Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella vulgaris and Its Blend with Different Vitamins
Abstract
1. Introduction
2. Results
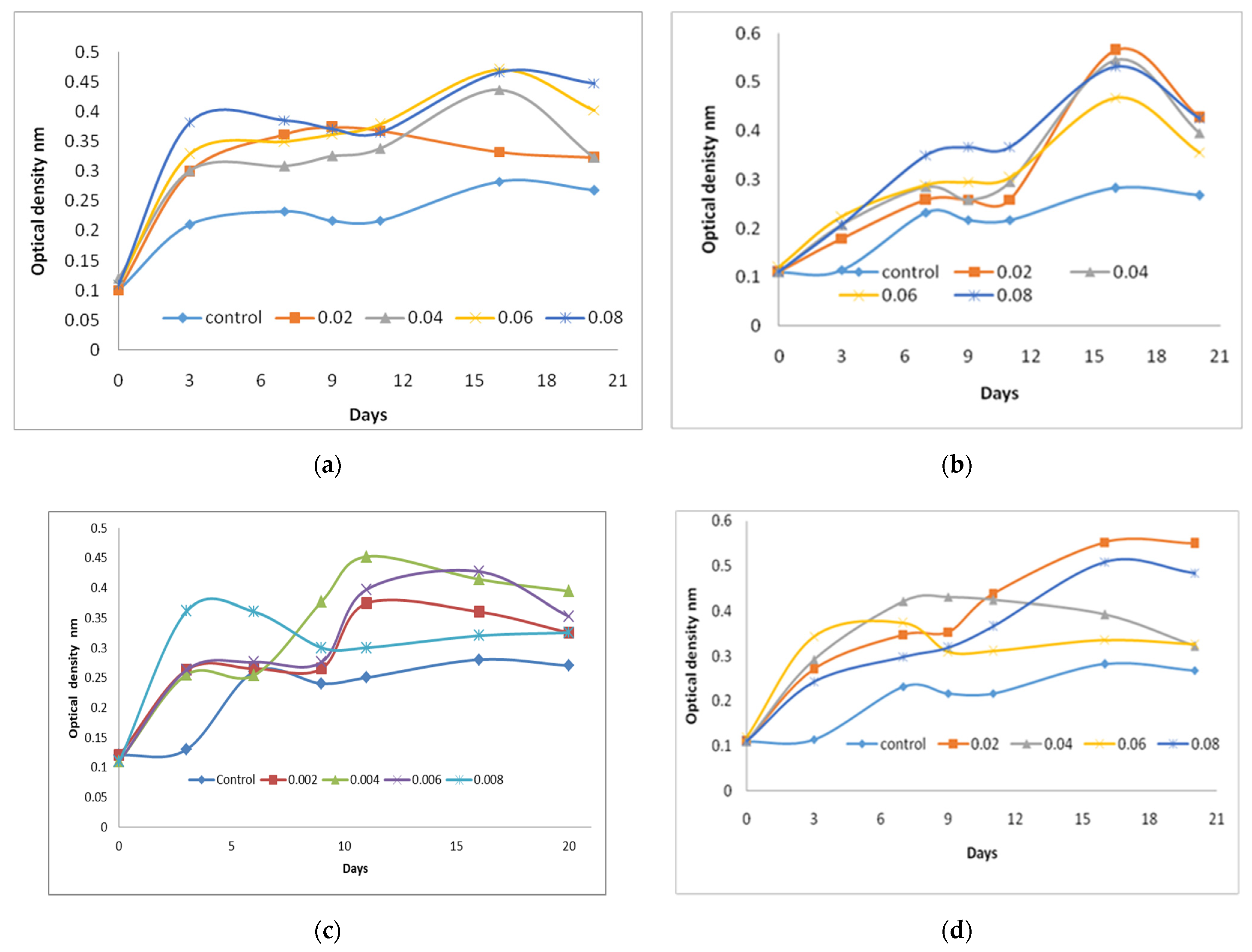
2.1. The Effect of Vitamins Supplementations on C. vulgaris Biomass Production
2.2. The Effect of Vitamins Supplementations on the Photosynthetic Pigments of C. vulgaris
2.3. Effect of Different Concentrations of the Tested Vitamins on Carbohydrate and Proteins Contents of C. vulgaris
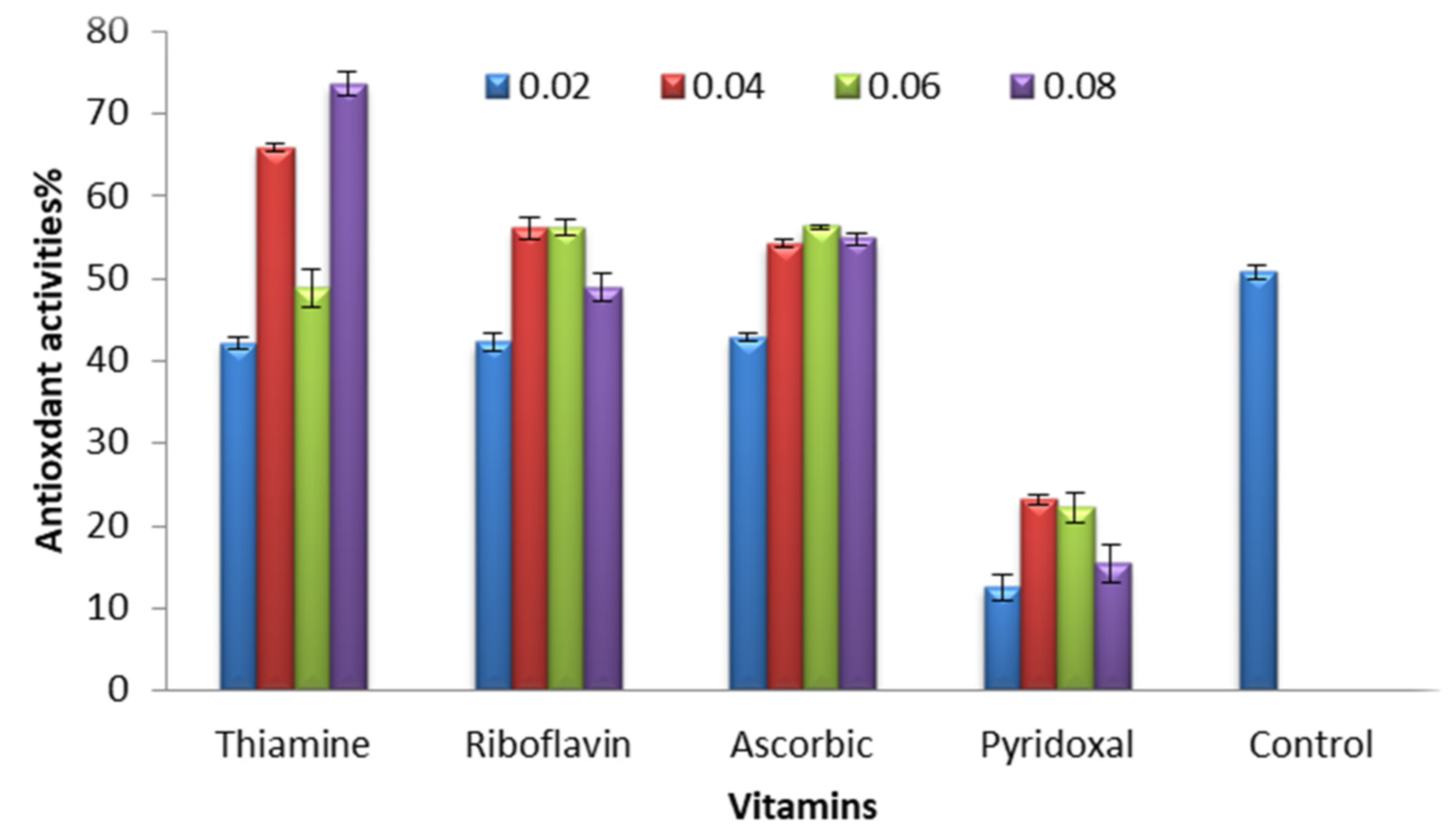
2.4. Antioxidant Activities of C. vulgaris and C. vulgaris Supplemented with Different Concentrations of the Tested Vitamins
2.5. Antitumor Effect of C. vulgaris and C. vulgaris Supplemented with Different Concentrations of Thiamine (Vit. B1)
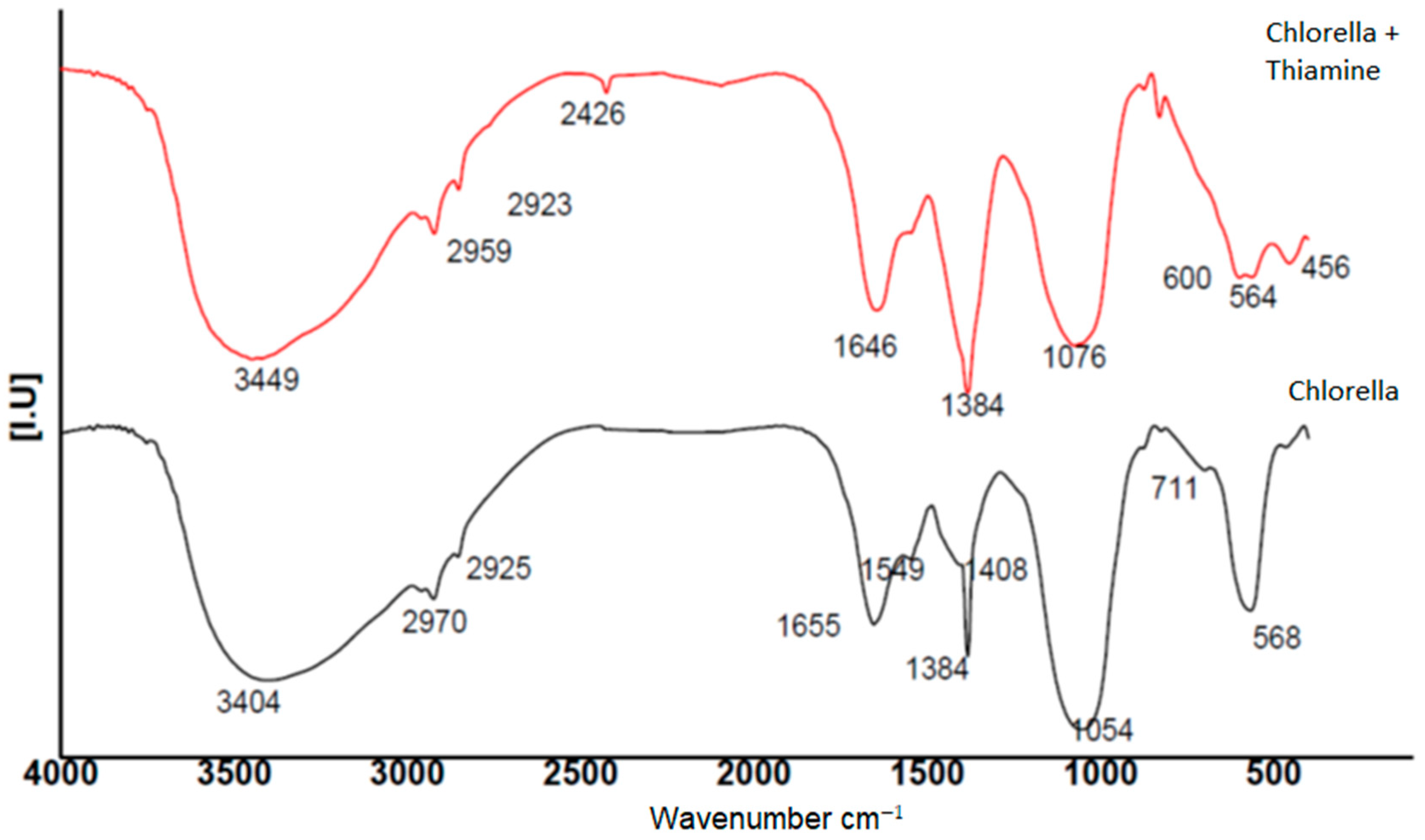
2.6. FT-IR
3. Discussion
4. Materials and Methods
4.1. Tested Alga
4.2. Preparation of Algal Extracts
4.3. Estimation of Pigments Content of C. vulgaris
4.4. Determination of Protein and Carbohydrate Content of C. vulgaris
4.5. Evaluation of the Antioxidant Activity of C. vulgaris In Vitro
4.5.1. DPPH Free Radicals Scavenging Assay
4.5.2. Determination of the Antitumor Activities of C. vulgaris
Cell Line
Chemical Reagents
MTT Assay
4.6. FT-IR Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Tony Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their drug ability. Anti-Cancer Agents Med. Chem. 2012, 12, 1281–1305. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Bai, L.; Zhu, L.; Yang, L.; Zhang, X. Marine algae-derived bioactive peptides for human nutrition and health. J. Agric. Food Chem. 2014, 62, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Guil-Guerrero, J.L.; Navarro-Juárez, R.; López-Martınez, J.C.; Campra-Madrid, P.; Rebolloso-Fuentes, M. Functional properties of the biomass of three microalgal species. J. Food Eng. 2004, 65, 511–517. [Google Scholar] [CrossRef]
- Kuda, T.; Kunii, T.; Goto, H.; Suzuki, T.; Yano, T. Changes of radical-scavenging capacity and ferrous reducing power in chub mackerel Scomber japonicus and Pacific saury Cololabissaira during 4 °C storage and retorting. Food. Chem. 2007, 5, 103–900. [Google Scholar]
- Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and cancer preventive properties of marine polysaccharides: Some results and prospects. Mar. Drugs 2013, 11, 4876–4901. [Google Scholar] [CrossRef]
- Specht, E.; Miyake-Stoner, S.; Mayfield, S. Micro-algae come of age as a platform for recombinant protein production. Biotechnol. Lett. 2010, 32, 1373–1383. [Google Scholar] [CrossRef]
- Sarkar, A.; Ghosh, U. Natural antioxidants-The key to safe and sustainable life. Int. J. Latest Trends Eng. Technol. 2016, 6, 460–466. [Google Scholar]
