Secondary Metabolites with Anti-Inflammatory from the Roots of Cimicifuga taiwanensis
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation of Compounds
2.2. Biological Studies
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation and Characterization of Secondary Metabolites
3.4. Determination of NO Production and Cell Viability Assay
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Chen, G.Y.; Nunez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed]
- Ross, R. Atherosclerosis—an inflammatory disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Weiner, H.L.; Frenkel, D. Immunology and immunotherapy of Alzheimer’s disease. Nat. Rev. Immunol. 2006, 6, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Feve, B.; Ferre, P. Diabetes and inflammation: Fundamental aspects and clinical implications. Diabetes Metab. 2010, 36, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancerrelated inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Brown, G.C. Mechanisms of inflammatory neurodegeneration: iNOS and NADPH oxidase. Biochem. Soc. Trans. 2007, 35, 1119–1121. [Google Scholar] [CrossRef]
- Schmidt, H.H.; Walter, U. NO at work. Cell 1994, 78, 919–925. [Google Scholar] [CrossRef]
- Bian, K.; Murad, F. Nitric oxide (NO)—biogeneration, regulation, and relevence to human diseases. Front. Biosci. 2003, 8, d264–d278. [Google Scholar] [CrossRef]
- Ohshima, H.; Bartsch, H. Chronic infections and inflammatory processes as cancer risk factors: Possible role of nitric oxide in carcinogenesis. Mutat. Res. 1994, 305, 253–264. [Google Scholar] [CrossRef]
- Kanwar, J.R.; Kanwar, R.K.; Burrow, H.; Baratchi, S. Recent advances on the roles of NO in cancer and chronic inflammatory disorders. Curr. Med. Chem. 2009, 16, 2373–2394. [Google Scholar] [CrossRef]
- Yang, T.-Y.; Huang, T.C.; Compton, J.A. Ranunculaceae L. In Flora of Taiwan, 2nd ed.; Editorial Committee: Taipei, Taiwan, 1996; Volume 2, pp. 506–574. [Google Scholar]
- Jiang, J.W.; Qi, C.D. Dictionary of Medicinal Plants; Tianjin Science and Technology: Tianjin, China, 2005; Volume 1, pp. 175–176. [Google Scholar]
- Yang, T.Y.A.; Huang, T.C. Additional Remarks on Ranunculaceae in Taiwan (8)—Revision of Ranunculaceae in Taiwan. Taiwania 2008, 53, 210–229. [Google Scholar]
