Efficient Heparin Recovery from Porcine Intestinal Mucosa Using Zeolite Imidazolate Framework-8
Abstract
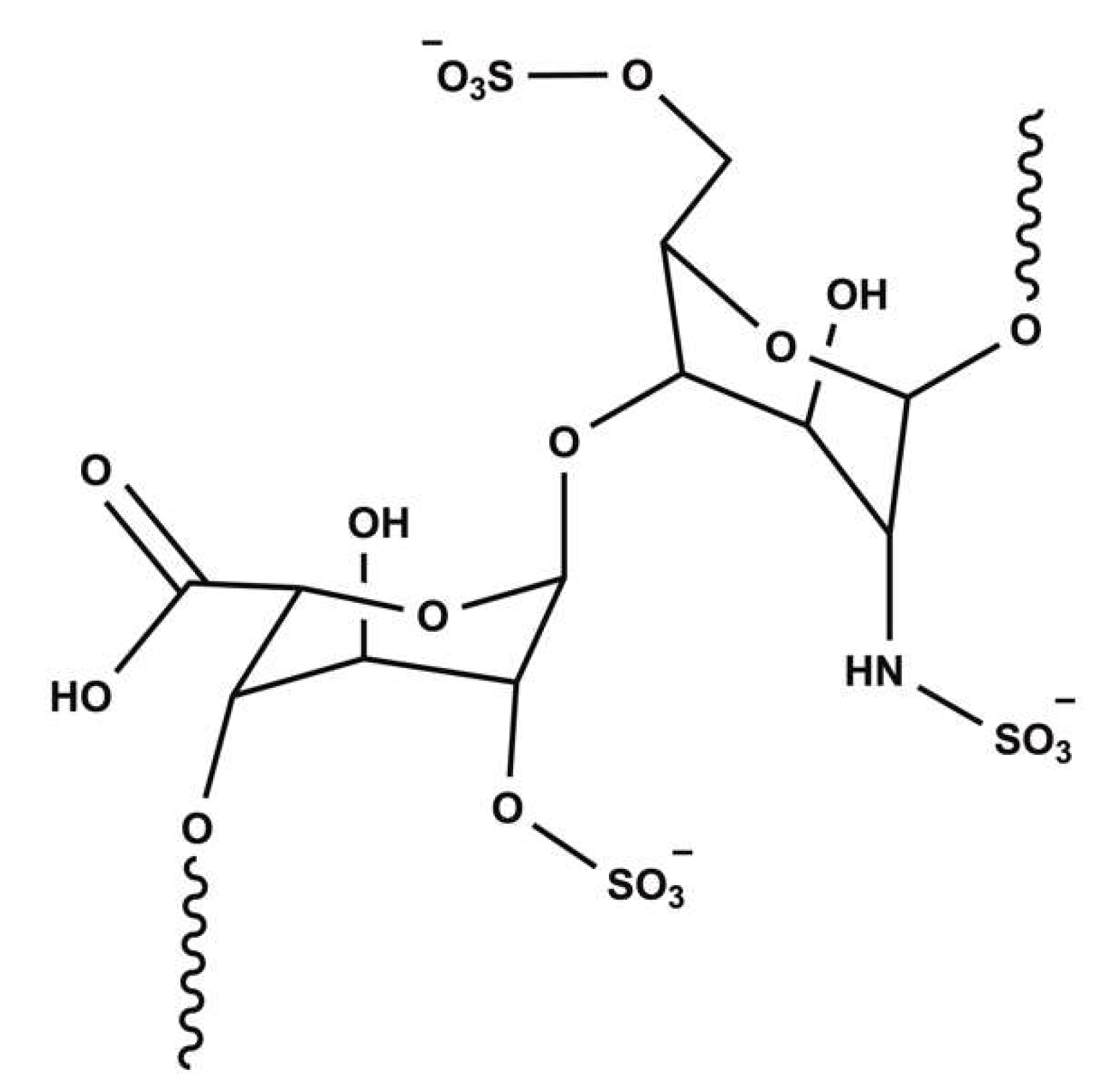
:1. Introduction
2. Result and Discussion
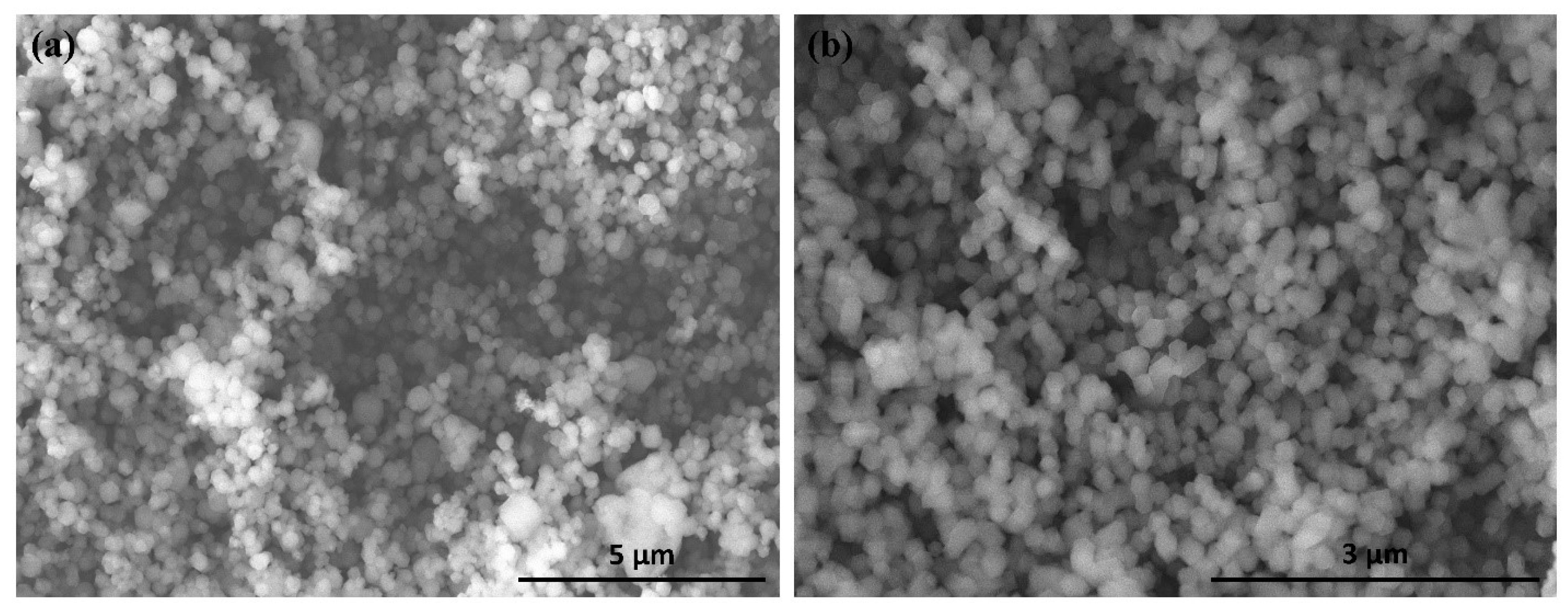
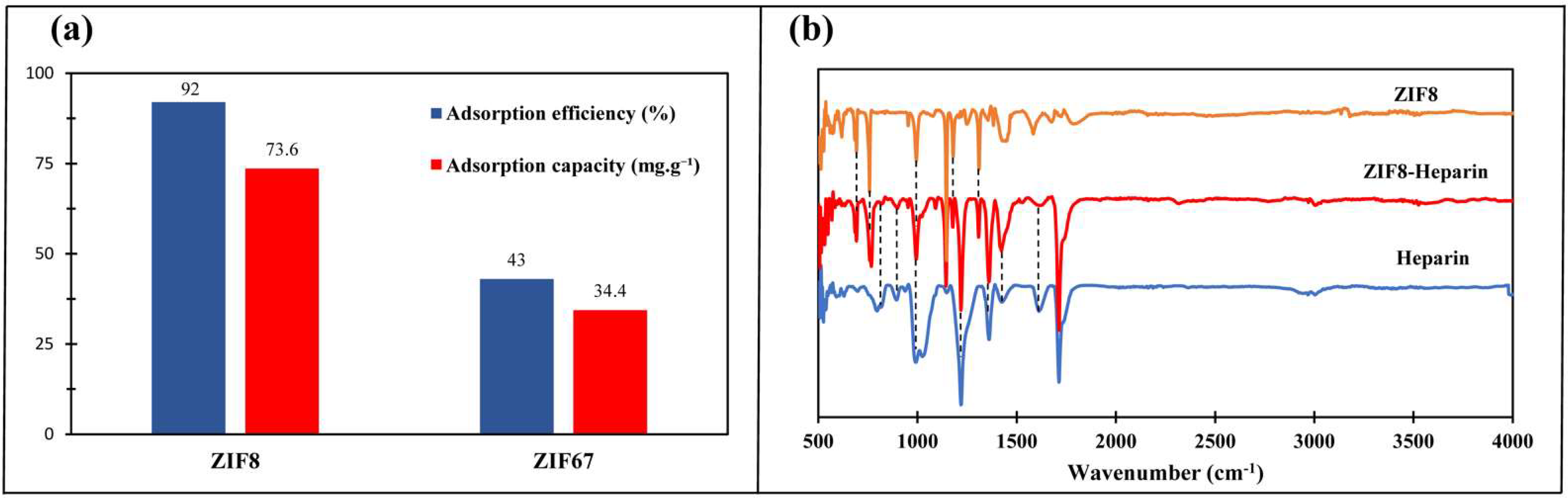
2.1. ZIF-8 and ZIF-67 Characterization
2.2. Heparin Adsorption Studies
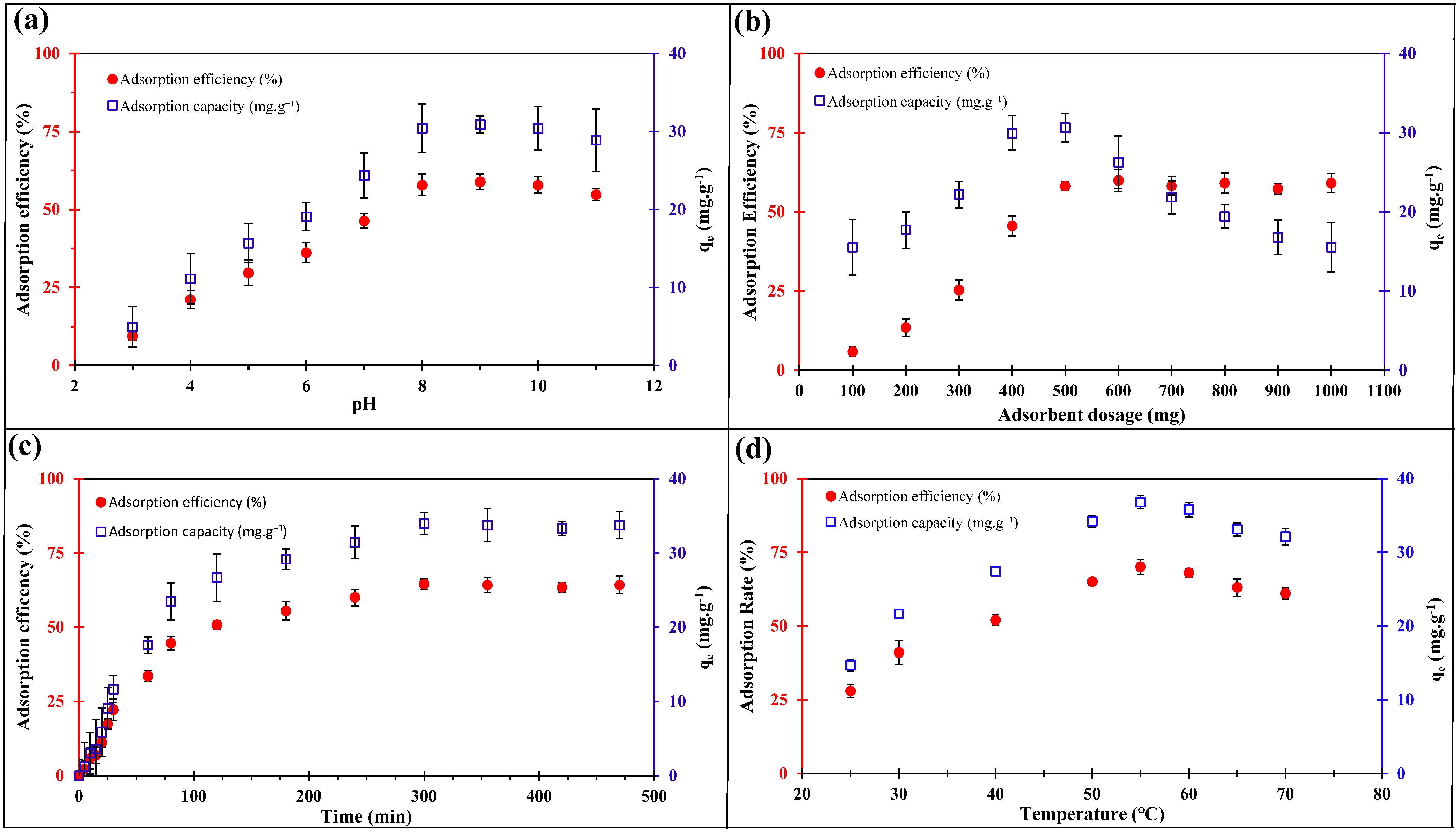
2.2.1. Effect of pH
2.2.2. Effect of the Adsorption Dosage
2.2.3. Adsorption Time and Temperature Effects
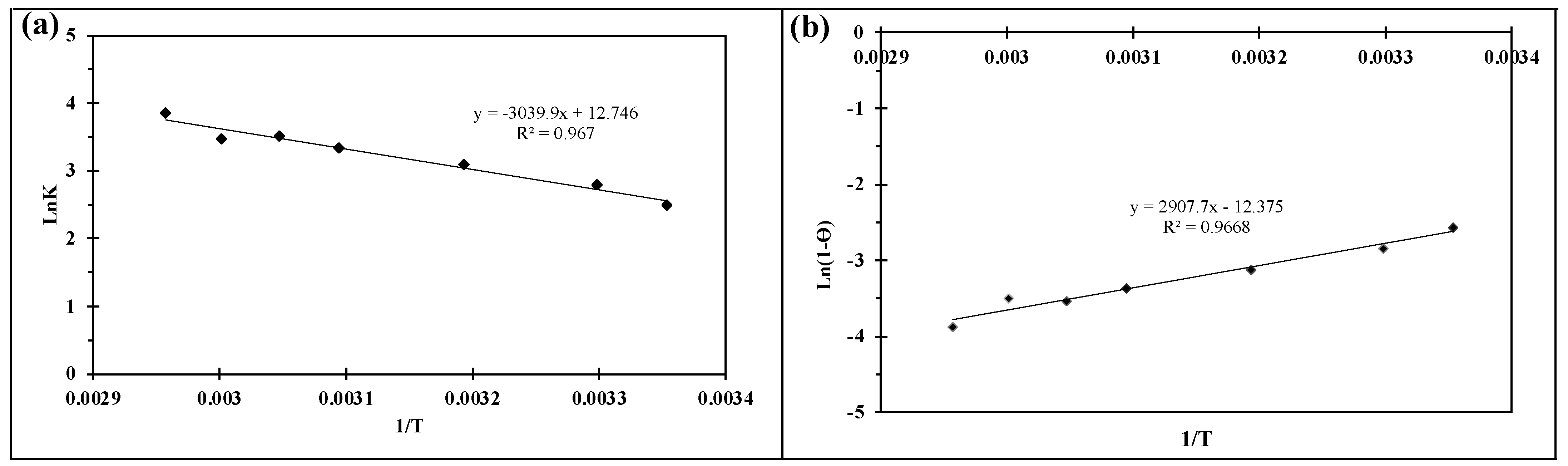
2.2.4. Kinetic and Thermodynamic Studies
2.2.5. Sorbent Reusability
2.2.6. Sheep Plasma Clotting Assay
3. Experimental Procedures
3.1. Materials
3.2. Instrumentation
3.3. Solutions and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Díaz-Torres, L.A. Heparin-based nanoparticles: An overview of their applications. J. Nanomater. 2018, 2018, 9780489. [Google Scholar] [CrossRef] [Green Version]
- Scott, L.J.; Perry, C.M. Tramadol: A review of its use in perioperative pain. Drugs 2000, 60, 139–176. [Google Scholar] [CrossRef] [PubMed]
- Turpie, A.G.G.; Hirsh, J. Heparin. Nova Scotia Med. Bull. 1979, 58, 25–29. [Google Scholar] [CrossRef]
- Urbinati, C.; Milanesi, M.; Lauro, N.; Bertelli, C.; David, G.; D’Ursi, P.; Rusnati, M.; Chiodelli, P. HIV-1 tat and heparan sulfate proteoglycans orchestrate the setup of in cis and in trans cell-surface interactions functional to lymphocyte trans-endothelial migration. Molecules 2021, 26, 7488. [Google Scholar] [CrossRef] [PubMed]
