Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study
Abstract
:1. Introduction
2. Results
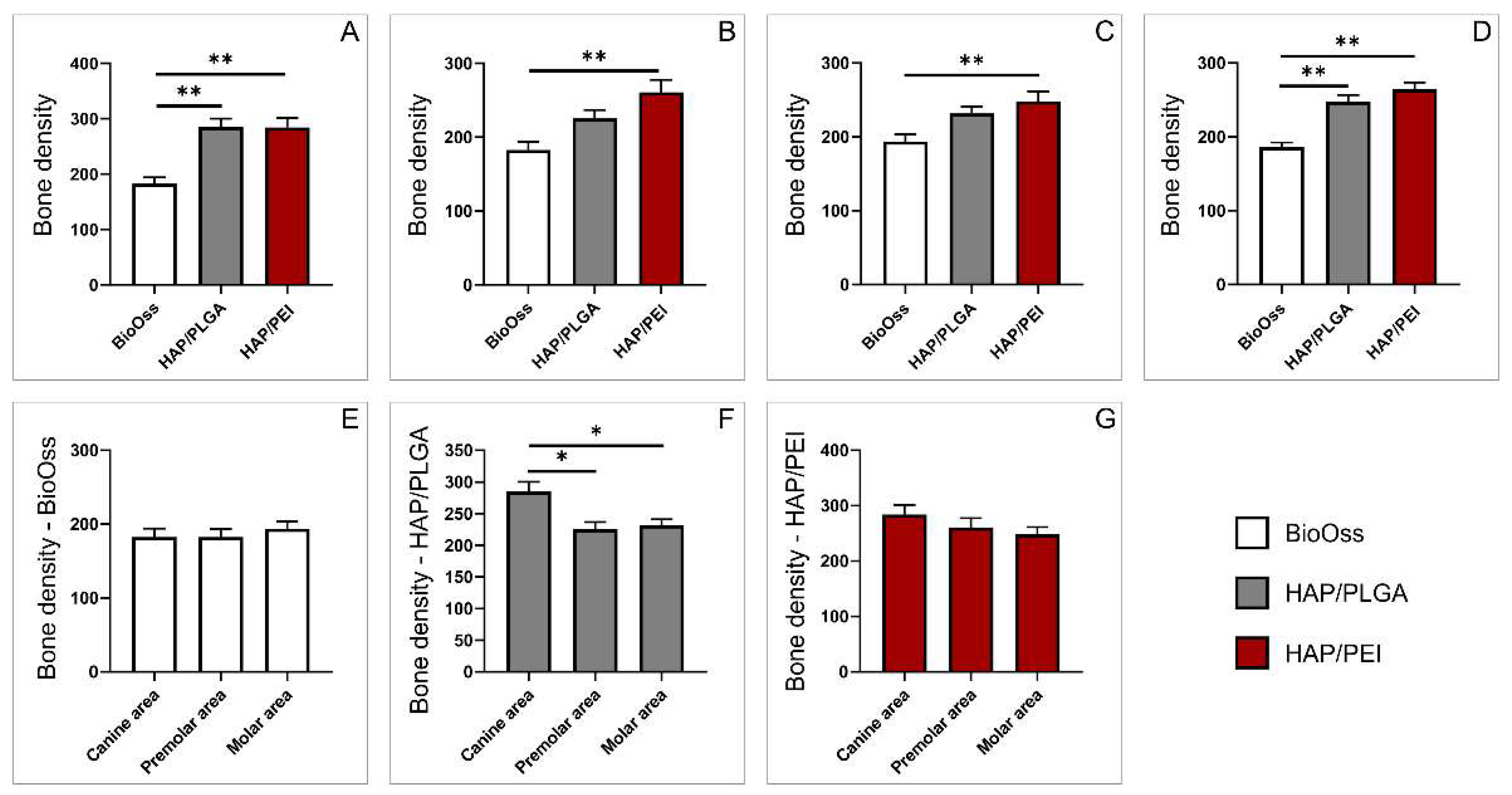
2.1. Bone Density
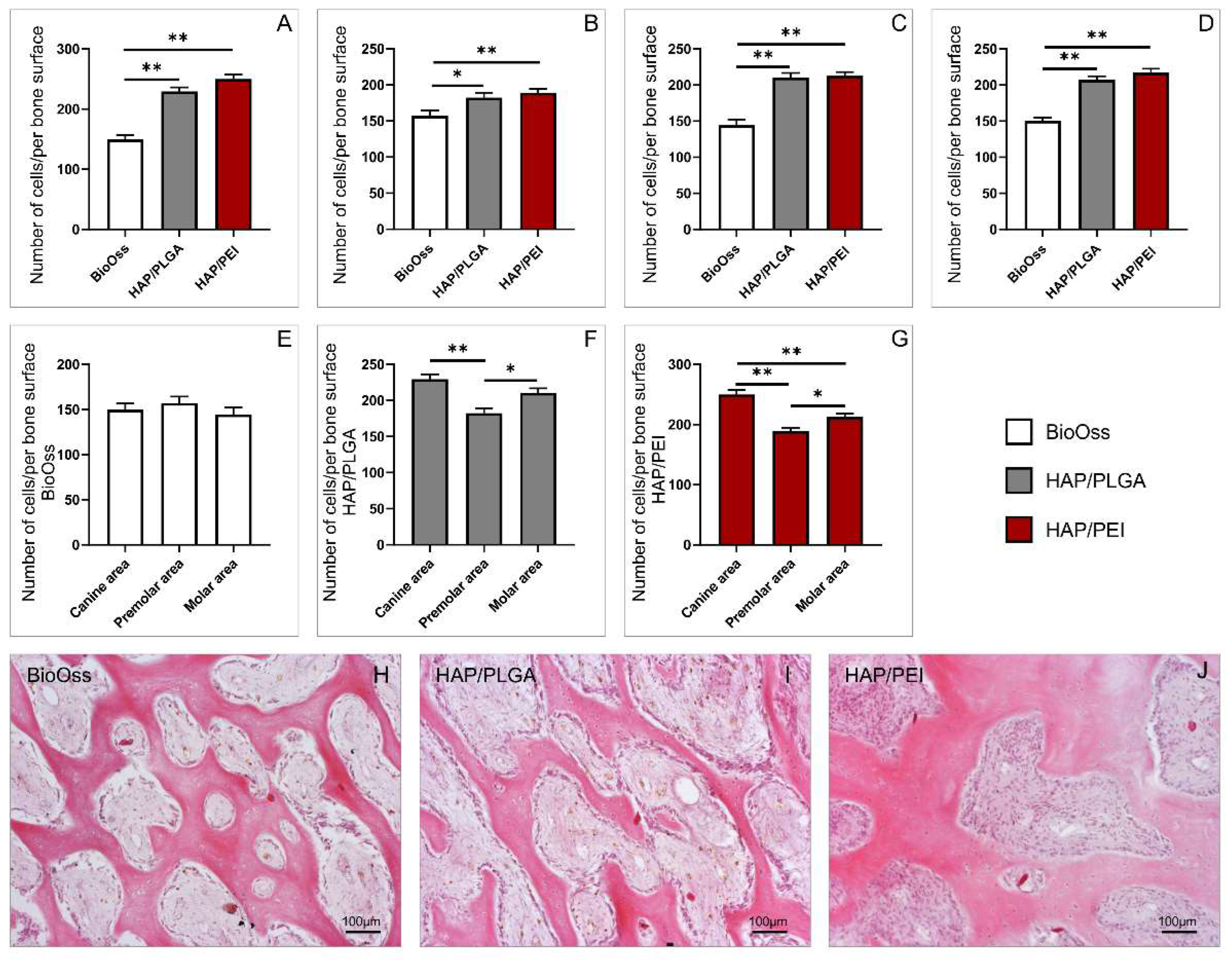
2.2. Number of Cells in the Investigated Regions
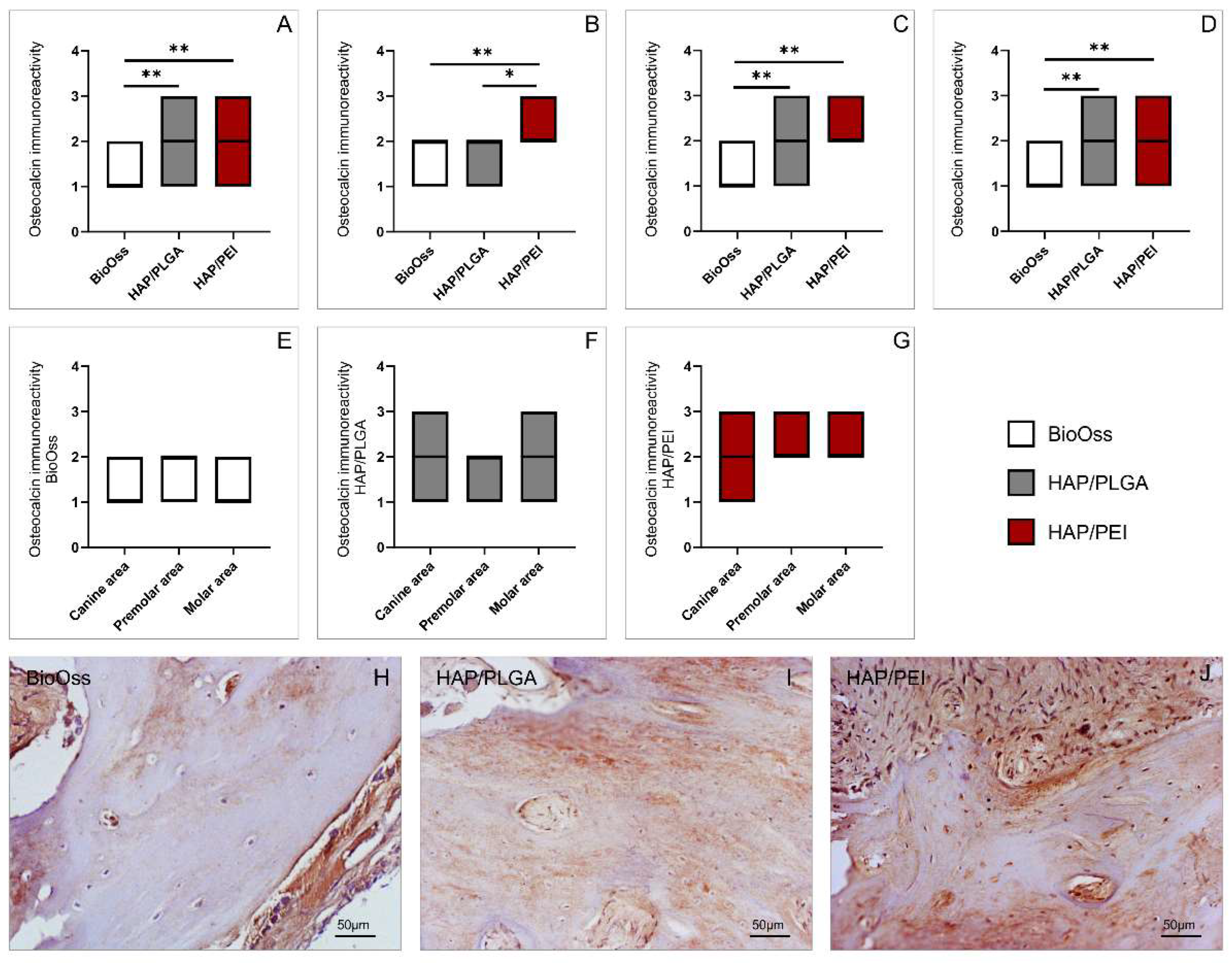
2.3. Immunoreactivity to Osteocalcin
2.4. Collagen Deposition in the Investigated Regions