- Chinese Pharmacopoeia Commission. The Pharmacopoeia of Chinese People’s Republic; The Chemical Industry Publishing House: Beijing, China, 2010; pp. 68–69. [Google Scholar]
- Hsiao, W.L.W.; Liu, L. The role of traditional Chinese herbal medicines in cancer therapy from TCM theory to mechanistic insights. Planta Med. 2010, 76, 1118–1131. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.R.; Qing, C.; Zhang, Y.L.; Ji, S.Y.; Li, Z.R.; Pei, S.J.; Qiu, M.H.; Gross, M.L.; Qiu, S.X. Cimicifoetisides A and B, two cytotoxic cycloartane triterpenoid glycosides from the rhizomes of Cimicifuga foetida, inhibit proliferation of cancer cells. Beilstein J. Org. Chem. 2007, 3, 3. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Chen, J.C.; Song, H.J.; Li, Y.; Nian, Y.; Qiu, M.H. Five new triterpene bisglycosides with acyclic side chains from the rhizomes of Cimicifuga foetida L. Chem. Pharm. Bull. 2009, 58, 729–733. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nian, Y.; Zhang, Y.L.; Chen, J.C.; Lu, L.; Qing, C.; Qiu, M.H. Cytotoxic chemical constituents from the roots of Cimicifuga foetida. J. Nat. Prod. 2010, 73, 93–98. [Google Scholar] [CrossRef]
- Nian, Y.; Zhang, X.M.; Li, Y.; Wang, Y.Y.; Chen, J.C.; Lu, L.; Zhou, L.; Qiu, M.H. Cycloartane triterpenoids from the aerial parts of Cimicifuga foetida Linnaeus. Phytochemistry 2011, 72, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Li, X.; Zhao, Z.; Lin, Y.; Zhu, Y.; Ma, G.; Zhao, X.; Xu, X.; Wu, M.; Wu, H.; et al. Two new cycloartane triterpenoid glycosides from the rhizomes of Actaea vaginata. Nat. Prod. Res. 2022, 25, 1–8. [Google Scholar] [CrossRef]
- Lu, N.H.; Yang, Y.R.; Li, X.F.; Liu, H.L.; Zhao, Z.R.; Du, Y.R. New cycloartane triterpenes from the roots of Cimicifuga foetida. Phytochem. Lett. 2021, 42, 109–116. [Google Scholar] [CrossRef]
- Chen, J.Y.; Li, P.L.; Tang, X.L.; Wang, S.J.; Jiang, Y.T.; Shen, L.; Xu, B.M.; Shao, Y.L.; Li, G.Q. Cycloartane triterpenoids and their glycosides from the rhizomes of Cimicifuga foetida. J. Nat. Prod. 2014, 77, 1997–2005. [Google Scholar] [CrossRef]
- Hao, Y.M.; Luo, W.; Jiang, G.Z.; Lv, C.N.; Lu, J.C. One new and seven known triterpene glycosides from the aerial parts of Cimicifuga dahurica. J. Asian. Nat. Prod. Res. 2020, 22, 788–793. [Google Scholar] [CrossRef]