- Vreeburg, J.-W.; Baauw, A. Method for Preparation of Heparin from Mucosa. Patent No. WO2010110654A12010, 30 September 2010. [Google Scholar]
- Lee, M.S.; Kong, J. Heparin: Physiology, pharmacology, and clinical application. Rev. Cardiovasc. Med. 2015, 16, 189–199. [Google Scholar] [CrossRef]
- Anderson, J.A.M.; Saenko, E.L. Heparin resistance. Br. J. Anaesth. 2002, 88, 467–469. [Google Scholar] [CrossRef] [Green Version]
- Flengsrud, R.; Larsen, M.L.; Ødegaard, O.R. Purification, characterization and in vivo studies of salmon heparin. Thromb. Res. 2010, 126, e409–e417. [Google Scholar] [CrossRef]
- Linhardt, R.J.; Ampofo, S.A.; Fareed, J.; Hoppensteadt, D.; Mulliken, J.B.; Folkman, J. Isolation and characterization of human heparin. Biochemistry 1992, 31, 12441–12445. [Google Scholar] [CrossRef]
- Hoke, D.E.; Carson, D.D.; Höök, M. A heparin binding synthetic peptide from human HIP/RPL29 fails to specifically differentiate between anticoagulantly active and inactive species of heparin. J. Negat. Results Biomed. 2003, 2, 1. [Google Scholar] [CrossRef] [Green Version]
- Shafiee, A.; Aibaghi, B.; Zhang, X. Reduced graphene oxide-cadmium sulfide quantum dots nanocomposite based dispersive solid phase microextraction for ultra-trace determination of carbamazepine and phenobarbital. J. Braz. Chem. Soc. 2021, 32, 833–841. [Google Scholar] [CrossRef]
