Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review
Abstract
1. Introduction
2. Harvesting of Cannabis
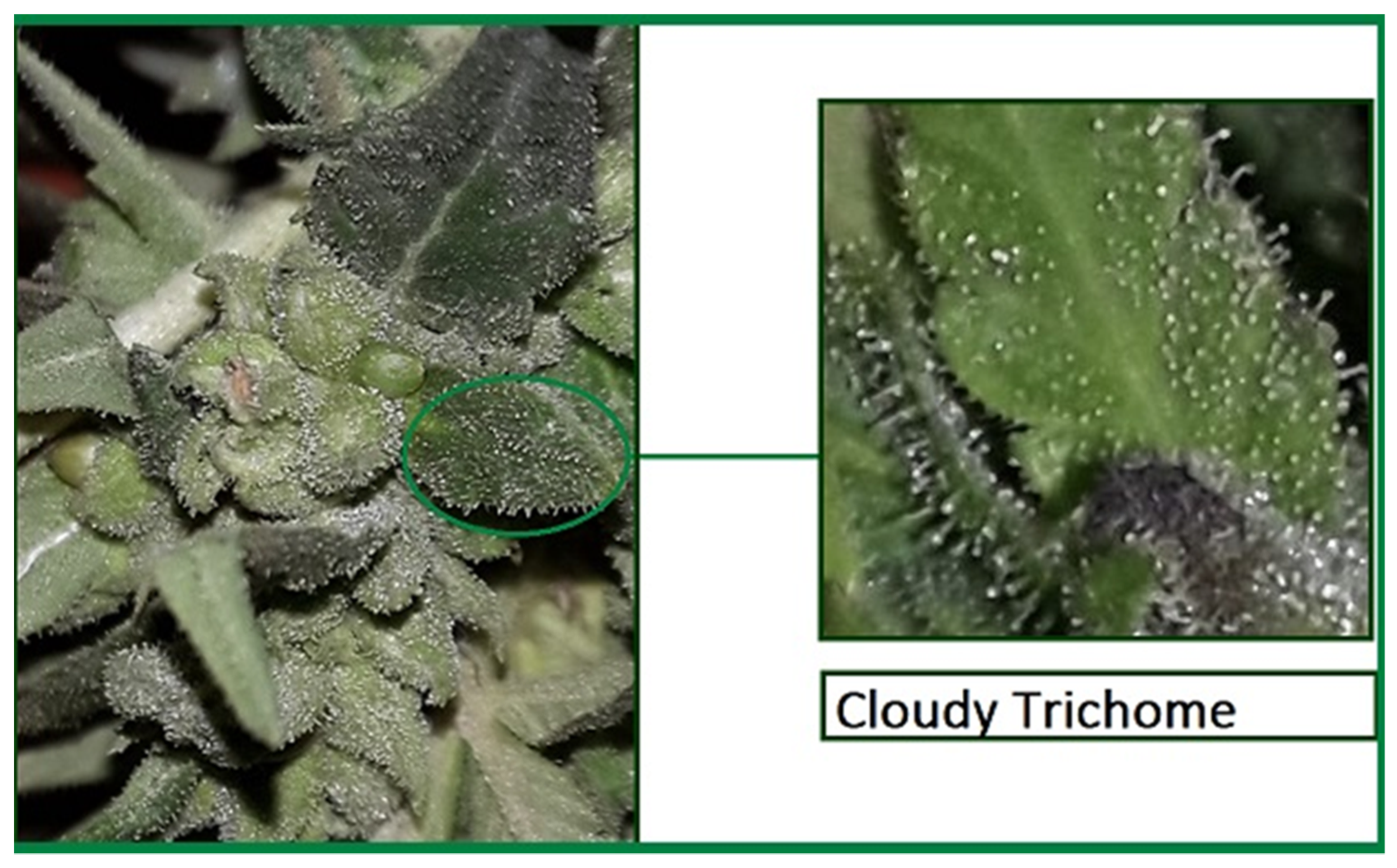
2.1. Determining the Time to Harvest
2.2. Harvesting Technology
3. Drying of Cannabis
3.1. Hot Air Drying or Hang Drying
3.2. Oven Drying
3.3. Microwave-Assisted Hot Air-Drying
3.4. Vacuum Freeze-Drying
3.5. Microwave-Assisted Freeze Drying (MFD)
4. Storage of Medicinal Cannabis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Abel, E.L. Cannabis in the ancient world. In Marihuana the First Twelve Thousand Years; Springer Science & Business Media: Boston, MA, USA, 1980; pp. 3–35. [Google Scholar]
- UNODC. World Drug Report; United Nations Publication: Vienna, Austria, 2008; pp. 95–111. [Google Scholar]
- Small, E.; Jui, P.Y.; Lefkovitch, L.P. A numerical taxonomic analysis of cannabis with special reference to species delimitation. Syst. Bot. 1976, 1, 67–84. [Google Scholar] [CrossRef]
- Small, E. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilisation. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Schultes, R.E.; Klein, W.M.; Plowman, T.; Lockwood, T.E. Cannabis: An example of taxonomic neglect. Cannabis Cult. 1975, 23, 21–38. [Google Scholar] [CrossRef]
- Aizpurua-Olaizola, O.; Soydaner, U.; Öztürk, E.; Schibano, D.; Simsir, Y.; Navarro, P.; Etxebarria, N.; Usobiaga, A. Evolution of the cannabinoid and terpene content during the growth of Cannabis sativa plants from different chemotypes. J. Nat. Prod. 2016, 79, 324–331. [Google Scholar] [CrossRef]
- Booth, J.K.; Bohlmann, J. Terpenes in Cannabis sativa–from plant genome to humans. Plant Sci. 2019, 284, 67–72. [Google Scholar] [CrossRef]
- AL Ubeed, H.M.S.; Bhuyan, D.J.; Alsherbiny, M.A.; Basu, A.; Vuong, Q.V. A comprehensive review on the techniques for extraction of bioactive compounds from medicinal cannabis. Molecules 2022, 27, 604. [Google Scholar] [CrossRef]
- Rong, C.; Lee, Y.; Carmona, N.E.; Cha, D.S.; Ragguett, R.-M.; Rosenblat, J.D.; Mansur, R.B.; Ho, R.C.; McIntyre, R.S. Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacol. Res. 2017, 121, 213–218. [Google Scholar] [CrossRef]
- Mohammed, N.; Ceprian, M.; Jimenez, L.; Ruth Pazos, M.; Martínez-Orgado, J. Neuroprotective effects of cannabidiol in hypoxic ischemic insult. The therapeutic window in newborn mice. CNS Neurol. Disord.-Drug Targets 2017, 16, 102–108. [Google Scholar] [CrossRef]
- Kwiatkoski, M.; Guimaraes, F.S.; Del-Bel, E. Cannabidiol-treated rats exhibited higher motor score after cryogenic spinal cord injury. Neurotox. Res. 2012, 21, 271–280. [Google Scholar] [CrossRef]
- Malfait, A.; Gallily, R.; Sumariwalla, P.; Malik, A.; Andreakos, E.; Mechoulam, R.; Feldmann, M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 2000, 97, 9561–9566. [Google Scholar] [CrossRef]
- Singh, K.; Nassar, N.; Bachari, A.; Schanknecht, E.; Telukutla, S.; Zomer, R.; Piva, T.J.; Mantri, N. The pathophysiology and the therapeutic potential of cannabinoids in prostate cancer. Cancers 2021, 13, 4107. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Jagerovic, N. Antitumor cannabinoid chemotypes: Structural insights. Front. Pharmacol. 2019, 10, 621. [Google Scholar] [CrossRef] [PubMed]