- Munir, N.; Sharif, N.; Naz, S.; Manzoor, F. Algae: A potent antioxidant source. Sky J. Microbiol. Res. 2013, 1, 22–31. [Google Scholar]
- Nick, G.L. Addressing human exposure to environmental toxins with Chlorella pyrenoidosa—Medicinal properties in whole foods. Town Send Lett. Dr. Patients 2003, 237, 28–32. [Google Scholar]
- Yaakob, Z.; Ali, E.; Zainal, A.; Mohamad, M.; Takriff, M.S. An overview: Biomolecules from microalgae for animal feed and aquaculture. J. Biol. Res.-Thessalon. 2014, 21, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yang, Q.; Wang, S. Antioxidant and hepatoprotective effects of a pigment–protein complex from Chlorella vulgaris on carbon tetrachloride-induced liver damage in vivo. RSC Adv. 2015, 5, 96097–96104. [Google Scholar] [CrossRef]
- Morris, H.J.; Almarals, A.; Carrill, O.; Bermudez, R.C. Utilisation of Chlorella vulgaris cell biomass for the production of enzymatic protein hydrolysates. Bioresour. Technol. 2008, 99, 7723–7729. [Google Scholar] [CrossRef] [PubMed]
- Pulz, O.; Groo, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biot. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Baianova, I.; Trubachev, I.N. Comparative evaluation of the vitamin composition of unicellular algae and higher plants grown under artificial conditions. Prikl. Biokhim. Mikrobiol. 1981, 17, 400–407. [Google Scholar] [PubMed]
- Fujiwara, Y. Effect of long term administration of Chlorella tablets on hyperlipemia. Nippon Eiyo Shokuryo Gakkaishi 1990, 43, 167–173. [Google Scholar] [CrossRef]
- Hasegawa, T.; Noda, K.; Kumamoto, S.; Ando, Y.; Yamada, A.; Yoshikai, Y. Chlorella vulgaris culture supernatant (CVS) reduces psychological stress induced apoptosis in thymocytes of mice. Int. J. Immunopharmacol. 2000, 22, 877–885. [Google Scholar] [CrossRef]
- Reddy, C.M.; Bhat, V.B.; Kiranmai, G.; Reddy, M.N.; Reddanna, P.; Madyastha, K.M. Selective inhibition of cyclooxygenase-2 by Cphycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000, 277, 599–603. [Google Scholar] [CrossRef]
- Khan, Z.; Bhadouria, P.; Bisen, P.S. Nutritional and therapeutic potential of Spirulina. Curr. Pharm. Biotechnol. 2005, 6, 373–379. [Google Scholar] [CrossRef]
- Basha, O.M.; Hafez, R.A.; El-Ayouty, Y.M.; Mahrous, K.F.; Bareedy, M.H.; Salama, A.M. C-Phycocyanin inhibits cell proliferation and may induce apoptosis in human HepG2 cells. Egypt J. Immunol. 2008, 15, 161–167. [Google Scholar]
- Sheih, I.C.; Fang, T.J.; Wu, T.K. Isolation and characterization of a novel angiotensin I-converting enzyme (ACE) inhibitory peptide from the algae protein waste. Food Chem. 2009, 115, 279–284. [Google Scholar] [CrossRef]