- Shafiee, A.; Aibaghi, B.; Zhang, X. Determination of ethambutol in biological samples using graphene oxide based dispersive solid-phase microextraction followed by ion mobility spectrometry. Int. J. Ion Mobil. Spectrom. 2020, 23, 19–27. [Google Scholar] [CrossRef]
- Reddy, D.H.K.; Yun, Y.-S. Spinel ferrite magnetic adsorbents: Alternative future materials for water purification? Coord. Chem. Rev. 2016, 315, 90–111. [Google Scholar] [CrossRef]
- Ghoraba, Z.; Aibaghi, B.; Soleymanpour, A. Application of cation-modified sulfur nanoparticles as an efficient sorbent for separation and preconcentration of carbamazepine in biological and pharmaceutical samples prior to its determination by high-performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1063, 245–252. [Google Scholar] [CrossRef]
- de Fátima Alpendurada, M. Solid-phase microextraction: A promising technique for sample preparation in environmental analysis. J. Chromatogr. A 2000, 889, 3–14. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Godec, M.; Arshadi, M.; Padilla-Zakour, O.I.; Abbaspourrad, A. Multi-porous quaternized chitosan/polystyrene microbeads for scalable, efficient heparin recovery. Chem. Eng. J. 2018, 348, 399–408. [Google Scholar] [CrossRef]