- Schley, M.; Legler, A.; Skopp, G.; Schmelz, M.; Konrad, C.; Rukwied, R. Delta-9-thc based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare and pain relief. Curr. Med. Res. Opin. 2006, 22, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Eubanks, L.M.; Rogers, C.J.; Beuscher IV, A.E.; Koob, G.F.; Olson, A.J.; Dickerson, T.J.; Janda, K.D. A molecular link between the active component of marijuana and alzheimer’s disease pathology. Mol. Pharm. 2006, 3, 773–777. [Google Scholar] [CrossRef]
- Zeissler, M.-L.; Eastwood, J.; McCorry, K.; Hanemann, C.O.; Zajicek, J.P.; Carroll, C.B. Delta-9-tetrahydrocannabinol protects against mpp+ toxicity in sh-sy5y cells by restoring proteins involved in mitochondrial biogenesis. Oncotarget 2016, 7, 46603. [Google Scholar] [CrossRef]
- Rajavashisth, T.B.; Shaheen, M.; Norris, K.C.; Pan, D.; Sinha, S.K.; Ortega, J.; Friedman, T.C. Decreased prevalence of diabetes in marijuana users: Cross-sectional data from the national health and nutrition examination survey (nhanes) iii. BMJ Open 2012, 2, e000494. [Google Scholar] [CrossRef]
- Eisohly, H.N.; Turner, C.E.; Clark, A.M.; Eisohly, M.A. Synthesis and antimicrobial activities of certain cannabichromene and cannabigerol related compounds. J. Pharm. Sci. 1982, 71, 1319–1323. [Google Scholar] [CrossRef]
- Shinjyo, N.; Di Marzo, V. The effect of cannabichromene on adult neural stem/progenitor cells. Neurochem. Int. 2013, 63, 432–437. [Google Scholar] [CrossRef]
- Usami, N.; Kobana, K.; Yoshida, H.; Kimura, T.; Watanabe, K.; Yoshimura, H.; Yamamoto, I. Synthesis and pharmacological activities in mice of halogenated δ9-tetrahydrocannabinol derivatives. Chem. Pharm. Bull. 1998, 46, 1462–1467. [Google Scholar] [CrossRef]
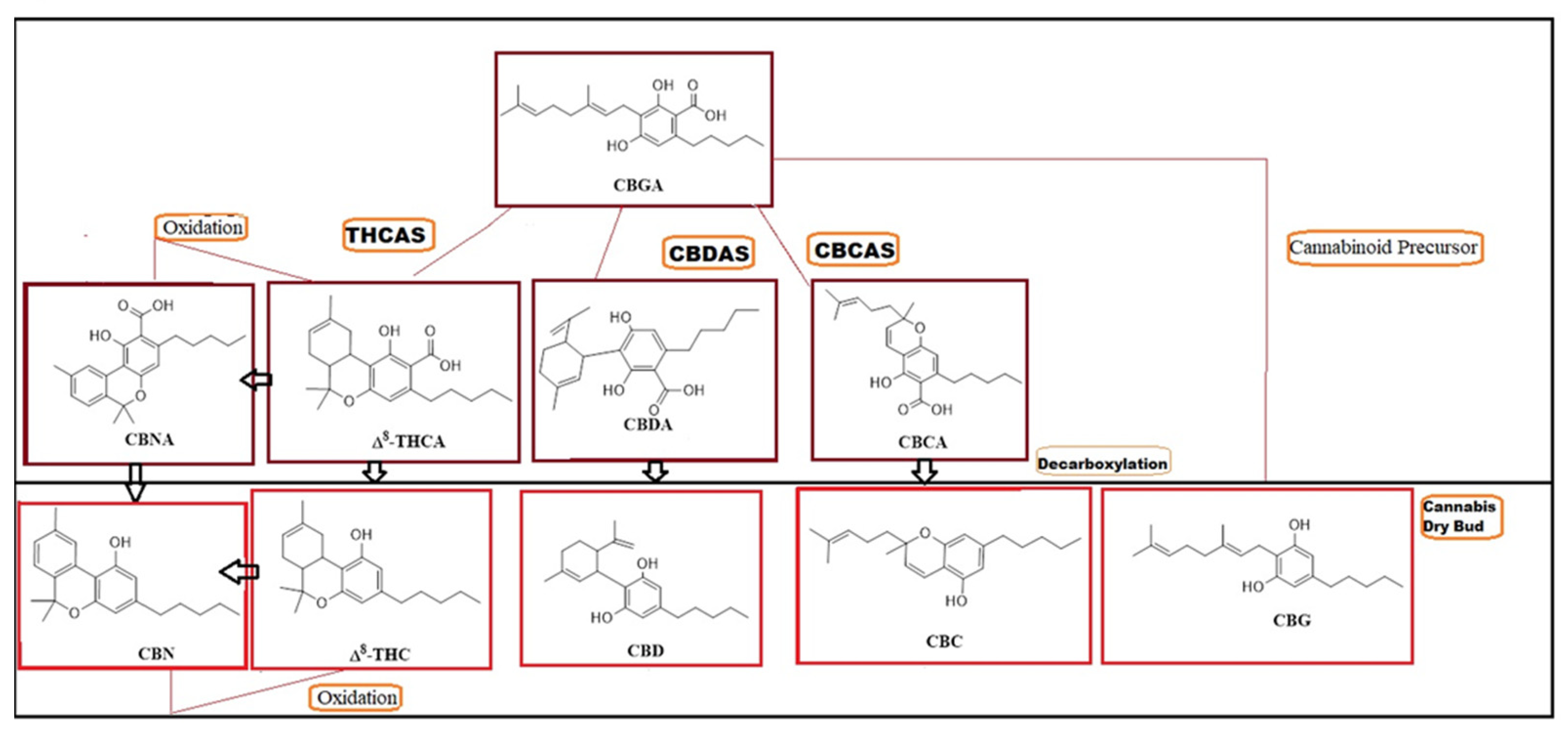
- Fellermeier, M.; Zenk, M.H. Prenylation of olivetolate by a hemp transferase yields cannabigerolic acid, the precursor of tetrahydrocannabinol. FEBS Lett. 1998, 427, 283–285. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Morimoto, S.; Shoyama, Y.; Ishikawa, Y.; Wada, Y.; Shoyama, Y.; Taura, F. The gene controlling marijuana psychoactivity: Molecular cloning and heterologous expression of δ1-tetrahydrocannabinolic acid synthase from Cannabis sativa L. J. Biol. Chem. 2004, 279, 39767–39774. [Google Scholar] [CrossRef] [PubMed]
- Taura, F.; Morimoto, S.; Shoyama, Y.; Mechoulam, R. First direct evidence for the mechanism of. Delta. 1-tetrahydrocannabinolic acid biosynthesis. J. Am. Chem. Soc. 1995, 117, 9766–9767. [Google Scholar] [CrossRef]
- Gagne, S.J.; Stout, J.M.; Liu, E.; Boubakir, Z.; Clark, S.M.; Page, J.E. Identification of olivetolic acid cyclase from Cannabis sativa reveals a unique catalytic route to plant polyketides. Proc. Natl. Acad. Sci. USA 2012, 109, 12811–12816. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.F.; Elsohly, M. The botany of Cannabis sativa L. In The Analytical Chemistry of Cannabis: Quality Assessment, Assurance and Regulation of Medicinal Marijuana and Cannabinoid Preparations; Elsevier: Oxford, UK, 2015; pp. 1–22. [Google Scholar]
- Radwan, M.M.; Chandra, S.; Gul, S.; ElSohly, M.A. Cannabinoids, phenolics, terpenes and alkaloids of cannabis. Molecules 2021, 26, 2774. [Google Scholar] [CrossRef] [PubMed]