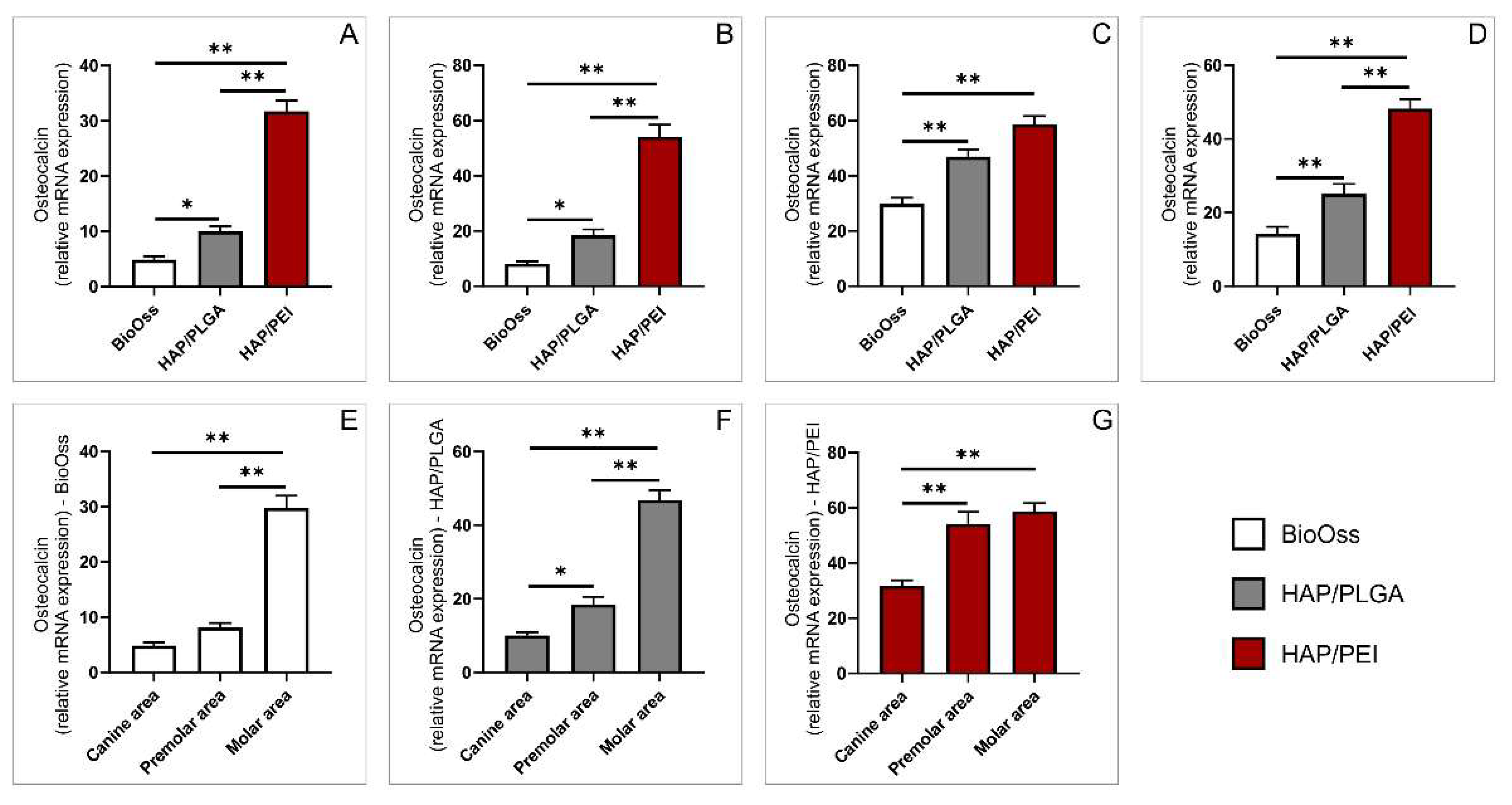
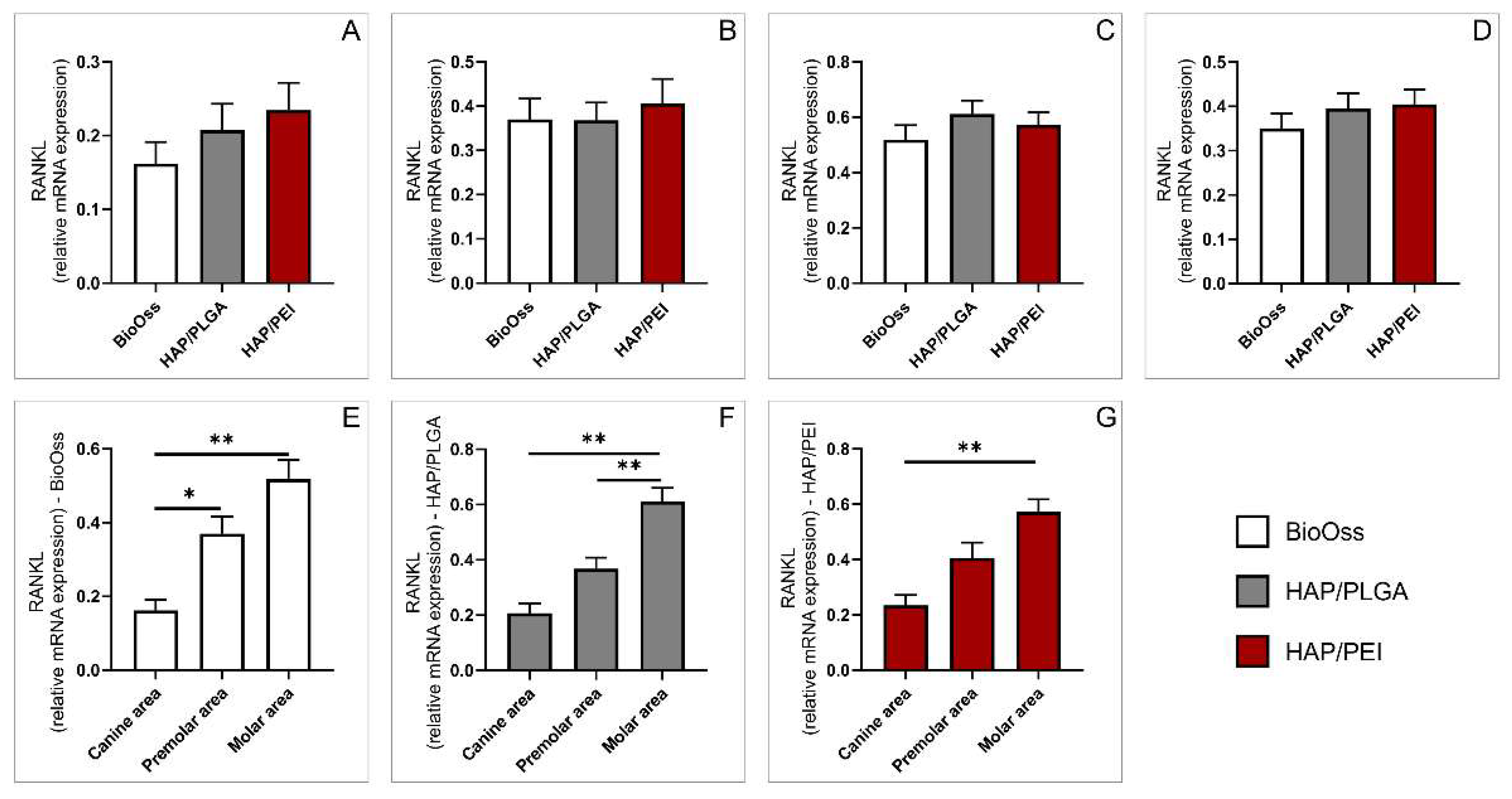
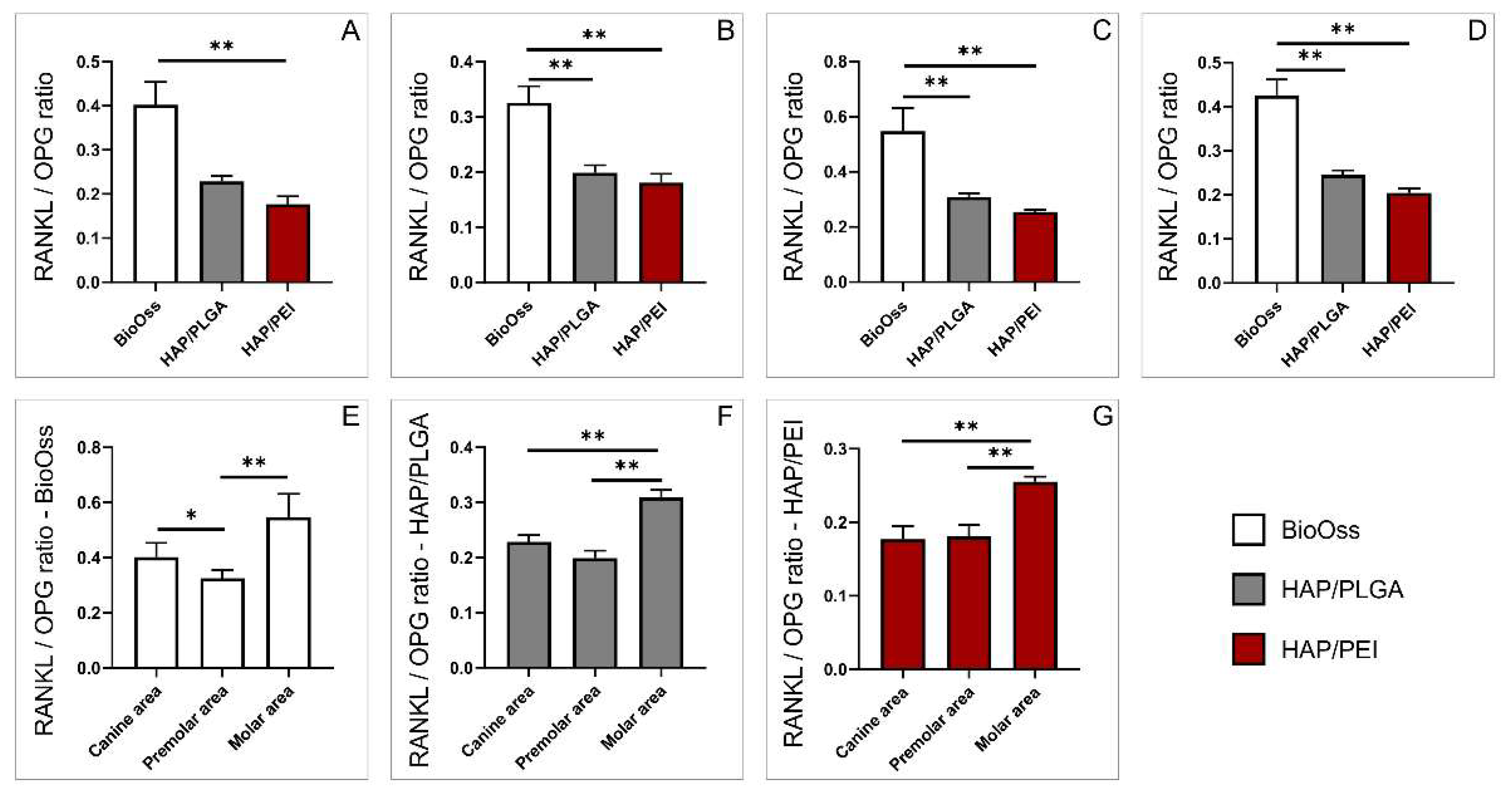
2.5. Expression of Genes Involved in Bone Remodeling
3. Discussion
4. Materials and Methods
4.1. Scaffold Preparation and Characterization
- Hydrothermal synthesis of HAP powder: The first stage of scaffold synthesis was the hydrothermal synthesis of HAP powder from a stoichiometric mixture of (NH4)2HPO4 and Ca(OH)2. After mixing the same volumes of aqueous solutions (500 mL of 3.02 cmol), the hydrothermal treatment was performed in autoclave at 150 °C under the pressure of 5·105 Pa for 8 h. The precipitate was decanted, dried at 80 °C for 48 h, washed with deionized water, and centrifuged. Then, 5 g of hydrothermally synthesized HAP and 1.5 g of poly(ethylene vinyl acetate)/poly(ethylene vinyl versatate) were mixed and further processed in the autoclave at 120 °C for 2 h.
- Synthesis of porous HAP granules: The obtained HAP powder was mixed with water to form ceramic slurry. Polyurethane foams with the required pore size were then dipped into the slurry to form scaffold porous structure. The ceramic slurry-coated polyurethane foams were left to dry at room temperature, then heated in an oven at 600 °C to burn away the foam, and finally sintered at 1200 °C for 4 h. The obtained porous HAP compact was further disintegrated into granules of sizes 300 µm−1 mm.