- Arshadi, M.; Eskandarloo, H.; Enayati, M.; Godec, M.; Abbaspourrad, A. Highly water-dispersible and antibacterial magnetic clay nanotubes functionalized with polyelectrolyte brushes: High adsorption capacity and selectivity toward heparin in batch and continuous system. Green Chem. 2018, 20, 5491–5508. [Google Scholar] [CrossRef]
- Enayati, M.; Abdolmaleki, M.K.; Abbaspourrad, A. Synthesis of cross-linked spherical polycationic adsorbents for enhanced heparin recovery. ACS Biomater. Sci. Eng. 2020, 6, 2822–2831. [Google Scholar] [CrossRef]
- Eskandarloo, H.; Arshadi, M.; Enayati, M.; Abbaspourrad, A. Highly efficient recovery of heparin using a green and low-cost quaternary ammonium functionalized halloysite nanotube. ACS Sustain. Chem. Eng. 2018, 6, 15349–15360. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to metal–organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Aunan, E.; Affolter, C.W.; Olsbye, U.; Lillerud, K.P. Modulation of the thermochemical stability and adsorptive properties of MOF-808 by the selection of non-structural ligands. Chem. Mater. 2021, 33, 1471–1476. [Google Scholar] [CrossRef]
- Luo, Y.; Estudillo-Wong, L.A.; Cavillo, L.; Granozzi, G.; Alonso-Vante, N. An easy and cheap chemical route using a MOF precursor to prepare Pd–Cu electrocatalyst for efficient energy conversion cathodes. J. Catal. 2016, 338, 135–142. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Na, J.; Kang, Y.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angew. Chem. Int. Ed. 2020, 59, 2066–2070. [Google Scholar] [CrossRef] [PubMed]