- Petrovska, B. Historical review of medicinal plants’ usage. Pharmacogn. Rev. 2012, 6, 1–5. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary metabolites profiled in cannabis inflorescences, leaves, stem barks and roots for medicinal purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Lozano, I. The therapeutic use of Cannabis sativa (L.) in arabic medicine. J. Cannabis Ther. 2001, 1, 63–70. [Google Scholar] [CrossRef]
- Stuart, G.; Smith, F. Part 1 vegetable kingdom. In Chinese Materia Medica; American Presbyterian Mission Press: Shanghai, China, 1911. [Google Scholar]
- Balant, M.; Gras, A.; Ruz, M.; Vallès, J.; Vitales, D.; Garnatje, T. Traditional uses of cannabis: An analysis of the cannuse database. J. Ethnopharmacol. 2021, 279, 114362. [Google Scholar] [CrossRef]
- Lima, K.S.B.; da Cruz Silva, M.E.G.; de Lima Araújo, T.C.; da Fonseca Silva, C.P.; Santos, B.L.; de Araújo Ribeiro, L.A.; Menezes, P.M.N.; Silva, M.G.; Lavor, É.M.; Silva, F.S. Cannabis roots: Pharmacological and toxicological studies in mice. J. Ethnopharmacol. 2021, 271, 113868. [Google Scholar] [CrossRef]
- Minghetti, P.; Marini, V.; Zaccara, V.; Casiraghi, A. Regulation for prescribing and dispensing system of cannabis: The italian case. Curr. Bioact. Compd. 2019, 15, 196–200. [Google Scholar] [CrossRef]
- Sirikantaramas, S.; Taura, F.; Tanaka, Y.; Ishikawa, Y.; Morimoto, S.; Shoyama, Y. Tetrahydrocannabinolic acid synthase, the enzyme controlling marijuana psychoactivity, is secreted into the storage cavity of the glandular trichomes. Plant Cell Physiol. 2005, 46, 1578–1582. [Google Scholar] [CrossRef]
- Morimoto, S.; Tanaka, Y.; Sasaki, K.; Tanaka, H.; Fukamizu, T.; Shoyama, Y.; Shoyama, Y.; Taura, F. Identification and characterisation of cannabinoids that induce cell death through mitochondrial permeability transition in cannabis leaf cells. J. Biol. Chem. 2007, 282, 20739–20751. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.; Kato, N.; Lawton, M. Programmed cell death, mitochondria and the plant hypersensitive response. Nature 2001, 411, 848–853. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R. Marihuana chemistry: Recent advances in cannabinoid chemistry open the area to more sophisticated biological research. Science 1970, 168, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Balk, J.; Chew, S.K.; Leaver, C.J.; McCabe, P.F. The intermembrane space of plant mitochondria contains a dnase activity that may be involved in programmed cell death. Plant J. 2003, 34, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Matile, P. Chloroplast senescence. In Crop Photosynthesis: Spatial Temporal Determinants; Baker, N.R., Thomas, H.C., Eds.; Elsevier: Amsterdam, The Netherlands, 1992; Volume 12, pp. 413–440. [Google Scholar]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W. Loss of cyclophilin d reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Gaoni, Y.; Mechoulam, R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Milay, L.; Berman, P.; Shapira, A.; Guberman, O.; Meiri, D. Metabolic profiling of cannabis secondary metabolites for evaluation of optimal post-harvest storage conditions. Front. Plant Sci. 2020, 11, 583605. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis sativa L. In Phytocannabinoids; Springer: Cham, Switzerland, 2017; pp. 1–36. [Google Scholar]
- Taschwer, M.; Schmid, M.G. Determination of the relative percentage distribution of thca and δ9-thc in herbal cannabis seized in austria–impact of different storage temperatures on stability. Forensic Sci. Int. 2015, 254, 167–171. [Google Scholar] [CrossRef]