- Cruz-López, R.; Maske, H. The vitamin B1 and B12 required by the marine dinoflagellate Lingulodinium polyedrum can be provided by its associated bacterial community in culture. Front. Microbiol. 2016, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, F.; Silbernagel, S.B. Effect of vitamin concentrations on growth and development of vitamin-requiring algae. J. Phycol. 1969, 5, 64–67. [Google Scholar] [CrossRef]
- Gobler, C.J.; Norman, C.; Panzeca, C.; Taylor, G.T.; Sanudo-Wilhelmy, S.A. Effect of B-vitamins (B-1, B-12) and inorganic nutrients on algal bloom dynamics in a coastal ecosystem. Aquat. Microb. Ecol. 2007, 49, 181–194. [Google Scholar] [CrossRef]
- Tandon, P.; Jin, Q.; Huang, L. A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb. Cell Factories 2017, 16, 219. [Google Scholar] [CrossRef]
- Hannon, M.; Gimpel, J.; Tran, M.; Rasala, B.; Mayfield, S. Biofuels from algae: Challenges and potential. Biofuels 2010, 1, 763–784. [Google Scholar] [CrossRef]
- Helliwell, K.E.; Scaife, M.A.; Sasso, S.; Araujo, A.P.U.; Purton, S.; Smith, A.G. Unraveling vitamin B-12-responsive gene regulation in algae. Plant Physiol. 2014, 165, 388–397. [Google Scholar] [CrossRef]
- Kirrolia, A.; Bishnoi, N.R.; Singh, R. Response surface methodology as a decision-making tool for optimization of culture conditions of green microalgae Chlorella spp. for biodiesel production. Ann. Microbiol. 2014, 64, 1133–1147. [Google Scholar] [CrossRef]
- Sylvander, P.; Häubner, N.; Snoeijs, P. The thiamine content of phytoplankton cells is affected by abiotic stress and growth rate. Microb. Ecol. 2013, 65, 566–577. [Google Scholar] [CrossRef]
- Monteverde, D.R.; Gómez-Consarnau, L.; Cutter, L.; Chong, L.; Berelson, W.; Sañudo-Wilhelmy, S.A. Vitamin B1 in marine sediments: Pore water concentration gradient drives benthic flux with potential biological implications. Front Microbiol. 2015, 6, 434. [Google Scholar] [CrossRef] [PubMed]
- Panzeca, C.; Tovar-Sanchez, A.; Agustí, S.; Reche, I.; Duarte, C.M.; Taylor, G.T.; Sañudo-Wilhelmy, S.A. B vitamins as regulators of phytoplankton dynamics. Eos Trans. Am. Geophys. Union 2006, 87, 593–596. [Google Scholar] [CrossRef]
- Croft, M.T.; Warren, M.J.; Smith, A.G. Algae need their vitamins. Eukaryot Cell 2006, 5, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Menzel, D.W.; Spaeth, J.P. Occurrence of vitamin B12 in the Sargasso Sea. Limnol. Oceanogr. 1962, 7, 151–154. [Google Scholar] [CrossRef]
- Lwoff, L.; Dusi, A.H. Le thiazol, facteur de croissance pour le flagella Polytoma ocellatum. CR Acad. Sci. 1937, 205, 205–882. [Google Scholar]