- Faust, T. MOFs move to market. Nat. Chem. 2016, 8, 990–991. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, U.; Jee, S.; Rao, P.C.; Shin, J.; Ko, C.; Yoon, M.; Park, K.S.; Choi, K.M. Recent advances in process engineering and upcoming applications of metal–organic frameworks. Coord. Chem. Rev. 2021, 426, 213544. [Google Scholar] [CrossRef]
- Chen, B.; Yang, Z.; Zhu, Y.; Xia, Y. Zeolitic imidazolate framework materials: Recent progress in synthesis and applications. J. Mater. Chem. A 2014, 2, 16811–16831. [Google Scholar] [CrossRef]
- Jiao, C.; Li, M.; Ma, R.; Wang, C.; Wu, Q.; Wang, Z. Preparation of a Co-doped hierarchically porous carbon from Co/Zn-ZIF: An efficient adsorbent for the extraction of trizine herbicides from environment water and white gourd samples. Talanta 2016, 152, 321–328. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Feng, P.; Chai, H.; Huang, Y. ZIF-67 derived hollow cobalt sulfide as superior adsorbent for effective adsorption removal of ciprofloxacin antibiotics. Chem. Eng. J. 2018, 344, 95–104. [Google Scholar] [CrossRef]
- Ganta, D.; Guzman, C.; Combrink, K.; Fuentes, M. Adsorption and Removal of Thymol from Water Using a Zeolite Imidazolate Framework-8 Nanomaterial. Anal. Lett. 2021, 54, 625–636. [Google Scholar] [CrossRef]