- Yue, G.G.; Xie, S.; Lee, J.K.; Kwok, H.F.; Gao, S.; Nian, Y.; Wu, X.X.; Wong, C.K.; Qiu, M.H.; Lau, C.B. New potential beneficial effects of actein, a triterpene glycoside isolated from Cimicifuga species, in breast cancer treatment. Sci. Rep. 2016, 6, 35263. [Google Scholar] [CrossRef] [PubMed]
- Jarry, H.; Stromeier, S.; Wuttke, W.; Nahrstedt, A. Petasiphenone, a phenol isolated from Cimicifuga racemosa, in vitro inhibits proliferation of the human prostate cancer cell line LNCaP. Planta Med. 2007, 73, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Yim, S.H.; Kim, H.J.; Park, S.H.; Kim, J.; Williams, D.R.; Jung, D.W.; Lee, I.S. Cytotoxic caffeic acid derivatives from the rhizomes of Cimicifuga heracleifolia. Arch. Pharm. Res. 2012, 35, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Kadota, S.; Hattori, M.; Yoshimachi, S.; Shiro, M.; Oogami, N.; Mizuno, H.; Namba, T. Isolation and characterization of ten new cycloartenol triterpenes from Cimicifuga heracleifolia Komarov. Chem. Pharm. Bull. 1993, 41, 832–841. [Google Scholar] [CrossRef]
- Kadota, S.; Li, J.X.; Tanaka, K.; Namba, T. Constituents of Cimicifugae rhizoma. II. Isolation and structures of new cycloartenol triterpenoids and related compounds from Cimicifuga foetida L. Tetrahedron 1995, 51, 1143–1166. [Google Scholar] [CrossRef]
- Nian, Y.; Wang, H.Y.; Zhou, L.; Su, J.; Li, Y.; Qiu, M.H. Cytotoxic cycloartane triterpenes of the traditional Chinese medicine “shengma” (Cimicifuga dahurica). Planta Med. 2013, 79, 60–69. [Google Scholar] [CrossRef]
- Fraga, B.M. Natural sesquiterpenoids. Nat. Prod. Rep. 1999, 16, 711–730. [Google Scholar] [CrossRef]
- Fang, Z.J.; Zhang, T.; Chen, S.X.; Wang, Y.L.; Zhou, C.X.; Mo, J.X.; Wu, Y.J.; Xu, Y.K.; Lin, L.G.; Gan, L.S. Cycloartane triterpenoids from Actaea vaginata with anti-inflammatory effects in LPS-stimulated RAW264.7 macrophages. Phytochemistry 2019, 160, 1–10. [Google Scholar] [CrossRef]
- Thao, N.P.; Lee, Y.S.; Luyen, B.T.T.; Oanh, H.V.; Ali, I.; Arooj, M.; Koh, Y.S.; Yang, S.Y.; Kim, Y.H. Chemicals from Cimicifuga dahurica and Their Inhibitory Effects on Pro-inflammatory Cytokine Production by LPS-stimulated Bone Marrow-derived Dendritic Cells. Nat. Prod. Sci. 2018, 24, 194–198. [Google Scholar] [CrossRef][Green Version]