- Sanudo-Wilhelmy, S.A.; Gobler, C.J.; Okbamichael, M.; Taylor, G.T. Regulation of phytoplankton dynamics by vitamin B12. Geophys. Res. Lett. 2006, 33, L04604. [Google Scholar] [CrossRef]
- Vishniac, H.S.; Riley, G.A. Cobalamin and thiamine in Long Island Sound: Patterns of distribution and ecological significance. Limnol. Oceanogr. 1961, 6, 36–41. [Google Scholar] [CrossRef]
- Tang, Y.Z.; Koch, F.; Gobler, C.J. Most harmful algal bloom species are vitamin B1 and B12 auxotrophs. Proc. Natl. Acad. Sci. USA 2010, 107, 20756–20761. [Google Scholar] [CrossRef]
- Provasoli, L. Algal nutrition and eutrophication. In Eutrophication: Causes, Consequences, Correctives; Rohlich, G.A., Ed.; National Academies Press: Washington, DC, USA, 1969; pp. 574–593. [Google Scholar]
- Pringsheim, E.G.; Pringsheim, O. Die Ernährung koloniebildender Volvocales. Biol. Zbl. 1959, 78, 937–971. [Google Scholar]
- Tarin, N.J.; Ali, N.M.; Chamon, A.S.; Mondol, M.N.; Rahman, M.M.; Aziz, A. Optimizing Chlorella Vulgaris and Anabaena Variabilis Growth Conditions for Use as Biofuel. Feed. Stock. J. Asiat. Soc. Bangladesh Sci. 2016, 42, 191–200. [Google Scholar] [CrossRef]
- Krichnavaruk, S.; Loataweesup, W.; Powtongsook, S.; Pavasant, P. Optimal Growth Conditions and the Cultivation of Chaetoceros calcitrans in Airlift Photobioreactor. Chem. Eng. J. 2005, 105, 91–98. [Google Scholar] [CrossRef]
- Li, L. Effect of vitamin-B12 and vitamin-H on the growth and astaxanthin content of Haematococcus pluvialis CH-1. Adv. J. Food Sci. Technol. 2013, 5, 1139–1142. [Google Scholar] [CrossRef]
- Desouky, S.A. Alleviation the toxicity effect of lead acetate by riboflavin on growth parameters, photosynthesis, respiration, carbohydrates, proteins, free amino acids and proline of Chlorella vulgaris Beijer cultures. Al-Azhar Bull. Sci. 2003, 25–27, 277–279. [Google Scholar]
- Berland, B.R.; Bonin, D.J.; Fiala, M.; Mastering, S.Y. Water and salt stress, kinetin and protein synthesis in tobacco leaves. Plant Physiol. 1978, 42, 361–365. [Google Scholar]
- Swift, D.G. Vitamins and phytoplankton growth. In The Physiological Ecology of Phytoplankton Morris; Oxford: London, UK, 1980; Volume 6, pp. 239–368. [Google Scholar]
- Petrov, S.A.; Zamorov, V.V.; Ustyanskaya, O.V.; Budnyak, O.K.; Chernadchuk, S.S.; Andrievskiy, O.M.; Kravchuk, I.O. Administration of Thiamine and Thiochrome Enhanced Reproduction of Chlorella, Drosophila melanogaster, and Danio. J. Nutr. Sci. Vitaminol. 1980, 62, 6–11. [Google Scholar] [CrossRef][Green Version]
- Desouky, S.A. Effect of Some Natural Organic Additives on the Growth and Photosynthesis of Pollutant—Chlorella Vulgaris Beijer. J. Appl. Sci. Res. 2011, 7, 23–32. [Google Scholar]