- The deposition of a PLGA and PEI film onto the surface of HAP granules: The final step in scaffold synthesis was the deposition of a thin film of PLGA or PEI onto the surface of HAP granules. PLGA pellets were dissolved in chloroform to obtain a 1% w/w solution, which was then poured over the HAP granules. After the solvent evaporation, thin PLGA film was formed on granule surfaces to form HAP/PLGA scaffold (signed in previous reports as ALBO OS). Branched PEI (3 g) was dissolved in 15 mL water by heating and stirring. To reduce amino content and cytotoxicity of PEI it was modified with carbon dioxide (CO2). Carbon dioxide was bubbled into this solution at ambient temperature and stirring was continued for 5 h until the reaction was complete. The contents were transferred to an Eppendorf tube, freeze dried to form solid PEI- CO2, and later dissolved in ethanol. HA/PEI coatings were obtained by immersion of HAP granules in the prepared solution.
4.2. Investigations on Animal Models
4.3. Surgical Procedure
- HAP/PLGA group—defects filled with HAP/PLGA scaffold (n = 30)
- HAP/PEI group—defects filled with HAP/PEI scaffold (n = 30)
- Control group—defects filled with BioOss scaffold (n = 30)
4.4. Analysis of Bone Density with Cone-Beam Computed Tomography (CBCT)
4.5. Histological Analysis
4.6. RT PCR Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Probst, F.A.; Fliefel, R.; Burian, E.; Probst, M.; Eddicks, M.; Cornelsen, M.; Riedl, C.; Seitz, H.; Aszódi, A.; Schieker, M.; et al. Bone regeneration of minipig mandibular defect by adipose derived mesenchymal stem cells seeded tri-calcium phosphate- poly(D,L-lactide-co-glycolide) scaffolds. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef]
- Chapekar, M.S. Tissue engineering: Challenges and opportunities. J. Biomed. Mater. Res. 2000, 53, 617–620. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Soldatović, I.; Antonijević, D.; Milosavljević, P.; Sjerobabin, N.; Sopta, J. Extraordinary biological properties of a new calcium hydroxyapatite/poly(lactide-co-glycolide)-based scaffold confirmed by in vivo investigation. Biomed. Eng. Biomed. Tech. 2017, 62, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: Recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Micic, M.; Antonijevic, D.; Milutinovic-Smiljanic, S.; Trisic, D.; Colovic, B.; Kosanovic, D.; Prokic, B.; Vasic, J.; Zivkovic, S.; Milasin, J.; et al. Developing a novel resorptive hydroxyapatite-based bone substitute for over-critical size defect reconstruction: Physicochemical and biological characterization and proof of concept in segmental rabbit’s ulna reconstruction. Biomed. Eng. Biomed. Tech. 2020, 65, 491–505. [Google Scholar] [CrossRef]
- Karadzic, I.; Vucic, V.; Jokanovic, V.; Debeljak-Martacic, J.; Markovic, D.; Petrovic, S.; Glibetic, M. Effects of novel hydroxyapatite-based 3D biomaterials on proliferation and osteoblastic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 350–357. [Google Scholar] [CrossRef]
- Liu, L.; Shi, G.; Cui, Y.; Li, H.; Li, Z.; Zeng, Q.; Guo, Y. Individual construction of freeform-fabricated polycaprolactone scaffolds for osteogenesis. Biomed. Eng. Biomed. Tech. 2017, 62, 467–479. [Google Scholar] [CrossRef]
- Jokanović, V.; Čolović, B.; Marković, D.; Petrović, M.; Jokanović, M.; Milosavljević, P.; Sopta, J. In Vivo investigation of ALBO-OS scaffold based on hydroxyapatite and PLGA. J. Nanomater. 2016, 2016, 3948768. [Google Scholar] [CrossRef] [Green Version]
- Furtos, G.; Rivero, G.; Rapuntean, S.; Abraham, G.A. Amoxicillin-loaded electrospun nanocomposite membranes for dental applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2017, 105, 966–976. [Google Scholar] [CrossRef]
- Mirică, I.-C.; Furtos, G.; Lucaciu, O.; Pascuta, P.; Vlassa, M.; Moldovan, M.; Campian, R.-S. Electrospun Membranes Based on Polycaprolactone, Nano-hydroxyapatite and Metronidazole. Materials 2021, 14, 931. [Google Scholar] [CrossRef]