- Grafström, K.; Andersson, K.; Pettersson, N.; Dalgaard, J.; Dunne, S.J. Effects of long term storage on secondary metabolite profiles of cannabis resin. Forensic Sci. Int. 2019, 301, 331–340. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Y.-H.; Avula, B.; Radwan, M.M.; Wanas, A.S.; van Antwerp, J.; Parcher, J.F.; ElSohly, M.A.; Khan, I.A. Decarboxylation study of acidic cannabinoids: A novel approach using ultra-high-performance supercritical fluid chromatography/photodiode array-mass spectrometry. Cannabis Cannabinoid Res. 2016, 1, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.R.; Bouchard, M.; Decorte, T. The globalisation of cannabis cultivation. In World Wide Weed; Routledge: New York, NY, USA, 2016; pp. 21–40. [Google Scholar]
- Rosenthal, E. Harvest and beyond In Ed Rosenthal’s Marijuana Grower’s Handbook: Your Complete Guide for Medical & Personal Marijuana Cultivation; Angela Bacca, H.L., Johnson-Igra, D., Eds.; Quick American Publishing: Oakland, CA, USA, 2010; pp. 393–411. [Google Scholar]
- Jin, D.; Jin, S.; Chen, J. Cannabis indoor growing conditions, management practices and post-harvest treatment: A review. Am. J. Plant Sci. 2019, 10, 925. [Google Scholar] [CrossRef]
- Xiao, K.; Mao, X.; Lin, Y.; Xu, H.; Zhu, Y.; Cai, Q.; Xie, H.; Zhang, J. Trichome, a functional diversity phenotype in plant. Mol. Biol. 2017, 6, 183. [Google Scholar] [CrossRef]
- Clarke, R.; Merlin, M. Ethnobotanical origins, early cultivation and evolution through human selection. In Cannabis: Evolution and Ethnobotany; University of California Press: London, UK, 2016; pp. 29–57. [Google Scholar]
- Upton, R.; ElSohly, M.; Craker, L.; Romm, A.; Russo, E.; Sexton, M. Commercial sources and handling. In Cannabis Inflorescence: CANNABIS spp.: Standards of Identity, Analysis and Quality Control; American Herbal Pharmacopoeia: Soquel, CA, USA, 2013; pp. 18–33. [Google Scholar]
- Rosenthal, E.; Downs, D. Marijuana Harvest: How to Maximize Quality and Yield in Your Cannabis Garden, illustrated ed.; Quick American: Piedmont: San Francisco, CA, USA, 2017. [Google Scholar]
- Tettey, J. Description of the cannabis plant and illicit cannabis products. In Recommended Methods for the Identification and Analysis of Cannabis and Cannabis Products; United Nations Office on Drugs and Crime: New York, NY, USA, 2009; p. 60. [Google Scholar]
- Crispim Massuela, D.; Hartung, J.; Munz, S.; Erpenbach, F.; Graeff-Hönninger, S. Impact of harvest time and pruning technique on total cbd concentration and yield of medicinal cannabis. Plants 2022, 11, 140. [Google Scholar] [CrossRef]
- Jin, D.; Henry, P.; Shan, J.; Chen, J. Identification of chemotypic markers in three chemotype categories of cannabis using secondary metabolites profiled in inflorescences, leaves, stem bark and roots. Front. Plant Sci. 2021, 12, 699530. [Google Scholar] [CrossRef]
- Bergman, R. Harvesting. In The Marijuana Grow Bible; Amazon Digital Services LLC-Kdp Print Us: Seattle, WA, USA, 2019; pp. 52–58. [Google Scholar]
- Chandra, S.; Lata, H.; ElSohly, M.A.; Walker, L.A.; Potter, D. Cannabis cultivation: Methodological issues for obtaining medical-grade product. Epilepsy Behav. 2017, 70, 302–312. [Google Scholar] [CrossRef]