- Moulin, M.; Nguyen, G.T.; Scaife, M.A.; Smith, A.G.; Fitzpatrick, T.B. Analysis of Chlamydomonas thiamin metabolism in vivo reveals riboswitch plasticity. Proc. Natl. Acad. Sci. USA 2013, 110, 14622–14627. [Google Scholar] [CrossRef] [PubMed]
- Desouky, S.A. Response of vitamins on the growth criteria and some metabolic activities of stressed Scenedesmus obliquus cultures. Aust. J. Basic Appl. Sci. 2011, 5, 89–99. [Google Scholar]
- Abd El Latif, A.; Assar, D.H.; Elkaw, E.M.; Hamza, H.A.; Alkhalifah, D.H.M.; Hozzein, W.N.; Hamouda, R.A. Protective role of Chlorella vulgaris with Thiamine against Paracetamol induced toxic effects on haematological, biochemical, oxidative stress parameters and histopathological changes in Wistar rats. Sci. Rep. 2021, 11, 3911. [Google Scholar]
- Ibrahim, M.R.; Hamouda, R.A.; Tayel, A.A.; Al-Saman, M.A. Anti-cholesterol and Antioxidant Activities of Independent and Combined Microalgae Aqueous Extracts In Vitro. Waste Biomass Valorization 2021, 12, 4845–4857. [Google Scholar] [CrossRef]
- Farghl, A.A. Thiamine and pyridoxine alleviate oxidative damage by copper stress in green alga Chlorella vulgaris. Egypt J. Microbiol. 2012, 47, 97–110. [Google Scholar]
- Malanga, G.; Puntarulo, S. Oxidative stress and antioxidant content in Chlorella vulgaris after exposure to ultraviolet-B radiation. Physiol. Plant. 1995, 94, 672–679. [Google Scholar] [CrossRef]
- Miranda, M.S.; Sato, S.; Mancini-Filho, J. Antioxidant activity of the microalga Chlorella vulgaris cultured on special conditions. Boll. Chim. Farm. 2001, 140, 165–168. [Google Scholar]
- Wang, H.Y.; Zeng, X.B.; Guo, S.Y.; Li, Z.T. Effects of magnetic field on the antioxidant defense system of recirculation-cultured Chlorella vulgaris. Bioelectromagn. J. Bioelectromagn. Society. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2008, 29, 39–46. [Google Scholar] [CrossRef]
- El-Fayoumy, E.A.; Shanab, S.M.M.; Gaballa, H.S.; Tantawy, M.A.; Shalaby, E.A. Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complement. Med. Ther. 2021, 21, 51. [Google Scholar] [CrossRef]
- Yusof, Y.A.M.; Saad, S.M.; Makpol, S.; Shamaan, N.A.; Ngah, W.Z.W. Hot water extract of Chlorella vulgaris induced DNA damage and apoptosis. Clinics 2010, 65, 1371–1377. [Google Scholar] [CrossRef]
- Hamouda, R.A.; Abd El Maksoud, A.I.; Wageed, M.; Alotaibi, A.S.; Elebeedy, D.; Khalil, H.; Abdella, A. Characterization and Anticancer Activity of Biosynthesized Au/Cellulose Nanocomposite from Chlorella vulgaris. Polymers 2021, 13, 3340. [Google Scholar] [CrossRef]
- Bramanti, E.; Benedetti, E. Determination of the secondary structure of isomeric forms of human serum albumin by a particular frequency deconvolution procedure applied to Fourier transform IR analysis. Biopolymers 1996, 38, 639–653. [Google Scholar] [CrossRef]