- Pang, Q.Q.; Mei, Y.D.; Zhang, Y.C.; Liu, L.X.; Shi, D.F.; Pan, D.B.; Yao, X.S.; Li, H.B.; Yu, Y. Three new cycloart-7-ene triterpenoid glycosides from Cimicifuga dahurica and their anti-inflammatory effects. Nat. Prod. Res. 2021, 35, 3634–3643. [Google Scholar] [CrossRef]
- Gieni, R.S.; Li, Y.; Hay Glass, K.T. Comparison of [3H]thymidine incorporation with MTT- and MTS-based bioassays for human and murine IL-2 and IL-4 analysis. Tetrazolium assays provide markedly enhanced sensitivity. J. Immunol. Methods 1995, 70, 85–93. [Google Scholar] [CrossRef]
- Johansson, M.; Kopcke, B.; Anke, H.; Sterner, O. Biologically active secondary metabolites from the ascomycete A111-95. 2. Structure elucidation. J. Antibiot. 2002, 55, 104–106. [Google Scholar] [CrossRef]
- Jain, C.; Khatana, S.; Vijayvergia, R. Bioactivity of secondary metabolites of various plants: A review. Int. J. Pharm. Sci. Res. 2019, 10, 494–504. [Google Scholar]
- Zhang, M.; Yang, Q.; Zhang, X.; Wu, H. A new cycloartane triterpene bisdesmoside from the rhizomes of Actaea vaginata. Nat. Prod. Res. 2021, 35, 3426–3431. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.Q.; Wang, W.H.; Lu, J.; Li, D.S.; Zhou, L.; Qiu, M.H. New Cytotoxic Cycloartane Triterpenes from the Aerial Parts of Actaea heracleifolia (syn. Cimicifuga heracleifolia). Planta Med. 2019, 85, 154–159. [Google Scholar]
- Lu, J.; Peng, X.R.; Li, D.S.; Shi, Q.Q.; Qiu, M.H. Cytotoxic Cycloartane Triterpenoid Saponins from the Rhizomes of Cimicifuga foetida. Nat. Prod. Bioprospect. 2019, 9, 303–310. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, C.J.; Ma, J.; Li, C.; Chen, F.Y.; Wang, N.; Shen, C.J.; Zhang, D.M. Cytotoxic 9,19-cycloartane type triterpenoid glycosides from the roots of Actaea dahurica. Phytochemistry 2019, 160, 48–55. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, C.J.; Ma, J.; Li, C.; Huang, J.W.; Wang, N.; Shen, C.J.; Zhang, D.M. Cytotoxic 9,19-cycloartane Triterpenoids from the Roots of Actaea dahurica. Fitoterapia 2019, 137, 104262. [Google Scholar] [CrossRef]
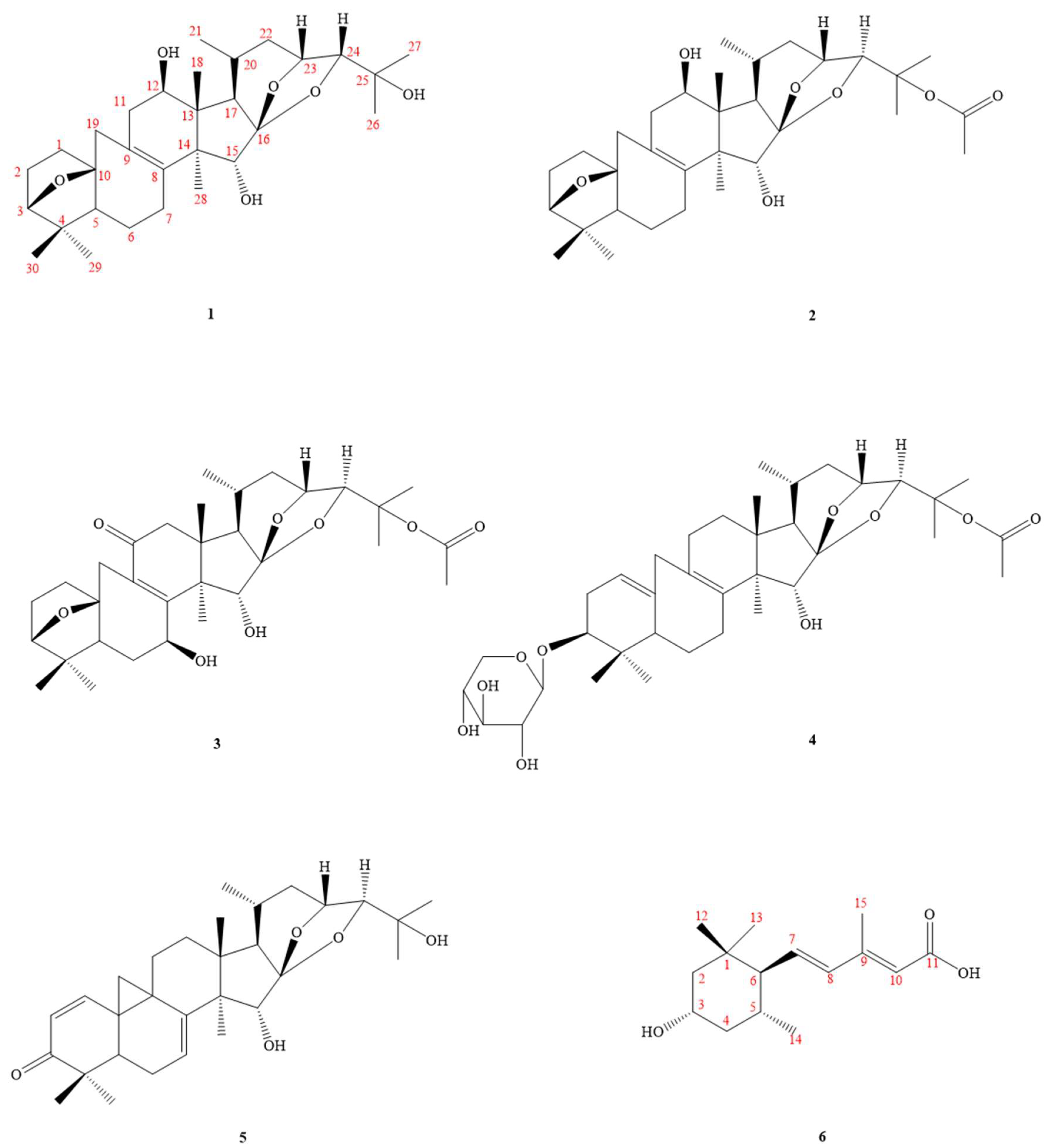

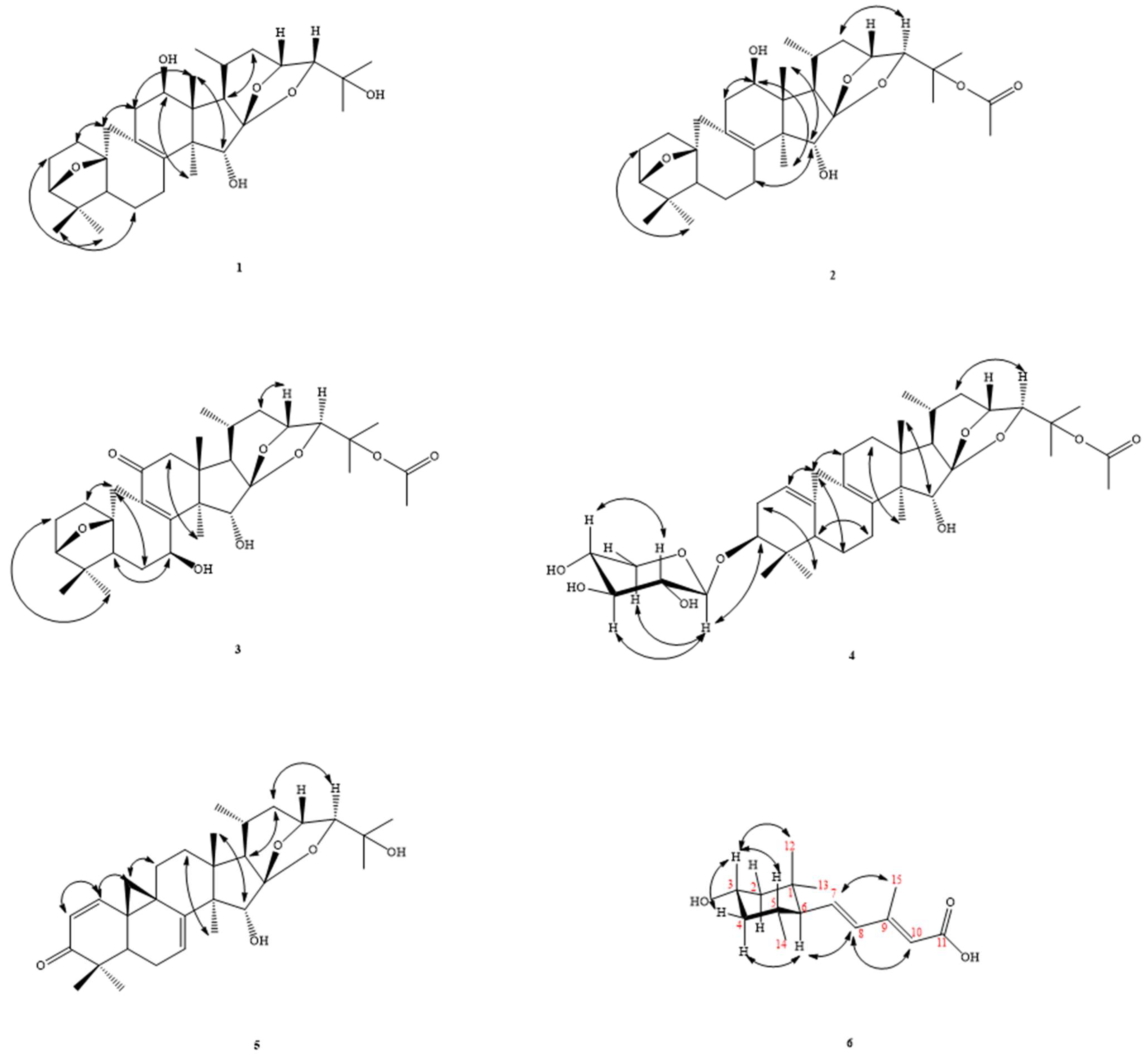
| No | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 1.40 (m, H-1β) 1.58 (m, H-1α) | 1.39 (m) 1.57 (m) | 1.52 (td, J = 12.0, 4.8, H-1β), 1.18 (m, H-1α) | 5.20 (br s) | 6.68 (d, J = 9.8) | |