- Clarke, R.C. Maturation and harvesting of cannabis In Marijuana Botany: An Advanced Study: The Propagation and Breeding of Distinctive Cannabis; Ronin Publishing: Berkeley, CA, USA, 1981; pp. 60–78. [Google Scholar]
- Vogelmann, A.F.; Turner, J.C.; Mahlberg, P.G. Cannabinoid composition in seedlings compared to adult plants of Cannabis sativa. J. Nat. Prod. 1988, 51, 1075–1079. [Google Scholar] [CrossRef]
- Pacifico, D.; Miselli, F.; Carboni, A.; Moschella, A.; Mandolino, G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica 2008, 160, 231–240. [Google Scholar] [CrossRef]
- De Backer, B.; Maebe, K.; Verstraete, A.G.; Charlier, C. Evolution of the content of thc and other major cannabinoids in drug-type cannabis cuttings and seedlings during growth of plants. J. Forensic Sci. 2012, 57, 918–922. [Google Scholar] [CrossRef]
- Davidson, M.; Reed, S.; Oosthuizen, J.; O’Donnell, G.; Gaur, P.; Cross, M.; Dennis, G. Occupational health and safety in cannabis production: An australian perspective. Int. J. Occup. Environ. Health 2018, 24, 75–85. [Google Scholar] [CrossRef]
- Zhang, J.-Q.; Chen, S.-L.; Wei, G.-F.; Ning, K.; Wang, C.-Q.; Wang, L.; Chen, H.; Dong, L.-L. Cultivars breeding and production of non-psychoactive medicinal cannabis with high cbd content. China J. Chin. Mater. Med. 2019, 44, 4772–4780. [Google Scholar]
- Russo, E.B.; Jiang, H.-E.; Li, X.; Sutton, A.; Carboni, A.; Del Bianco, F.; Mandolino, G.; Potter, D.J.; Zhao, Y.-X.; Bera, S. Phytochemical and genetic analyses of ancient cannabis from central asia. J. Exp. Bot. 2008, 59, 4171–4182. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, B.; Huang, J.; Tian, K.; Liu, H.; Li, X.; Yin, G. Research status and suggestions of mechanical harvesting technology for high-stalk bast-fiber crops. Int. Agric. Eng. J. 2020, 29, 269–284. [Google Scholar]
- Fike, J. Industrial hemp: Renewed opportunities for an ancient crop. Crit. Rev. Plant Sci. 2016, 35, 406–424. [Google Scholar] [CrossRef]
- Sausserde, R.; Adamovics, A.; Ivanovs, S.; Bulgakov, V. Investigations into growing and harvesting industrial hemp. J. Res. Appl. Agric. Eng. 2013, 58, 150–154. [Google Scholar]
- Cheng, S.; Bin, Z.; Xianwang, L.; Guodong, Y.; Qiaomin, C.; Chunhua, X. Bench cutting tests and analysis for harvesting hemp stalk. Int. J. Agric. Biol. Eng. 2017, 10, 56–67. [Google Scholar] [CrossRef]
- Rodriguez, G.; Munir, Z. Good manufacturing practices (gmp) approach to post-harvest activities for cannabis. J GXP Compl 2019, 23, 6. [Google Scholar]
- Ilikj, M.; Brchina, I.; Ugrinova, L.; Karcev, V.; Grozdanova, A. Gmp/gacp-new standards for quality assurance of cannabis. Maced. Pharm. Bull. 2021, 66, 91–101. [Google Scholar] [CrossRef]
- Farag, S.; Kayser, O. Cultivation and breeding of Cannabis sativa L. For preparation of standardised extracts for medicinal purposes. In Medicinal and Aromatic Plants of the World; Springer: Dordrecht, The Netherlands, 2015; pp. 165–186. [Google Scholar]
- Green, G.; Kryptonite, S.; Chimera, B.; Ralpheme, R. Harvesting and Curing Your Bud in the Cannabis Grow Bible, 4th ed.; Green Candy Press: San Francisco, CA, USA, 2001; pp. 280–284. [Google Scholar]
- Hawes, M.D.; Cohen, M.R. Method of Drying Cannabis Materials. U.S. Patent 20150096189A1, 9 April 2015. Available online: https://patents.google.com/patent/US20150096189A1/en (accessed on 2 March 2022).