- Bizenjima, T.; Takeuchi, T.; Seshima, F.; Saito, A. Effect of poly(lactide-co-glycolide) (PLGA)-coated beta-tricalcium phosphate on the healing of rat calvarial bone defects: A comparative study with pure-phase beta-tricalcium phosphate. Clin. Oral Implant. Res. 2016, 27, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Ku, I.-N. Application of polyethyleneimine-modified scaffolds to the regeneration of cartilaginous tissue. Biotechnol. Prog. 2009, 25, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.M.; Hatti-Kaul, R. Protein stabilising effect of polyethyleneimine. J. Biotechnol. 1999, 72, 21–31. [Google Scholar] [CrossRef]
- Guerra-Crespo, M.; Charli, J.L.; Rosales-García, V.H.; Pedraza-Alva, G.; Pérez-Martínez, L. Polyethylenimine improves the transfection efficiency of primary cultures of post-mitotic rat fetal hypothalamic neurons. J. Neurosci. Methods 2003, 127, 179–192. [Google Scholar] [CrossRef]
- Tetè, S.; Vinci, R.; Zizzari, V.; Zara, S.; La Scala, V.; Cataldi, A.; Gherlone, E.; Piattelli, A. Maxillary sinus augmentation procedures through equine-derived biomaterial or calvaria autologous bone: Immunohistochemical evaluation of OPG/RANKL in humans. Eur. J. Histochem. 2013, 57, 10. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, M.C.; Pieters, B.C.H.; Guimarães, P.B.; Duffles, L.F.; Heredia, J.E.; Silveira, A.L.M.; Oliveira, A.C.C.; Teixeira, M.M.; Ferreira, A.V.M.; Silva, T.A.; et al. Bovine milk extracellular vesicles are osteoprotective by increasing osteocyte numbers and targeting RANKL/OPG system in experimental models of bone loss. Front. Bioeng. Biotechnol. 2020, 8, 891. [Google Scholar] [CrossRef]
- Jura-Półtorak, A.; Szeremeta, A.; Olczyk, K.; Zoń-Giebel, A.; Komosińska-Vassev, K. Bone metabolism and RANKL/OPG ratio in rheumatoid arthritis women treated with TNF-α inhibitors. J. Clin. Med. 2021, 10, 2905. [Google Scholar] [CrossRef]
- Van Tuyl, L.H.D.; Voskuyl, A.E.; Boers, M.; Geusens, P.; Landewé, R.B.M.; Dijkmans, B.A.C.; Lems, W.F. Baseline RANKL:OPG ratio and markers of bone and cartilage degradation predict annual radiological progression over 11 years in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 1623–1628. [Google Scholar] [CrossRef]
- Sheikh, Z.; Javaid, M.; Abdallah, M. Bone replacement graft materials in dentistry. In Dental Biomaterials (Principles & Applications), 2nd ed.; Khurshid, Z., Sheikh, Z., Eds.; Paramount Publishing Enterprise: Karachi, Pakistan, 2013; pp. 252–254. [Google Scholar]
- Giannoudis, P.V.; Dinopoulos, H.; Tsiridis, E. Bone substitutes: An update. Injury 2005, 36 (Suppl. S3), S20–S27. [Google Scholar] [CrossRef]
- Khan, W.S.; Rayan, F.; Dhinsa, B.S.; Marsh, D. An osteoconductive, osteoinductive, and osteogenic tissue-engineered product for trauma and orthopaedic surgery: How far are we? Stem Cells Int. 2011, 2012, 236231. [Google Scholar] [CrossRef]
- Shah, A.F.; Saima, S.; Jan, S.M.; Yousuf, A.; Batra, M. Bone grafts and bone substitutes in dentistry. J. Oral Res. Rev. 2016, 8, 36. [Google Scholar] [CrossRef]
- Cypher, T.J.; Grossman, J.P. Biological principles of bone graft healing. J. Foot Ankle Surg. 1996, 35, 413–417. [Google Scholar] [CrossRef]
- Milutinovic-Smiljanic, S.; Antonijevic, D.; Micic, M.; Biocanin, V.; Sjerobabin, N.; Petrovic, B.; Danilovic, V.; Jokanovic, V. The influence of various coatings of hydroxyapatite bone carrier on the success of bone regeneration in rabbit calvarial defects: Histomorphometric and histological analysis. Vojn. Pregl. 2021, 72. [Google Scholar] [CrossRef]
- Kumar, S.; Raj, S.; Sarkar, K.; Chatterjee, K. Engineering a multi-biofunctional composite using poly(ethylenimine) decorated graphene oxide for bone tissue regeneration. Nanoscale 2016, 8, 6820–6836. [Google Scholar] [CrossRef]
- Zhao, R.; Yang, X.; Chen, C.; Chen, K.; Wang, S.; Xie, C.; Ren, X.; Kong, X. The anti-tumor effect of p53 gene-loaded hydroxyapatite nanoparticles in vitro and in vivo. J. Nanoparticle Res. 2014, 16, 2353. [Google Scholar] [CrossRef]
- Ignjatovic, N.; Uskoković, V.; Ajduković, Z.; Uskokovic, D. Multifunctional hydroxyapatite and poly(d,l-lactide-co-glycolide) nanoparticles for the local delivery of cholecalciferol. Mater. Sci. Eng. C 2013, 33, 943–950. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Mu, Z.; Yu, Y.; Zhang, L.; Hu, J. Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery. RSC Adv. 2016, 6, 101790–101799. [Google Scholar] [CrossRef]