| 2 | 1.71 (m, H-2β) 1.93 (m, H-2α) | 1.68 (m, H-2β) 1.91 (m, H-2α) | 1.70 (m, H-2β) 1.88 (m, H-2α) | 2.10 (m, H-2β) 2.33 (m, H-2α) | 5.99 (d, J = 9.8) | 1.14 (t, J = 12.0, Hax-1α), 1.75 (ddd, J = 12.0, 4.4, 2.0, Heq-1β) |
| 3 | 3.72 (d, J = 5.6) | 3.71 (d, J = 5.6) | 3.80 (d, J = 5.2) | 3.41 (dd, J = 8.6, 5.4) | 3.81 (tt, J = 12.0, 4.4, Hax-3β) | |
| 4 | 0.94 (q-like, J = 12.0, Hax-4α), 2.03 (dtd, J = 12.0, 4.4, 2.0, Heq-4β) | |||||
| 5 | 1.15 (m) | 1.15 (d, J = 12.0) | 0.93 (m) | 1.95 (m) | 1.92 (m) | 1.61 (m) |
| 6 | 1.68 (m, H-6β) 1.52 (m, H-6α) | 1.48 | 1.84 (m, H-6β) 2.00 (m, H-6α) | 1.46 (m, H-6β) 1.78 (m, H-6α) | 1.76 (m, H-6β) 2.03 (m, H-6α) | 1.45 (t, J = 10.0) |
| 7 | 2.42 (m) | 2.42 (m) | 4.79 (dd, J = 10.2, 7.6) | 2.58 (m, H-7β) 2.43 (m, H-7α) | 5.74 (dd, J = 7.4, 1.8) | 5.85 (dd, J = 15.6, 10.0) |
| 8 | 6.08 (d, J = 15.6) | |||||
| 9 | ||||||
| 10 | 5.73 (br s) | |||||
| 11 | 1.84 (m, H-11β) 2.59 (m, H-11α) | 1.82 (m, H-11β) 2.59 (m, H-11α) | 2.03 (m, H-11β) 1.24 (m, H-11α) | 1.44 (m, H-11β) 2.30 (m, H-11α) | ||
| 12 | 4.10 (t, J = 7.6, H-12α) | 4.19 (m) | 2.43/2.72 (each d, J = 17.6, H-12β/α) | 1.48 (m, H-12β) 1.84 (m, H-12α) | 1.78 (m, H-12β) 1.86 (m, H-12α) | 0.86 (s) |
| 13 | 0.87 (s) | |||||
| 14 | 0.81 (d, J = 6.8) | |||||
| 15 | 4.02 (d, J = 7.6, H-15β) 2.77 (d, J = 7.6, OH) | 4.10 (d, J = 7.6) | 4.74 (br s) | 4.07 (s) | 4.06 (s) | 2.28 (d, J = 1.2) |
| 16 | ||||||
| 17 | 1.47 (d, J = 1.4) | 1.54 (m) | 1.56 (d, J = 11.2) | 1.34 (m) | 1.33 (d, J = 10.9) | |
| 18 | 0.83 (s) | 0.82 (s) | 1.05 (s) | 0.83 (s) | 1.03 (s) | |
| 19 | 1.69 (br d, J = 13.6), 3.14 (br d, J = 13.6) | 1.67 (br d, J = 13.6), 3.12 (br d, J = 13.6) | 2.69/3.15 (each d, J = 13.2), | 2.41 (br d, J = 14.8), 2.83 (br d, J = 14.8) | 1.11/1.66 (each d, J = 4.0) | |
| 20 | 1.51 (m) | 1.52 (m) | 1.68 (m) | 1.64 (m) | 1.61 (m) | |
| 21 | 1.08 (d, J = 6.4) | 1.08 (d, J = 5.2) | 0.91 (d, J = 6.8) | 0.91-(d, J = 6.8) | 0.90 (d, J = 6.8) | |
| 22 | 2.00 (m, H-22β) 2.15 (m, H-22α) | 2.35 (m, H-22β) 1.01 (m, H-22α) | 2.35 (m, H-22β) 1.01 (m, H-22α) | 2.31 (m, H-22β) 0.97 (m, H-22α) | 1.96 (m, H-22β) 2.16 (m, H-22α) | |
| 23 | 4.42 (ddd, J = 9.6, 4.0, 3.2) | 4.37 (d, J = 9.2) | 4.40 (br d, J = 9.2) | 4.37 (d, J = 8.4) | 4.45 (ddd, J = 9.8, 4.4, 2.6) | |
| 24 | 3.54 (d, J = 4.0) | 3.90 (s) | 3.93 (s) | 3.90 (s) | 3.57 (s) | |
| 25 | ||||||