- Challa, S.K.R.; Misra, N.; Martynenko, A. Drying of cannabis—State of the practices and future needs. Dry. Technol. 2021, 39, 2055–2064. [Google Scholar] [CrossRef]
- Ross, S.A.; ElSohly, M.A. The volatile oil composition of fresh and air-dried buds of Cannabis sativa. J. Nat. Prod. 1996, 59, 49–51. [Google Scholar] [CrossRef]
- Coffman, C.; Gentner, W. Cannabis sativa L.: Effect of drying time and temperature on cannabinoid profile of stored leaf tissue. Bull. Narc. 1974, 26, 68–70. [Google Scholar]
- Turner, J.C.; Mahlberg, P.G. Effects of sample treatment on chromatographic analysis of cannabinoids in Cannabis sativa L. (cannabaceae). J. Chromatogr. A 1984, 283, 165–171. [Google Scholar] [CrossRef]
- Dev, S.; Geetha, P.; Orsat, V.; Gariépy, Y.; Raghavan, G. Effects of microwave-assisted hot air drying and conventional hot air drying on the drying kinetics, color, rehydration and volatiles of moringa oleifera. Dry. Technol. 2011, 29, 1452–1458. [Google Scholar] [CrossRef]
- Chasiotis, V.; Tsakirakis, A.; Termentzi, A.; Machera, K.; Filios, A. Drying and quality characteristics of Cannabis sativa L. Inflorescences under constant and time-varying convective drying temperature schemes. Therm. Sci. Eng. Prog. 2022, 28, 101076. [Google Scholar] [CrossRef]
- Kwaśnica, A.; Pachura, N.; Masztalerz, K.; Figiel, A.; Zimmer, A.; Kupczyński, R.; Wujcikowska, K.; Carbonell-Barrachina, A.A.; Szumny, A.; Różański, H. Volatile composition and sensory properties as quality attributes of fresh and dried hemp flowers (Cannabis sativa L.). Foods 2020, 9, 1118. [Google Scholar] [CrossRef]
- Tang, X.C.; Pikal, M.J. Design of freeze-drying processes for pharmaceuticals: Practical advice. Pharm. Res. 2004, 21, 191–200. [Google Scholar] [CrossRef]
- Tambunan, A.; Yudistira; Kisdiyani; Hernani. Freeze drying characteristics of medicinal herbs. Dry. Technol. 2001, 19, 325–331. [Google Scholar] [CrossRef]
- Kasper, J.C.; Friess, W. The freezing step in lyophilisation: Physico-chemical fundamentals, freezing methods and consequences on process performance and quality attributes of biopharmaceuticals. Eur. J. Pharm. Biopharm. 2011, 78, 248–263. [Google Scholar] [CrossRef]
- Patel, S.M.; Doen, T.; Pikal, M.J. Determination of end point of primary drying in freeze-drying process control. Aaps Pharmscitech 2010, 11, 73–84. [Google Scholar] [CrossRef]
- Mujumdar, A.S.; Woo, M.W. Effects of electric and magnetic field on freezing. In Drying Technologies for Biotechnology Pharmaceutical Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 283–301. [Google Scholar]
- Duan, X.; Zhang, M.; Mujumdar, A.; Wang, R. Trends in microwave-assisted freeze drying of foods. Dry. Technol. 2010, 28, 444–453. [Google Scholar] [CrossRef]
- Liapis, A.; Bruttini, R. Exergy analysis of freeze drying of pharmaceuticals in vials on trays. Int. J. Heat Mass Transf. 2008, 51, 3854–3868. [Google Scholar] [CrossRef]
- Zhang, M.; Jiang, H.; Lim, R.-X. Recent developments in microwave-assisted drying of vegetables, fruits and aquatic products—Drying kinetics and quality considerations. Dry. Technol. 2010, 28, 1307–1316. [Google Scholar] [CrossRef]
- Chen, C.; Wongso, I.; Putnam, D.; Khir, R.; Pan, Z. Effect of hot air and infrared drying on the retention of cannabidiol and terpenes in industrial hemp (Cannabis sativa L.). Ind. Crops Prod. 2021, 172, 114051. [Google Scholar] [CrossRef]
- Lazarjani, M.P.; Young, O.; Kebede, L.; Seyfoddin, A. Processing and extraction methods of medicinal cannabis: A narrative review. J. Cannabis Res. 2021, 3, 32. [Google Scholar] [CrossRef] [PubMed]
- Müller, J. Convective drying of medicinal, aromatic and spice plants: A review. Stewart Post-harvest Rev. 2007, 3, 1–6. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave food processing—A review. Food Res. Int. 2013, 52, 243–261. [Google Scholar] [CrossRef]
- Wray, D.; Ramaswamy, H.S. Novel concepts in microwave drying of foods. Dry. Technol. 2015, 33, 769–783. [Google Scholar] [CrossRef]
- Feng, H.; Yin, Y.; Tang, J. Microwave drying of food and agricultural materials: Basics and heat and mass transfer modeling. Food Eng. Rev. 2012, 4, 89–106. [Google Scholar] [CrossRef]