- Bayati, B.; Ghorbani, A.; Ghasemzadeh, K.; Iulianelli, A.; Basile, A. Study on the separation of H2 from CO2 using a ZIF-8 membrane by molecular simulation and Maxwell-Stefan model. Molecules 2019, 24, 4350. [Google Scholar] [CrossRef] [Green Version]
- Sahoo, T.R.; Prelot, B. Adsorption processes for the removal of contaminants from wastewater: The perspective role of nanomaterials and nanotechnology. In Micro and Nano Technologies, Nanomaterials for the Detection and Removal of Wastewater Pollutant; Bonelli, B., Freyria, F.S., Rossetti, I., Sethi, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 161–222. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Alizadeh, A.; Malekhosseini, Z.; Ranjbar, M. Removal of bromocresol green from aqueous solution via adsorption on Ziziphus nummularia as a new, natural, and low-cost adsorbent: Kinetic and thermodynamic study of removal process. J. Chem. Eng. Data 2011, 56, 3738–3746. [Google Scholar] [CrossRef]
- Ghadim, E.E.; Manouchehri, F.; Soleimani, G.; Hosseini, H.; Kimiagar, S.; Nafisi, S. Adsorption properties of tetracycline onto graphene oxide: Equilibrium, kinetic and thermodynamic studies. PLoS ONE 2013, 8, e79254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaedi, M.; Shokrollahi, A.; Hossainian, H.; Kokhdan, S.N. Comparison of activated carbon and multiwalled carbon nanotubes for efficient removal of eriochrome cyanine R (ECR): Kinetic, isotherm, and thermodynamic study of the removal process. J. Chem. Eng. Data 2011, 56, 3227–3235. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Simonin, J.P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef] [Green Version]
- Robati, D. Pseudo-second-order kinetic equations for modeling adsorption systems for removal of lead ions using multi-walled carbon nanotube. J. Nanostruct. Chem. 2013, 3, 55. [Google Scholar] [CrossRef] [Green Version]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green synthesis of Zinc oxide nanoparticles for enhanced adsorption of lead ions from aqueous solutions: Equilibrium, kinetic and thermodynamic studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Juang, R.-S. Initial behavior of intraparticle diffusion model used in the description of adsorption kinetics. Chem. Eng. J. 2009, 153, 1–8. [Google Scholar] [CrossRef]
- Chien, S.H.; Clayton, W.R. Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci. Soc. Am. J. 1980, 44, 265–268. [Google Scholar] [CrossRef]
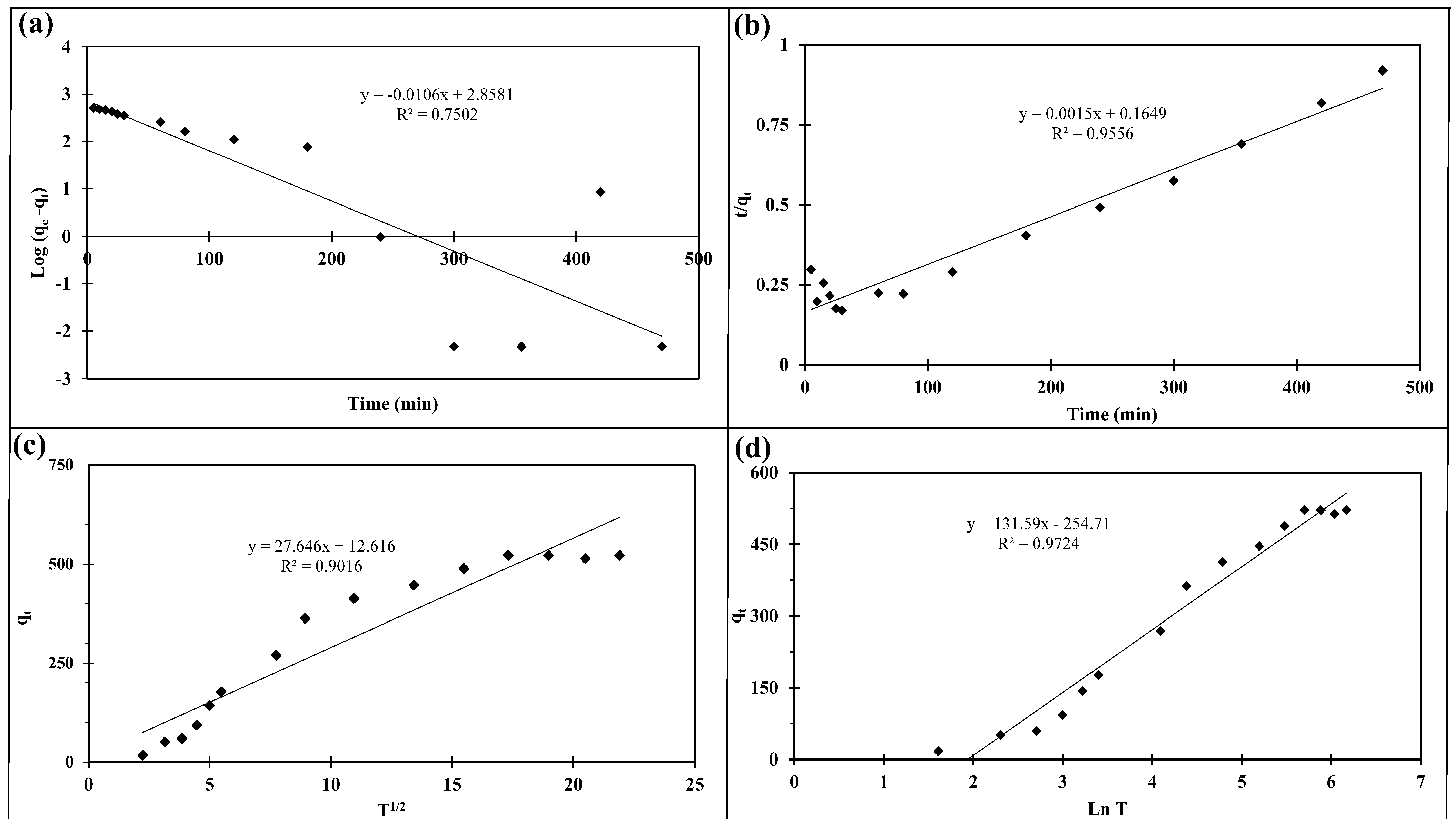

| Model | Parameter | Value |
|---|---|---|
| Pseudo-first-order | 0.024 | |
| 721.31 | ||
| 0.8502 | ||
| Pseudo-second-order | 1.34 10−5 | |
| 672.22 | ||
| 0.9556 | ||
| Intraparticle Diffusion | 27.64 | |
| 12.61 | ||
| 0.901 | ||
| Elovich | 0.0076 | |