- Wetzel, R.; Becker, M.; Behlke, J.; Billwitz, H.; Bohn, S.; Ebert, B.; Hamann, H.; Krumbiegel, J.; Lassmann, G. Temperature behaviour of human serum albumin. Eur. J. Biochem. 1980, 104, 469–478. [Google Scholar] [CrossRef]
- Jebsen, C.; Norici, A.; Wagner, H.; Palmucci, M.; Giordano, M.; Wilhelm, C. FTIR spectra of algal species can be used as physiological fingerprints to assess their actual growth potential. Physiol. Plant. 2012, 146, 427–438. [Google Scholar] [CrossRef]
- Lowry, H.; Rosebrough, N.J.; Farr, A.L.; Randall, R. Protein determination by a modified Folin phenol method. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate Chemistry 17; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Theivandran, G.; Ibrahim, S.M.; Murugan, M. Fourier Transform Infrared (Ft-Ir) Spectroscopic Analysis of Spirulina fusiformis. J. Med. Plants Stud. 2015, 3, 30–32. [Google Scholar]
- Duncan, D.B. Multiple range and multiple F-test. In Biometrics; SAS Institute Inc.: Cary, NC, USA, 1955; Volume 11, pp. 1–42. [Google Scholar]
| Vitamins Conc. µg/L | Pigments | Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 9 | 11 | 16 | 20 | p-Value | ||
| Control | Chl a | 1.17 ± 0.06 bB | 3.12 ± 0.02 aB | 1.53 ± 0.08 bB | 3.06 ± 0.49 aB | 1.81 ± 0.05 bB | 1.79 ± 0.07 bB | ** |
| Chl b | 1.04 ± 0.05 B | 1.28 ± 0.09 C | 1.26 ± 0.06 B | 1.12 ± 0.35 B | 1.61 ± 0.14 B | 1.23 ± 0.04 B | NS | |
| Carotenoid | 10.65 ± 1.50 cA | 5.36 ± 0.29 dA | 10.88 ± 0.88 cbA | 17.48 ± 0.83 aA | 16.93 ± 0.29 aA | 13.53 ± 0.83 bA | ** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.02 | Chl a | 2.25 ± 0.48 abB | 1.63 ± 0.20 bB | 1.55 ± 0.03 bB | 2.83 ± 0.38 aB | 2.03 ± 0.27 abB | 2.24 ± 0.09 abB | * |
| Chl b | 0.93 ± 0.17 bB | 1.96 ± 0.26 bB | 2.10 ± 0.04 bB | 3.63 ± 0.43 bB | 6.42 ± 1.69 aB | 3.50 ± 0.43 bB | ** | |
| Carotenoid | 10.50 ± 1.24 bA | 7.75 ± 0.98 bA | 12.68 ± 0.34 bA | 24.12 ± 2.73 aA | 19.21 ± 2.96 abA | 12.42 ± 1.85 bA | *** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.04 | Chl a | 0.54 ± 0.14 cB | 1.30 ± 0.04 cbB | 1.24 ± 0.24 cbB | 2.57 ± 0.68 aB | 2.85 ± 0.36 abB | 2.27 ± 0.59 abB | ** |
| Chl b | 0.92 ± 0.37 cB | 1.45 ± 0.28 cbB | 1.43 ± 0.009 cbB | 2.81 ± 0.47 aB | 2.32 ± 0.18 abB | 1.38 ± 0.50 cbB | * | |
| Carotenoid | 9.19 ± 1.64 bA | 10.24 ± 1.72 bA | 10.32 ± 0.95 bA | 19.60 ± 1.50 aA | 15.42 ± 1.10 aA | 17.89 ± 2.32 aA | *** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.06 | Chl a | 0.28 ± 0.03 dC | 1.03 ± 0.07 cdB | 1.55 ± 0.08 cbB | 2.58 ± 0.55 aB | 2.18 ± 0.33 abB | 1.81 ± 0.14 abcB | ** |
| Chl b | 2.06 ± 0.63 abB | 1.46 ± 0.10 bB | 2.23 ± 0.17 abB | 2.54 ± 0.63 abB | 2.92 ± 0.122 aB | 2.51 ± 0.35 abB | * | |