- Andrés, A.; Bilbao, C.; Fito, P. Drying kinetics of apple cylinders under combined hot air–microwave dehydration. J. Food Eng. 2004, 63, 71–78. [Google Scholar] [CrossRef]
- Pham, N.D.; Khan, M.; Karim, M. A mathematical model for predicting the transport process and quality changes during intermittent microwave convective drying. Food Chem. 2020, 325, 126932. [Google Scholar] [CrossRef]
- Abo Bakr, T.M. Microwave applications in food processing: An overview. Alex. J. Food Sci. 2020, 17, 11–22. [Google Scholar] [CrossRef]
- Ratti, C. Hot air and freeze-drying of high-value foods: A review. J. Food Eng. 2001, 49, 311–319. [Google Scholar] [CrossRef]
- Bantle, M.; Kolsaker, K.; Eikevik, T.M. Modification of the weibull distribution for modeling atmospheric freeze-drying of food. Dry. Technol. 2011, 29, 1161–1169. [Google Scholar] [CrossRef]
- Rahman, S.; Mujumdar, A. A novel atmospheric freeze-drying system using a vibro-fluidised bed with adsorbent. Dry. Technol. 2008, 26, 393–403. [Google Scholar] [CrossRef]
- Claussen, I.; Ustad, T.; Str⊘ Mmen, I.; Walde, P. Atmospheric freeze drying—A review. Dry. Technol. 2007, 25, 947–957. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Wang, Z.H.; Shi, M.H. Microwave freeze drying characteristics of beef. Dry. Technol. 1999, 17, 434–447. [Google Scholar] [CrossRef]
- Rosenthal, E. Marijuana Pest and Disease Control; Quick American: Oakland, CA, USA, 2012. [Google Scholar]
- Charoux, C.M.; O’Donnell, C.P.; Tiwari, B.K. Effect of airborne ultrasonic technology on microbial inactivation and quality of dried food ingredients. Ultrason. Sonochem. 2019, 56, 313–317. [Google Scholar] [CrossRef]
- Offers, J. Cannabis: Beyond Potency: Fungi, Mold and Mycotoxins. 2019. Available online: https://manoxblog.com/2019/12/29/cannabis-beyond-potency-fungi-mold-and-mycotoxins/ (accessed on 8 February 2022).
- Llamas, R.; Hart, D.R.; Schneider, N.S. Allergic bronchopulmonary aspergillosis associated with smoking moldy marihuana. Chest 1978, 73, 871–872. [Google Scholar] [CrossRef]
- Hamadeh, R.; Ardehali, A.; Locksley, R.M.; York, M.K. Fatal aspergillosis associated with smoking contaminated marijuana, in a marrow transplant recipient. Chest 1988, 94, 432–433. [Google Scholar] [CrossRef]
- Remington, T.L.; Fuller, J.; Chiu, I. Chronic necrotizing pulmonary aspergillosis in a patient with diabetes and marijuana use. CMAJ 2015, 187, 1305–1308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scott, C.; Punja, Z.K. Evaluation of disease management approaches for powdery mildew on Cannabis sativa L.(marijuana) plants. Can. J. Plant Pathol. 2021, 43, 394–412. [Google Scholar] [CrossRef]
- McKernan, K.; Spangler, J.; Zhang, L.; Tadigotla, V.; Helbert, Y.; Foss, T.; Smith, D. Cannabis microbiome sequencing reveals several mycotoxic fungi native to dispensary grade cannabis flowers. FResearch 2015, 4, 1422. [Google Scholar]
- Winston, M.E.; Hampton-Marcell, J.; Zarraonaindia, I.; Owens, S.M.; Moreau, C.S.; Gilbert, J.A.; Hartsel, J.; Kennedy, S.J.; Gibbons, S.M. Understanding cultivar-specificity and soil determinants of the cannabis microbiome. PLoS ONE 2014, 9, e99641. [Google Scholar] [CrossRef]
- Punja, Z.K.; Collyer, D.; Scott, C.; Lung, S.; Holmes, J.; Sutton, D. Pathogens and molds affecting production and quality of Cannabis sativa L. Front. Plant Sci. 2019, 10, 1120. [Google Scholar] [CrossRef]
- McPartland, J. A review of cannabis diseases. J. Int. Hemp Assoc. 1996, 3, 19–23. [Google Scholar]
- Taylor, D.N.; Wachsmuth, I.K.; Shangkuan, Y.-h.; Schmidt, E.V.; Barrett, T.J.; Schrader, J.S.; Scherach, C.S.; McGee, H.B.; Feldman, R.A.; Brenner, D. Salmonellosis associated with marijuana: A multistate outbreak traced by plasmid fingerprinting. N. Engl. J. Med. 1982, 306, 1249–1253. [Google Scholar] [CrossRef]
- Ungerleider, J.; Andrysiak, T.; Tashkin, D.; Gale, R. Contamination of marihuana cigarettes with pathogenic bacteria--possible source of infection in cancer patients. Cancer Treat. Rep. 1982, 66, 589–591. [Google Scholar]
