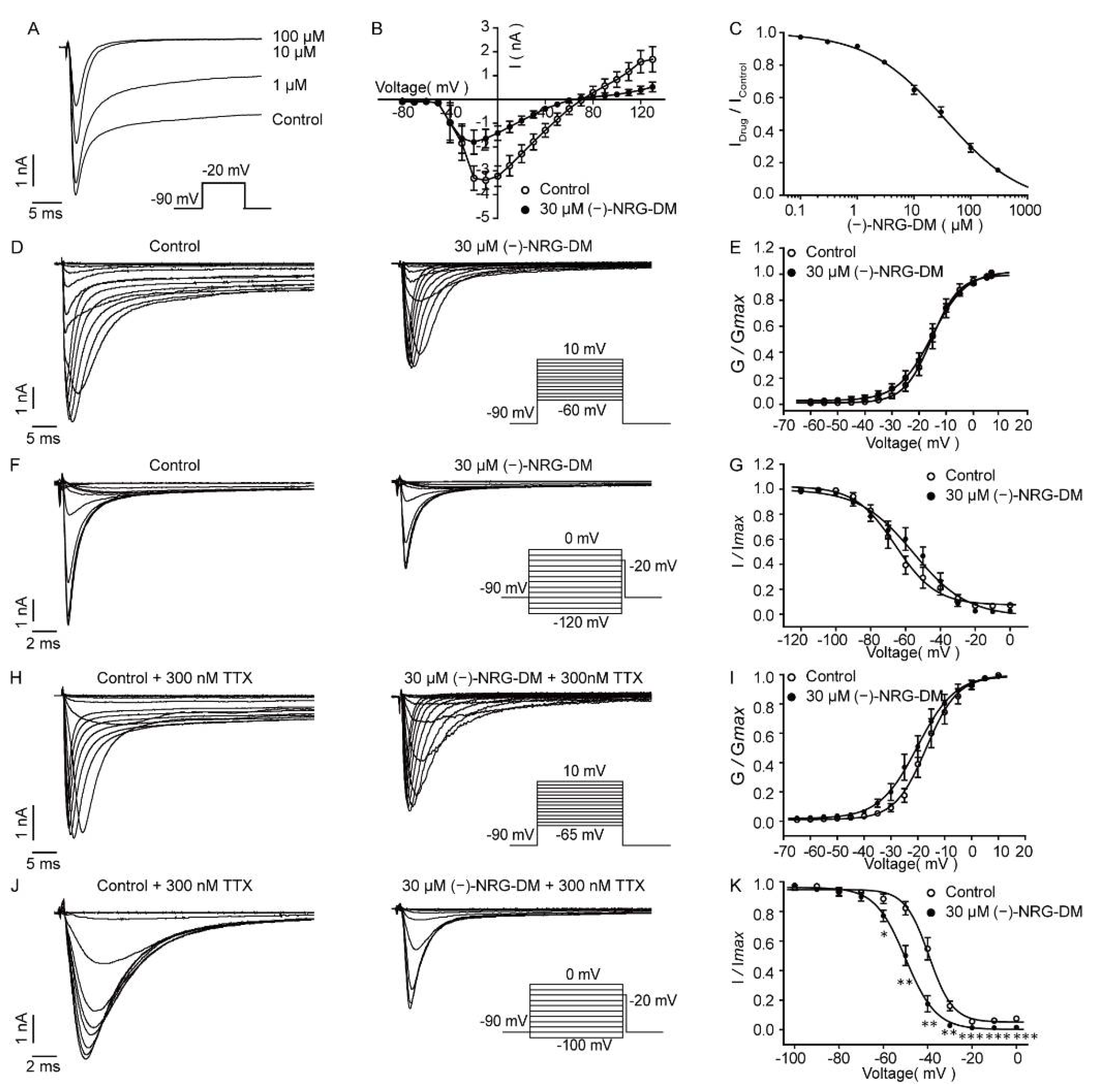
2.6. Inhibition of (−)-NRG-DM on Neuronal Sodium Currents
2.7. Inhibitory Effects of (−)-NRG-DM on Heterologously Expressed Kv2.1 Channel
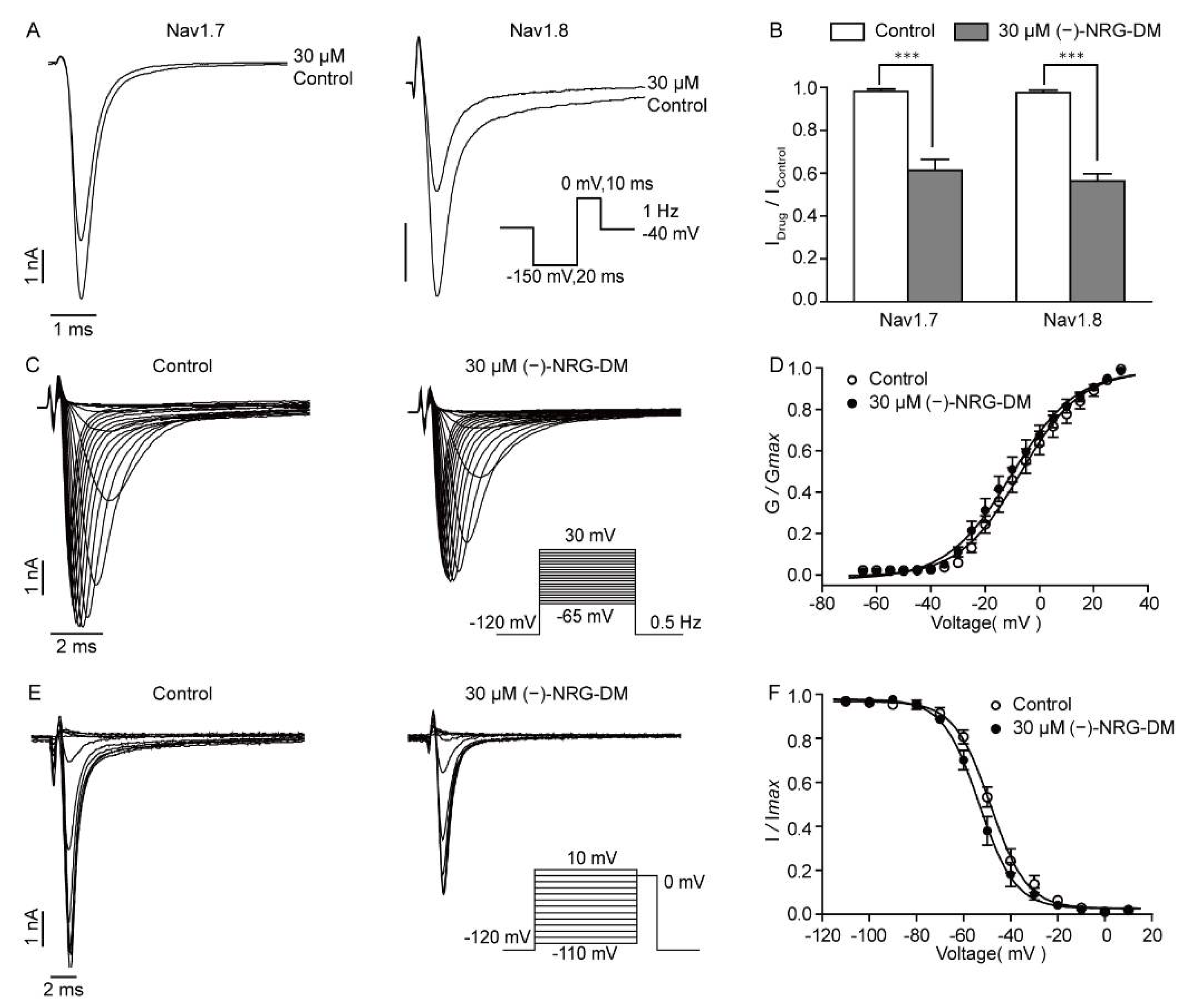
2.8. (−)-NRG-DM Inhibits Nav Channels
3. Discussion
4. Materials and Methods
4.1. Chemical Compounds
4.2. Animals
4.3. Formalin-Induced Inflammatory Model
4.4. Mustard Oil-Induced Mouse Colorectal Pain Model
4.5. Rotarod Test
4.6. Pharmacokinetic Study
4.7. Preparation of Dorsal Root Ganglion Neurons
4.8. Cell Culture and Transfection
4.9. Electrophysiological Recordings
4.10. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Okifuji, A.; Skinner, M. Chapter 70—Psychological Aspects of Pain. Curr. Ther. Pain. 2009, 24, 513–518. [Google Scholar]
- Cohen, S.P.; Vase, L.; Hooten, W.M. Chronic pain: An update on burden, best practices, and new advances. Lancet 2021, 397, 2082–2097. [Google Scholar] [CrossRef]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An overview of treatment approaches for chronic pain management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, E.J. Acute Pain Management Pharmacology for the Patient with Concurrent Renal or Hepatic Disease. Anaesth. Intensive Care 2005, 33, 311–322. [Google Scholar] [CrossRef]
- Cahill, C.M.; Lueptow, L.; Kim, H.; Shusharla, R.; Bishop, A.; Evans, C.J. Kappa Opioid Signaling at the Crossroads of Chronic Pain and Opioid Addiction. Handb. Exp. Pharmacol. 2022, 271, 315–350. [Google Scholar]
- Mercadante, S.; Arcuri, E.; Santoni, A. Opioid-Induced Tolerance and Hyperalgesia. CNS Drugs 2019, 33, 943–955. [Google Scholar] [CrossRef]
- Esposito, M.F.; Malayil, R.; Hanes, M.; Deer, T. Unique Characteristics of the Dorsal Root Ganglion as a Target for Neuromodulation. Pain Med. 2019, 20 (Suppl. S1), S23–S30. [Google Scholar] [CrossRef]
- Liem, L.; Dongen, E.V.; Huygen, F.J.; Staats, P.; Kramer, J. The Dorsal Root Ganglion as a Therapeutic Target for Chronic Pain. Reg. Anesth. Pain Med. 2016, 41, 511–519. [Google Scholar] [CrossRef]
- Li, H.-S.; Zhao, Z.Q. Small sensory neurons in the rat dorsal root ganglia express functional NK-1 tachykinin receptors. Eur. J. Neurosci. 1998, 12, 1292–1299. [Google Scholar] [CrossRef]