- Stein, G.S.; Lian, J.B.; Stein, J.L.; Van Wijnen, A.J.; Montecino, M. Transcriptional control of osteoblast growth and differentiation. Physiol. Rev. 1996, 76, 593–629. [Google Scholar] [CrossRef]
- Gundberg, C.M. Biochemical markers of bone formation. Clin. Lab. Med. 2000, 20, 489–502. [Google Scholar] [CrossRef]
- Dworetzky, S.I.; Fey, E.G.; Penman, S.; Lian, J.B.; Stein, J.L.; Stein, G.S. Progressive changes in the protein composition of the nuclear matrix during rat osteoblast differentiation. Proc. Natl. Acad. Sci. USA 1990, 87, 4605–4609. [Google Scholar] [CrossRef] [Green Version]
- Chesnutt, B.M.; Yuan, Y.; Buddington, K.; Haggard, W.O.; Bumgardner, J.D. Composite chitosan/nano-hydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation In Vivo. Tissue Eng. Part A 2009, 15, 2571–2579. [Google Scholar] [CrossRef]
- Moriishi, T.; Ozasa, R.; Ishimoto, T.; Nakano, T.; Hasegawa, T.; Miyazaki, T.; Liu, W.; Fukuyama, R.; Wang, Y.; Komori, H.; et al. Osteocalcin is necessary for the alignment of apatite crystallites, but not glucose metabolism, testosterone synthesis, or muscle mass. PLoS Genet. 2020, 16, e1008586. [Google Scholar] [CrossRef]
- Komori, T. What is the function of osteocalcin? J. Oral Biosci. 2020, 62, 223–227. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Goldring, S.R. The osteocyte: Key player in regulating bone turnover. RMD Open 2015, 1, e000049. [Google Scholar] [CrossRef]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Lacey, D.L.; Tan, H.L.; Lu, J.; Kaufman, S.; Van, G.; Qiu, W.; Rattan, A.; Scully, S.; Fletcher, F.; Juan, T.; et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am. J. Pathol. 2000, 157, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Jokanović, V.; Marković, D.; Živojinović, V.; Pašalić, S.; Izvonar, D.; Plavšić, M. Kinetics and sintering mechanisms of hydro-thermally obtained hydroxyapatite. Mater. Chem. Phys. 2008, 111, 180–185. [Google Scholar] [CrossRef]
- Biological Evaluation of Medical Devices—Part 2: Animal Welfare Requirements. International Standard ISO 10993-3 2006, ISO 10993-2:2006(E). Available online: https://www.iso.org/standard/36405.html (accessed on 20 February 2022).
- Solakoglu, Önder; Götz, W.; Heydecke, G.; Schwarzenbach, H. Histological and immunohistochemical comparison of two different allogeneic bone grafting materials for alveolar ridge reconstruction: A prospective randomized trial in humans. Clin. Implant Dent. Relat. Res. 2019, 21, 1002–1016. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Target Gene | Forward | Reverse | Genbank Accession No. |
|---|---|---|---|
| β-actin | TTGCTGACAGGATGCAGAAG | GAGCCTCCAATCCAGACAGA | ABF19863.1 |
| OPG | CCAAGGTATCGACCTCTGTGA | GGGCAAGCTTTGCATTAAGA | XM_003481346.4 |
| Osteocalcin | GAAGAGACTCAGGCGCTACC | GGGTTGAGCTCACACACCTC | NM_001164004.1 |
| RANKL | ACACGGATTTGCAAGACACA | CTGCATTTCCTTTTGCACAG | XM_001925694.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stevanovic, M.; Selakovic, D.; Vasovic, M.; Ljujic, B.; Zivanovic, S.; Papic, M.; Zivanovic, M.; Milivojevic, N.; Mijovic, M.; Tabakovic, S.Z.; et al. Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study. Molecules 2022, 27, 1694. https://doi.org/10.3390/molecules27051694
Stevanovic M, Selakovic D, Vasovic M, Ljujic B, Zivanovic S, Papic M, Zivanovic M, Milivojevic N, Mijovic M, Tabakovic SZ, et al. Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study. Molecules. 2022; 27(5):1694. https://doi.org/10.3390/molecules27051694
Chicago/Turabian StyleStevanovic, Momir, Dragica Selakovic, Miroslav Vasovic, Biljana Ljujic, Suzana Zivanovic, Milos Papic, Marko Zivanovic, Nevena Milivojevic, Milica Mijovic, Sasa Z. Tabakovic, and et al. 2022. "Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study" Molecules 27, no. 5: 1694. https://doi.org/10.3390/molecules27051694
APA StyleStevanovic, M., Selakovic, D., Vasovic, M., Ljujic, B., Zivanovic, S., Papic, M., Zivanovic, M., Milivojevic, N., Mijovic, M., Tabakovic, S. Z., Jokanovic, V., Arnaut, A., Milanovic, P., Jovicic, N., & Rosic, G. (2022). Comparison of Hydroxyapatite/Poly(lactide-co-glycolide) and Hydroxyapatite/Polyethyleneimine Composite Scaffolds in Bone Regeneration of Swine Mandibular Critical Size Defects: In Vivo Study. Molecules, 27(5), 1694. https://doi.org/10.3390/molecules27051694