- Victory, K.; Lowe, B.; Burton, N.C.; Green, B.J.; Nayak, A.; Lemons, A.R.; Beezhold, D. Evaluation of Potential Hazards during Harvesting and Processing Cannabis at an Outdoor Organic Farm; US Department of Health Human Services Centers for Disease Control Prevention: Cinncinati, OH, USA, 2017. [Google Scholar]
- Caplan, D.M. Propagation and Root Zone Management for Controlled Environment Cannabis Production. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2018. [Google Scholar]
- Scholten, W.K. Guidelines for cultivating cannabis for medicinal purposes [voorschriften voor de verbouw van cannabis voor medicinale doeleinden]. Annex to the regulation of the minister of health, welfare and sport of 9 january 2003, gmt/bmc 2340685, containing policy guidelines for the decision on applications for opium act exemptions (policy guidelines opium act exemptions)(authorised english translation). J. Cannabis Ther. 2003, 3, 51–61. [Google Scholar]
- Hazekamp, A.; Sijrier, P.; Verpoorte, R. An evaluation of the quality of medicinal grade cannabis in the netherlands. Cannabinoids 2006, 1, 1–9. [Google Scholar]
- Brenneisen, R. Chemistry and analysis of phytocannabinoids and other cannabis constituents. In Marijuana and the Cannabinoids; Springer: Totowa, NJ, USA, 2007; pp. 17–49. [Google Scholar]
- PharmOut. New Revision of PIC/S GMP GUIDE (PIC/S PE 009-13) Now Live. 2017. Available online: https://www.pharmout.net/new-revision-of-pics-pe-009-13/ (accessed on 4 February 2022).
- Therapeutic Goods Administration. Labelling & Packaging. 2021. Available online: https://www.tga.gov.au/labelling-packaging (accessed on 10 February 2022).
| Drying Technique | Drying Conditions/Procedures | Advantages and Disadvantage | References |
|---|---|---|---|
| Hot Air Drying | The plant materials were hanged on either string lines, wire cages, or static wires upside-down to allow for air circulation and uniform drying by control system has been set between 18–21 °C, relative humidity at 50–55% and air circulation using a small fan under these controlled conditions. Trimmed flowers take only 4–5 days, but the whole plant takes up to 14 days. | A simple technique, but required regularly maintain optimal conditions. | [59,75,76,77,78] |
| Oven Drying | Buds were hanging upside down in the oven and oven must be preheated at 37 °C for 24 h to prevent decarboxylation for Phyto cannabinoids | A simple technique, but under optimal conditions and difficult for commercial production. | [75,78,79] |
| Microwave-assisted hot air-drying | Samples were dried by applied volumetric heating and creating a temperature gradient and standard microwaves frequency set at 915 MHz and 240 W to maintain high-quality medicinal cannabis | An advanced technique, but under optimal conditions. | [80,81,82] |
| Vacuum Freeze-Drying | Vacuum freezing the cannabis bud by reducing the temperature to approximately −40 °C before drying the buds to retain a high quality of phytochemicals. | Quite effective and most suitable advanced technique, but prohibitive operational cost. | [83,84,85,86,87] |
| Microwave-Assisted Freeze Drying | Circulates cold, dry air over the frozen material at a temperature below −40 °C to −45 °C, pressure at 100 Pa, microwave frequency 2450 MHz. | An advanced technique, but under optimal conditions. | [76,88,89,90] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AL Ubeed, H.M.S.; Wills, R.B.H.; Chandrapala, J. Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review. Molecules 2022, 27, 1719. https://doi.org/10.3390/molecules27051719
AL Ubeed HMS, Wills RBH, Chandrapala J. Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review. Molecules. 2022; 27(5):1719. https://doi.org/10.3390/molecules27051719
Chicago/Turabian StyleAL Ubeed, Hebah Muhsien Sabiah, Ronald B. H. Wills, and Jayani Chandrapala. 2022. "Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review" Molecules 27, no. 5: 1719. https://doi.org/10.3390/molecules27051719
APA StyleAL Ubeed, H. M. S., Wills, R. B. H., & Chandrapala, J. (2022). Post-Harvest Operations to Generate High-Quality Medicinal Cannabis Products: A Systemic Review. Molecules, 27(5), 1719. https://doi.org/10.3390/molecules27051719