- Maljevic, S.; Lerche, H. Potassium channels: A review of broadening therapeutic possibilities for neurological diseases. J. Neurol. 2013, 260, 2201–2211. [Google Scholar] [CrossRef]
- Bennett, D.L.; Clark, A.J.; Huang, J.; Waxman, S.G.; Dib-Hajj, S.D. The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol Rev. 2019, 99, 1079–1151. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, G.; McMahon, S.B. The physiological function of different voltage-gated sodium channels in pain. Nat. Rev. Neurosci. 2021, 22, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S. Nav1.7 and Nav1.8: Role in the pathophysiology of pain. Mol. Pain. 2019, 15, 1744806919858801. [Google Scholar] [CrossRef] [Green Version]
- Labro, A.J.; Priest, M.F.; Lacroix, J.J.; Snyders, D.J.; Bezanilla, F. Kv3.1 uses a timely resurgent K+ current to secure action potential repolarization. Nat. Commun. 2015, 6, 10173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mara, A.; Alon, K. A Quantitative Description of Dendritic Conductances and Its Application to Dendritic Excitation in Layer 5 Pyramidal Neurons. J. Neurosci. 2014, 34, 182–196. [Google Scholar]
- Nie, A.; Wei, C.; Meng, Z. Sodium metabisulfite modulation of potassium channels in pain-sensing dorsal root ganglion neurons. Neurochem. Res. 2009, 34, 2233–2242. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Qiu, J.; Wang, X.; Zhang, G. Characteristics of voltage-gated potassium currents in monosodium urate induced gouty arthritis in mice. Inflamm. Res. 2020, 69, 589–598. [Google Scholar] [CrossRef]
- Murakoshi, H.; Trimmer, J.S. Identification of the Kv2.1 K+ Channel as a Major Component of the Delayed Rectifier K+ Current in Rat Hippocampal Neurons. J. Neurosci. 1999, 19, 1728–1735. [Google Scholar] [CrossRef] [Green Version]
- Bocksteins, E.; Raes, A.L.; Vijver, G.V.; Bruyns, T.; Bogaert, P.V.; Snyders, D.J. Kv2.1 and silent Kv subunits underlie the delayed rectifier K+ current in cultured small mouse DRG neurons. Am. J. Physiol. Cell Physiol. 2009, 296, C1271–C1278. [Google Scholar] [CrossRef] [Green Version]
- Boris, V. Safronov, Ulrike Bischoff and Werner Vogel. Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurons of rat. J. Physiol. 1996, 493, 393–408. [Google Scholar]