| 26 | 1.22 (s) | 1.41 (s) | 1.41 (s) | 1.41 (s) | 1.21 (s) | |
| 27 | 1.32 (s) | 1.45 (s) | 1.46 (s) | 1.45 (s) | 1.32 (s) | |
| 28 | 0.92 (s) | 0.93 (s) | 1.15 (s) | 0.94 (s) | 1.07 (s) | |
| 29 | 0.99 (s) | 0.97 (s) | 0.99 (s) | 0.99 (s) | 1.13 (s) | |
| 30 | 0.94 (s) | 0.91 (s) | 0.98 (s) | 0.78 (s) | 1.02 (s) | |
| OAc | 1.97 (s) | 1.99 (s) | 1.98 (s) | |||
| OAc | ||||||
| 1′ | 4.43, (d, J = 5.9) | |||||
| 2′ | 3.45, (dd, J = 7.2, 5.9) | |||||
| 3′ | 3.58 (t, J = 7.2) | |||||
| 4′ | 3.72 (td, J = 7.2, 4.4) | |||||
| 5′ | 4.02 (dd, J = 12.2, 4.4, H-5′β) 3.33 (m, J = 12.2, 7.2, H-5′α) |
| No | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1 | 36.2 | 36.4 | 35.3 | 116.9 | 151.6 | 36.0 |
| 2 | 25.4 | 25.8 | 25.4 | 31.1 | 126.9 | 50.4 |
| 3 | 84.9 | 84.8 | 86.0 | 85.1 | 203.9 | 66.9 |
| 4 | 45.2 | 45.4 | 44.9 | 38.1 | 45.2 | 44.8 |
| 5 | 54.6 | 54.7 | 50.3 | 50.0 | 40.1 | 31.5 |
| 6 | 22.5 | 22.9 | 32.9 | 25.2 | 21.9 | 58.2 |
| 7 | 30.2 | 30.5 | 69.7 | 29.0 | 115.3 | 138.5 |
| 8 | 136.4 | 136.1 | 159.0 | 136.3 | 145.7 | 135.6 |
| 9 | 124.6 | 124.1 | 130.1 | 126.8 | 26.2 | 154.0 |
| 10 | 89.6 | 89.5 | 88.3 | 137.8 | 32.8 | 116.6 |
| 11 | 43.4 | 43.7 | 198.5 | 29.4 | 26.7 | 170.6 |
| 12 | 71.0 | 71.1 | 49.2 | 31.9 | 33.6 | 31.6 |
| 13 | 46.0 | 46.3 | 42.2 | 41.4 | 41.1 | 22.0 |
| 14 | 52.2 | 52.2 | 49.9 | 49.4 | 50.9 | 21.6 |
| 15 | 74.8 | 74.5 | 73.9 | 74.8 | 77.5 | 14.6 |
| 16 | 112.2 | 111.9 | 111.0 | 112.0 | 111.5 | |
| 17 | 59.3 | 57.2 | 57.1 | 57.0 | 61.1 | |
| 18 | 9.7 | 10.3 | 18.8 | 17.6 | 21.4 | |
| 19 | 35.0 | 35.2 | 25.6 | 41.6 | 30.6 | |
| 20 | 22.7 | 23.6 | 23.7 | 24.2 | 23.2 | |
| 21 | 20.4 | 21.6 | 19.4 | 20.4 | 19.6 | |
| 22 | 29.5 | 38.0 | 37.3 | 38.0 | 29.3 | |
| 23 | 73.3 | 71.8 | 72.0 | 72.0 | 73.6 | |
| 24 | 82.3 | 86.0 | 86.8 | 86.1 | 83.2 | |
| 25 | 68.7 | 82.3 | 82.5 | 82.5 | 68.8 | |
| 26 | 24.1 | 22.4 | 22.4 | 22.1 | 24.4 | |
| 27 | 31.3 | 23.3 | 22.9 | 23.4 | 31.7 | |
| 28 | 16.2 | 16.6 | 18.1 | 17.9 | 18.1 | |
| 29 | 25.1 | 25.4 | 25.3 | 25.6 | 21.7 | |
| 30 | 23.3 | 23.6 | 23.5 | 16.8 | 19.2 | |
| OAc | 22.8 | 22.5 | 22.8 | |||
| OAc | 169.6 | 170.4 | 169.6 | |||
| 1′ | 104.1 | |||||
| 2′ | 72.5 | |||||
| 3′ | 74.1 | |||||
| 4′ | 69.7 | |||||
| 5′ | 63.9 |