| Carotenoid | 6.80 ± 0.47 bA | 12.31 ± 0.54 abA | 12.03 ± 0.59 abA | 12.10 ± 4.18 abA | 16.71 ± 1.39 aA | 15.43 ± 0.79 aA | *** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.08 | Chl a | 0.19 ± 0.06 cB | 2.58 ± 0.43 aB | 1.58 ± 0.62 abB | 1.41 ± 0.31 bB | 1.81 ± 0.60 abB | 1.64 ± 0.08 abB | *** |
| Chl b | 0.84 ± 0.40 dB | 1.17 ± 0.09 cdB | 1.99 ± 0.05 cbB | 2.45 ± 0.36 bB | 3.63 ± 0.65 aB | 2.72 ± 0.065 abB | *** | |
| Carotenoid | 5.20 ± 1.55 bA | 9.68 ± 1.53 abA | 11.25 ± 1.46 abA | 16.85 ± 4.50 aA | 11.28 ± 2.20 abA | 9.32 ± 1.30 abA | * | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| Vitamin Conc. µg/L | Pigments | Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 9 | 11 | 16 | 20 | p-Value | ||
| Control | Chl a | 1.17 ± 0.06 bB | 3.12 ± 0.02 aB | 1.53 ± 0.08 bB | 3.06 ± 0.49 aB | 1.81 ± 0.05 bB | 1.79 ± 0.07 bB | ** |
| Chl b | 1.04 ± 0.05 B | 1.28 ± 0.09 C | 1.26 ± 0.06 B | 1.12 ± 0.35 B | 1.61 ± 0.14 B | 1.23 ± 0.04 B | NS | |
| Carotenoid | 10.65 ± 1.50 cA | 5.36 ± 0.29 dA | 10.88 ± 0.88 cbA | 17.48 ± 0.83 aA | 16.93 ± 0.29 aA | 13.53 ± 0.83 bA | ** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.02 | Chl a | 2.08 ± 0.06 cAB | 2.82 ± 0.22 cbB | 2.39 ± 0.23 cbB | 4.02 ± 0.37 abB | 5.65 ± 1.06 abB | 4.13 ± 0.68 aB | ** |
| Chl b | 0.36 ± 0.189 cB | 1.79 ± 0.13 abB | 1.58 ± 0.06 abB | 2.14 ± 0.71 aC | 0.90 ± 0.13 bcC | 1.27 ± 0.35 abcC | * | |
| Carotenoid | 9.65 ± 4.15 bA | 9.12 ± 2.59 bA | 10.12 ± 1.73 bA | 16.19 ± 0.08 abA | 22.91 ± 1.82 aA | 15.35 ± 0.49 bA | ** | |
| p-Value | * | * | *** | *** | *** | *** | ||
| 0.04 | Chl a | 1.13 ± 0.43 bB | 2.42 ± 0.25 abB | 2.43 ± 0.32 abB | 1.71 ± 1.01 bB | 4.52 ± 1.48 aAB | 2.29 ± 0.28 abB | * |
| Chl b | 0.67 ± 0.10 bB | 1.55 ± 0.61 bB | 1.64 ± 0.054 bB | 4.56 ± 1.05 aB | 2.07 ± 0.05 bB | 1.76 ± 0.06 bB | *** | |
| Carotenoid | 11.92 ± 2.08 abA | 8.86 ± 1.53 bA | 11.51 ± 1.06 abA | 19.62 ± 2.98 aA | 13.87 ± 5.19 abA | 10.34 ± 2.55 abA | * | |
| p-Value | *** | ** | *** | *** | * | ** | ||
| 0.06 | Chl a | 1.10 ± 0.54 B | 2.87 ± 0.89 B | 1.78 ± 0.07 B | 1.96 ± 0.44 B | 1.93 ± 0.79 B | 2.14 ± 0.53 B | NS |
| Chl b | 0.62 ± 0.31 B | 1.84 ± 0.56 B | 1.83 ± 0.09 B | 2.65 ± 0.81 B | 2.52 ± 0.93 B | 1.69 ± 0.41 B | NS | |
| Carotenoid | 14.38 ± 6.28 A | 8.30 ± 1.71 A | 11.30 ± 0.86 A | 16.83 ± 0.40 A | 16.04 ± 1.004 A | 10.75 ± 2.15 A | NS | |
| p-Value | * | ** | *** | *** | *** | ** | ||
| 0.08 | Chl a | 1.26 ± 0.50 B | 2.75 ± 0.57 B | 2.07 ± 0.07 B | 2.45 ± 0.88 B | 2.72 ± 0.35 B | 2.65 ± 0.33 B | NS |
| Chl b | 0.36 ± 0.138 dB | 1.76 ± 0.36 bcB | 1.32 ± 0.12 cB | 2.90 ± 0.53 aB | 2.66 ± 0.22 abB | 1.09 ± 0.14 cdB | *** | |