- Dhiman, N.; Bhattacharya, A. Nardostachys jatamansi (D.Don) DC.-Challenges and opportunities of harnessing the untapped medicinal plant from the Himalayas. J. Ethnopharmacol. 2020, 246, 112211. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Betancur, I.; Cortés, N.; Benjumea, D.; Osorio, E.; León, F.; Cutler, S.J. Antinociceptive activity of extracts and secondary metabolites from wild growing and micropropagated plants of Renealmia alpinia. J. Ethnopharmacol. 2015, 165, 191–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, M.; Yousuf, S.; Khan, M.B.; Hoda, M.N.; Ahmad, A.S.; Ansari, M.A.; Ansari, T.; Agrawal, A.K.; Islam, F. Attenuation by Nardostachys jatamansi of 6-hydroxydopamine-induced parkinsonism in rats: Behavioral, neurochemical, and immunohistochemical studies. Pharmacol. Biochem. Behav. 2006, 83, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.C.; Park, J.S.; Yoon, C.S.; Kim, Y.C.; Oh, H. Nardostachin from Nardostachys jatamansi exerts anti-neuroinflammatory effects through TLR4/MyD88related suppression of the NF-κB and JNK MAPK signaling pathways in lipopolysaccharide-induced BV2 and primary microglial cells. Mol. Med. Rep. 2021, 23, 82. [Google Scholar] [CrossRef]
- Yoon, C.S.; Kim, D.C.; Park, J.S.; Kim, K.W.; Kim, Y.C.; Oh, H. Isolation of Novel Sesquiterpeniods and Anti-neuroinflammatory Metabolites from Nardostachys jatamansi. Molecules 2018, 23, 2367. [Google Scholar] [CrossRef] [Green Version]
- Yoon, C.S.; Kim, K.W.; Lee, S.C.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of sesquiterpenoids isolated from Nardostachys jatamansi. Bioorg. Med. Chem. Lett. 2018, 28, 140–144. [Google Scholar] [CrossRef]
- Singh, M.; Khan, M.A.; T, K.Y.; Ahmad, J.; Fahmy, U.A.; Kotta, S.; Alhakamy, N.A.; Ahmad, S. Effect of Nardostachys jatamansi DC. On Apoptosis, Inflammation and Oxidative Stress Induced by Doxorubicin in Wistar Rats. Plants 2020, 9, 1579. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [Green Version]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Manchope, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Jr, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [Green Version]
- Xue, X.; Chen, Y.; Wang, Y.; Zhan, J.; Chen, B.; Wang, X.; Pan, X. Troxerutin suppresses the inflammatory response in advanced glycation end-product-administered chondrocytes and attenuates mouse osteoarthritis development. Food Funct. 2019, 10, 5059–5069. [Google Scholar] [CrossRef]