| No | 1 | 2 | 3 | 4 | 5 | 6 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COSY | HMBC | NOESY | COSY | HMBC | NOESY | COSY | HMBC | NOESY | COSY | HMBC | NOESY | COSY | HMBC | NOESY | COSY | HMBC | NOESY | |
| 1 | 3, 5, 19 | 2β, 19 | 2 | 3, 19 | 2 | 3, 19 | 2, 19 | 2, 19 | 2 | 19 | 2, 11 | 2, 6, 12, 13 | ||||||
| 2 | 1 | 1, 3 | 1, 3 | 5 | 1, 3 | 1 | 3 | 1′, 3 | 1 | 1 | 3 | 12, 13 | 12, 13 | |||||
| 3 | 2 | 2, 29, 30 | 2, 29, 30 | 2 | 29, 30 | 2β | 2 | 29, 30 | 1′ | 1′, 2α, 5, | 1, 29, 30 | 2, 4 | 2, 4 | 12 | ||||
| 4 | 2, 5, 18, 28 | 5, 29, 30 | 29, 30 | 2, 29, 30 | 2, 5, 29, 30 | 3 | 2, 14 | |||||||||||
| 5 | 1, 3, 19, 29, 30 | 6α | 1, 3, 19, 29 | 3, 6, 19, 29, 30 | 1α | 3, 19, 29, 30 | 7α, 29 | 1, 6, 7, 19, 29, 30 | 6 | 4, 6 | 12 | |||||||
| 6 | 7 | 5 | 7 | 5, 7 | 7 | 5 | 7 | 19, 29 | 5, 7 | 2, 7, 12, 13 | 2β, 4β | |||||||
| 7 | 6 | 5 | 6 | 5, 19 | 6 | 5, 6 | 5, 6α, 28 | 6 | 6 | 6α, 15 | 6, 8 | 6 | 15 | |||||
| 8 | 6, 11, 15, 19, 28 | 19, 28 | 6, 7, 15, 19, 28 | 15, 19, 28 | 6, 11, 15, 19, 28 | 7 | 10, 15 | 6, 10 | ||||||||||
| 9 | 11, 19 | 11, 19 | 17, 19 | 11, 12, 19 | 1, 7, 11, 12, 19 | 7, 15 | ||||||||||||
| 10 | 1, 3, 5, 19 | 3, 5, 19 | 1, 3, 6, 19 | 1, 5, 6, 19 | 2, 5, 6, 19 | 15 | 15 | 8 | ||||||||||
| 11 | 11 | 12 | 19 | 12, 19 | 12 | 12, 19 | 12β | 12 | 12 | 12, 19 28 | 10, 15 | |||||||
| 12 | 11 | 11, 17, 18 | 11α, 28 | 11 | 17, 18 | 11α, 17, 28 | 17, 18 | 11 | 18, 20 | 11 | 17, 18 | 6, 13 | ||||||
| 13 | 12, 17, 18, 28 | 12, 17, 18, 20 | 12, 17, 18, 28 | 12, 17, 18, 20, 28 | 11, 12, 17, 18, 28 | 2, 6, 12 | ||||||||||||
| 14 | 18, 28 | 18, 28 | 12, 15, 18, 28 | 12, 15, 18, 28 | 7, 12, 15, 18, 28 | 5 | 4, 6 | |||||||||||
| 15 | 28 | 28 | 7β, 18 | 28 | 28 | 28 | 7, 28 | 10 | 8, 10 | 7 | ||||||||
| 16 | 15, 17 | 12, 17, 23, 24 | 15, 17, 23, 24 | 15, 17, 23, 24 | 15, 17 | |||||||||||||
| 17 | 12, 18, 21, 22 | 22α | 12, 18, 21, 22 | 18, 21, 22 | 12α, 22α | 20 | 18, 22, 28 | 21, 22 | 12α, 20, 22α, 28 | |||||||||
| 18 | 12, 17 | 11β, 15 | 12, 17 | 15β | 12β | 17 | 11β, 12β, 15, 20 | 12 | 12β, 20 | |||||||||
| 19 | 1 | 11β | 6β, 1β | 1, 11β, 6β | 11 | 11β | ||||||||||||
| 20 | 21 | 17, 21 | 22 | 17, 21, 22, 23 | 17, 21 | 17, 21, 22 | 22 | 22α | 21, 22 | |||||||||
| 21 | 17 | 22 | 20 | 22α | 20, 22 | 17, 20, 22α | ||||||||||||
| 22 | 20, 21, 24 | 21, 24 | 20, 21, 24 | 23 | 20, 21, 24 | 24 (22α) 20 (22β) | 20, 21, 24 | 20, 21, 17, 26 | ||||||||||