| 1.58 | ||
| 0.9724 | ||
| 522.11 |
| ΔH° (J/mol·K) | ΔS° (KJ/mol) | Ea (KJ/mol) | Temperature (K) | ||||||
| 298 | 303 | 313 | 323 | 328 | 333 | 338 | |||
| 25.27 | 105.97 | 19.41 | ΔG° (KJ/mol) | ||||||
| −31.57 | −32.10 | −33.16 | −34.22 | −34.74 | −35.27 | −35.80 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimi Abdolmaleki, M.; Ganta, D.; Shafiee, A.; Velazquez, C.A.; Khambhati, D.P. Efficient Heparin Recovery from Porcine Intestinal Mucosa Using Zeolite Imidazolate Framework-8. Molecules 2022, 27, 1670. https://doi.org/10.3390/molecules27051670
Karimi Abdolmaleki M, Ganta D, Shafiee A, Velazquez CA, Khambhati DP. Efficient Heparin Recovery from Porcine Intestinal Mucosa Using Zeolite Imidazolate Framework-8. Molecules. 2022; 27(5):1670. https://doi.org/10.3390/molecules27051670
Chicago/Turabian StyleKarimi Abdolmaleki, Mahmood, Deepak Ganta, Ali Shafiee, Carlo Alberto Velazquez, and Devang P. Khambhati. 2022. "Efficient Heparin Recovery from Porcine Intestinal Mucosa Using Zeolite Imidazolate Framework-8" Molecules 27, no. 5: 1670. https://doi.org/10.3390/molecules27051670
APA StyleKarimi Abdolmaleki, M., Ganta, D., Shafiee, A., Velazquez, C. A., & Khambhati, D. P. (2022). Efficient Heparin Recovery from Porcine Intestinal Mucosa Using Zeolite Imidazolate Framework-8. Molecules, 27(5), 1670. https://doi.org/10.3390/molecules27051670