- Hu, Y.; Gui, Z.; Zhou, Y.; Xia, L.; Lin, K.; Xu, Y. Quercetin alleviates rat osteoarthritis by inhibiting inflammation and apoptosis of chondrocytes, modulating synovial macrophages polarization to M2 macrophages. Free Radic. Biol. Med. 2019, 145, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Pinho-Ribeiro, F.A.; Zarpelon, A.C.; Fattori, V.; Manchope, M.F.; Mizokami, S.S.; Casagrande, R.; Verri, W.A. Naringenin reduces inflammatory pain in mice. Neuropharmacology 2016, 105, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, G.; Huang, Z.; Zhong, X.; Xu, X.; Yuan, J. Identification of compounds in alien invasive plant Chromolaena odorata. Chin. Pharm. J. 2016, 51, 698–702. [Google Scholar]
- Oyama, K.; Kondo, T. Total synthesis of flavocommelin, a component of the blue supramolecular pigment from Commelina communis, on the basis of direct 6-C-glycosylation of flavan. J. Org. Chem. 2004, 69, 5240–5246. [Google Scholar] [CrossRef] [PubMed]
- Zainullin, R.A.; Kunakova, R.V.; Gareev, V.F.; Galyautdinov, I.V.; Sadretdinova, Z.R.; Muslimov, Z.S.; Odinokov, V.N. Flavanones and flavones from Bashkir propolis. Chem. Nat. Compd. 2018, 54, 975–977. [Google Scholar] [CrossRef]
- Mogil, J.S. Animal models of pain: Progress and challenges. Nat. Rev. Neurosci. 2009, 10, 283–294. [Google Scholar] [CrossRef]
- Laird, J.M.A.; Martinez-Caro, L.; Garcia-Nicas, E.; Cervero, F. A new model of visceral pain and referred hyperalgesia in the mouse. Pain 2001, 92, 335–342. [Google Scholar] [CrossRef]
- Bean, B.P. The action potential in mammalian central neurons. Nat. Rev. Neurosci. 2007, 8, 451–465. [Google Scholar] [CrossRef]
- Du, J.; Wang, Q.; Hu, F.; Wang, J.; Ding, H.; Gao, R.; Xiao, H.; Wang, L. Effects of estradiol on voltage-gated potassium channels in mouse dorsal root ganglion neurons. J. Membr. Biol. 2014, 247, 541–548. [Google Scholar] [CrossRef]
- Chahine, M.; O’Leary, M.E. Regulation/modulation of sensory neuron sodium channels. Handb. Exp. Pharmacol. 2014, 221, 111–135. [Google Scholar]
- Zhu, J.; Zang, S.; Chen, X.; Jiang, L.; Gu, A.; Cheng, J.; Zhang, L.; Wang, J.; Xiao, H. Involvement of the delayed rectifier outward potassium channel Kv2.1 in methamphetamine-induced neuronal apoptosis via the p38 mitogen-activated protein kinase signaling pathway. J. Appl. Toxicol. 2018, 38, 696–704. [Google Scholar] [CrossRef]