| 23 | 22β, 24 | 22, 24 | 22 | 22 | 20, 22 | 22β | 22 | 22 | 22β | 22 | 22 | 22, 24 | 22, 24, 26 | |||||
| 24 | 23 | 22, 26, 27 | 22, 26, 27 | 22α | 22, 26, 27 | 22, 26, 27 | 17, 22α | 22, 26, 27 | 23, 26, 27 | |||||||||
| 25 | 24, 26, 27 | 24, 26, 27 | 26, 27 | 24, 26, 27 | 24, 26, 27 | |||||||||||||
| 26 | 27 | 24, 27 | 27 | 27 | ||||||||||||||
| 27 | 24, 26 | 26 | 26 | 26 | ||||||||||||||
| 28 | 15 | 12 | 2α | 12α | 15 | 7α, 12α, 17 | 15 | 7, 11α, 12α | ||||||||||
| 29 | 3, 5, 30 | 2α | 30 | 2α | 30 | 2α | 30 | 1′, 2α, 5, 6α,13 | 30 | |||||||||
| 30 | 3, 29 | 6β | 29 | 3, 29 | 3, 29 | 6 | 5, 29 | 6β, 19 | ||||||||||
| OAc | ||||||||||||||||||
| OAc | OAc | OAc | OAc | |||||||||||||||
| 1′ | 2′, 3′, 5′, 3 | 3′, 5′α, 3 | ||||||||||||||||
| 2′ | 3′ | 4′ | ||||||||||||||||
| 3′ | 2′, 4′, 5′ | 1′ | ||||||||||||||||
| 4′ | 3′ 5′ | 2′ | ||||||||||||||||
| 5′ | 1′, 3′ | |||||||||||||||||
| Compounds | IC50 (μM) (a) | |
|---|---|---|
| NO | Cell Viability (% Control) | |
| Cimicitaiwanin B (2) | 8.37 ± 3.25 * | 69.14 ± 2.87 |
| Cimicitaiwanin C (3) | 6.54 ± 0.87 * | 95.53 ± 3.39 |
| Cimicitaiwanin D (4) | 10.11 ± 0.47 * | 91.12 ± 4.22 |
| Cimicitaiwanin E (5) | 24.58 ± 4.97 ** | 88.54 ± 1.50 |
| Cimicitaiwanin F (6) | 15.36 ± 0.85 ** | 86.84 ± 2.95 |
| Quercetin (b) | 34.58 ± 2.34 * | 95.56 ± 1.91 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-J.; Cheng, M.-J.; Lee, T.-H.; Kuo, Y.-H.; Lu, C.-T. Secondary Metabolites with Anti-Inflammatory from the Roots of Cimicifuga taiwanensis. Molecules 2022, 27, 1657. https://doi.org/10.3390/molecules27051657
Chen J-J, Cheng M-J, Lee T-H, Kuo Y-H, Lu C-T. Secondary Metabolites with Anti-Inflammatory from the Roots of Cimicifuga taiwanensis. Molecules. 2022; 27(5):1657. https://doi.org/10.3390/molecules27051657
Chicago/Turabian StyleChen, Jih-Jung, Ming-Jen Cheng, Tzong-Huei Lee, Yueh-Hsiung Kuo, and Chao-Tsen Lu. 2022. "Secondary Metabolites with Anti-Inflammatory from the Roots of Cimicifuga taiwanensis" Molecules 27, no. 5: 1657. https://doi.org/10.3390/molecules27051657
APA StyleChen, J.-J., Cheng, M.-J., Lee, T.-H., Kuo, Y.-H., & Lu, C.-T. (2022). Secondary Metabolites with Anti-Inflammatory from the Roots of Cimicifuga taiwanensis. Molecules, 27(5), 1657. https://doi.org/10.3390/molecules27051657