- Arora, V.; Campbell, J.N.; Chung, M.K. Fight fire with fire: Neurobiology of capsaicin-induced analgesia for chronic pain. Pharmacol. Ther. 2021, 220, 107743. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Hoferova, Z.; Falk, M. Brief Story on Prostaglandins, Inhibitors of their Synthesis, Hematopoiesis, and Acute Radiation Syndrome. Molecules 2019, 24, 4019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engels, N.S.; Waltenberger, B.; Michalak, B.; Huynh, L.; Tran, H.; Kiss, A.K.; Stuppner, H. Inhibition of Pro-Inflammatory Functions of Human Neutrophils by Constituents of Melodorum fruticosum Leaves. Chem. Biodivers. 2018, 15, e1800269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, A.; Zhang, Y.; Li, G.; Zhang, G. Inhibitory Effects of Honokiol on the Voltage-Gated Potassium Channels in Freshly Isolated Mouse Dorsal Root Ganglion Neurons. Neurochem. Res. 2018, 43, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, D.; Zhang, Y.; Jiang, X.; Wang, F.; Tao, J. Neuromedin U type 1 receptor stimulation of A-type K+ current requires the betagamma subunits of Go protein, protein kinase A, and extracellular signal-regulated kinase 1/2 (ERK1/2) in sensory neurons. J. Biol. Chem. 2012, 287, 18562–18572. [Google Scholar] [CrossRef] [Green Version]
- Clark, R.B.; Hatano, N.; Kondo, C.; Belke, D.D.; Brown, B.S.; Kumar, S.; Votta, B.J.; Votta, W.R. Voltage-gated K+ currents in mouse articular chondrocytes regulate membrane potential. Channels 2010, 4, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Takeda, M.; Tsubo, Y.; Kitagawa, J.; Nakagawa, K.; Iwata, K.; Matsumoto, S. Potassium channels as a potential therapeutic target for trigeminal neuropathic and inflammatory pain. Mol. Pain. 2011, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.C.; Nahorski, M.S.; Hockley, J.R.F.; Lu, V.B.; Ison, G.; Pattison, L.A.; Callejo, G.; Stouffer, K.; Fletcher, E.; Brown, C.; et al. Human Labor Pain Is Influenced by the Voltage-Gated Potassium Channel KV6.4 Subunit. Cell Rep. 2020, 32, 107941. [Google Scholar] [CrossRef]
- Leipold, E.; Liebmann, L.; Korenke, G.C.; Heinrich, T.; Heinrich, S.; Baets, J.; Ebbinghaus, M.; Goral, R.O.; Stödberg, T.; Hennings, J.C.; et al. A de novo gain-of-function mutation in SCN11A causes loss of pain perception. Nat. Genet. 2013, 45, 1399–1404. [Google Scholar] [CrossRef]
- Guo, R.; Shi, A.M.; Deng, L.; Li, L.; Wang, L.; Oteng, A.B.; Wei, M.P.; Zhao, Z.H.; Hooiveld, G.; Zhang, C.; et al. Flavonoid-Like Components of Peanut Stem and Leaf Extract Promote Sleep by Decreasing Neuronal Excitability. Mol. Nutr. Food Res. 2021, 66, e2100210. [Google Scholar] [CrossRef] [PubMed]

| Compound | Dosage (mg/kg) | Time of Test (h) | Fall nFall /nTest | Motor Impairment (%) |
|---|---|---|---|---|
| Vehicle | - | 0.5 | 0/10 | 0 |
| 1 | 0/10 | 0 | ||
| (−)-NRG-DM | 100 | 0.5 | 0/10 | 0 |
| 1 | 0/10 | 0 | ||
| 200 | 0.5 | 1/10 | 10 | |
| 1 | 0/10 | 0 | ||
| 400 | 0.5 | 0/10 | 0 | |
| 1 | 0/10 | 0 |
| Compound | Dosage (mg/kg) | Time of Test (h) | Concentration (ng/mL) | Concentration (μM/L) |
|---|---|---|---|---|
| 0.5 | 2293.3 ± 183.4 | 7.64 ± 0.61 | ||
| (−)-NRG-DM | 50 | 1 | 2216.0 ± 1252.0 | 7.39 ± 4.17 |
| 1.5 | 716.7 ± 222.5 | 2.39 ± 0.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, R.-R.; Meng, X.-H.; Zhang, Y.; Xu, H.-Y.; Zhan, L.; Gao, Z.-B.; Yang, J.-L.; Zheng, Y.-M. (−)-Naringenin 4′,7-dimethyl Ether Isolated from Nardostachys jatamansi Relieves Pain through Inhibition of Multiple Channels. Molecules 2022, 27, 1735. https://doi.org/10.3390/molecules27051735
Gu R-R, Meng X-H, Zhang Y, Xu H-Y, Zhan L, Gao Z-B, Yang J-L, Zheng Y-M. (−)-Naringenin 4′,7-dimethyl Ether Isolated from Nardostachys jatamansi Relieves Pain through Inhibition of Multiple Channels. Molecules. 2022; 27(5):1735. https://doi.org/10.3390/molecules27051735
Chicago/Turabian StyleGu, Ru-Rong, Xian-Hua Meng, Yin Zhang, Hai-Yan Xu, Li Zhan, Zhao-Bing Gao, Jun-Li Yang, and Yue-Ming Zheng. 2022. "(−)-Naringenin 4′,7-dimethyl Ether Isolated from Nardostachys jatamansi Relieves Pain through Inhibition of Multiple Channels" Molecules 27, no. 5: 1735. https://doi.org/10.3390/molecules27051735
APA StyleGu, R.-R., Meng, X.-H., Zhang, Y., Xu, H.-Y., Zhan, L., Gao, Z.-B., Yang, J.-L., & Zheng, Y.-M. (2022). (−)-Naringenin 4′,7-dimethyl Ether Isolated from Nardostachys jatamansi Relieves Pain through Inhibition of Multiple Channels. Molecules, 27(5), 1735. https://doi.org/10.3390/molecules27051735