| Carotenoid | 13.50 ± 4.50 A | 11.01 ± 0.53 A | 11.29 ± 1.13 A | 17.97 ± 2.64 A | 15.20 ± 0.77 A | 14.17 ± 1.33 A | NS | |
| p-Value | * | *** | *** | *** | *** | *** | ||
| Vitamin Conc. µg/L | Pigments | Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 9 | 11 | 16 | 20 | p-Value | ||
| Control | Chl a | 1.17 ± 0.06 bB | 3.12 ± 0.02 aB | 1.53 ± 0.08 bB | 3.06 ± 0.49 aB | 1.81 ± 0.05 bB | 1.79 ± 0.07 bB | ** |
| Chl b | 1.04 ± 0.05 B | 1.28 ± 0.09 C | 1.26 ± 0.06 B | 1.12 ± 0.35 B | 1.61 ± 0.14 B | 1.23 ± 0.04 B | NS | |
| Carotenoid | 10.65 ± 1.50 cA | 5.36 ± 0.29 dA | 10.88 ± 0.88 cbA | 17.48 ± 0.83 aA | 16.93 ± 0.29 aA | 13.53 ± 0.83 bA | ** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.02 | Chl a | 0.63 ± 0.21 d | 2.26 ± 0.34 cbB | 1.28 ± 0.19 cdB | 4.28 ± 0.56 aB | 3.41 ± 0.90 ab | 3.26 ± 0.32 abB | ** |
| Chl b | 0.08 ± 0.13 d | 2.17 ± 0.42 cbB | 1.51 ± 0.07 cB | 4.26 ± 0.78 aB | 3.47 ± 0.58 ab | 2.20 ± 0.18 bcB | * | |
| Carotenoid | 9.05 ± 5.32 | 8.22 ± 1.51 A | 14.68 ± 0.74 A | 19.001 ± 3.58 A | 9.48 ± 4.53 | 12.36 ± 0.66 A | NS | |
| p-Value | NS | *** | *** | *** | NS | *** | ||
| 0.04 | Chl a | 0.42 ± 0.37 b | 2.29 ± 0.03 abB | 2.02 ± 0.035 abB | 3.64 ± 1.58 aB | 4.01 ± 0.43 a | 4.05 ± 0.63 aB | ** |
| Chl b | 1.24 ± 0.89 | 2.50 ± 0.31 B | 1.54 ± 0.09 B | 2.85 ± 0.61 B | 3.07 ± 0.60 | 1.54 ± 0.27 C | NS | |
| Carotenoid | 3.77 ± 2.18 b | 7.30 ± 0.59 abA | 9.80 ± 1.78 abA | 15.60 ± 2.49 aA | 8.94 ± 4.84 ab | 7.56 ± 0.78 abA | ** | |
| p-Value | NS | ** | ** | *** | NS | ** | ||
| 0.06 | Chl a | 0.76 ± 0.27 bB | 2.42 ± 0.34 aB | 2.02 ± 0.039 abB | 2.68 ± 0.57 a | 2.05 ± 0.78 ab | 2.15 ± 0.11 abB | ** |
| Chl b | 0.93 ± 0.62 cB | 1.93 ± 0.23 cbB | 2.08 ± 0.14 cbB | 3.85 ± 0.41 a | 3.09 ± 0.60 ab | 2.23 ± 0.29 bcB | ** | |
| Carotenoid | 10.54 ± 2.00 A | 10.96 ± 1.77 A | 8.57 ± 1.23 A | 7.48 ± 4.09 | 4.66 ± 0.92 | 8.86 ± 1.44 A | NS | |
| p-Value | *** | *** | *** | NS | NS | *** | ||
| 0.08 | Chl a | 0.036 ± 0.15 c | 3.47 ± 0.172 aB | 1.98 ± 0.109 bB | 1.78 ± 0.400 bB | 1.93 ± 0.50 bB | 1.66 ± 0.46 bB | *** |
| Chl b | 2.23 ± 0.96 | 1.74 ± 0.08 B | 2.10 ± 0.20 B | 2.92 ± 0.18 B | 2.24 ± 0.39 B | 2.14 ± 0.52 B | NS | |
| Carotenoid | 5.34 ± 2.83 b | 8.97 ± 1.001 bA | 10.83 ± 3.05 bA | 29.27 ± 2.40 aA | 12.43 ± 3.02 bA | 8.11 ± 0.47 bA | *** | |
| p-Value | * | ** | ** | *** | *** | *** | ||
| Vitamin Conc. µg/L | Pigments | Days | ||||||
|---|---|---|---|---|---|---|---|---|
| 3 | 7 | 9 | 11 | 16 | 20 | p-Value | ||
| Control | Chl a | 1.17 ± 0.06 bB | 3.12 ± 0.02 aB | 1.53 ± 0.08 bB | 3.06 ± 0.49 aB | 1.81 ± 0.05 bB | 1.79 ± 0.07 bB | ** |
| Chl b | 1.04 ± 0.05 B | 1.28 ± 0.09 C | 1.26 ± 0.06 B | 1.12 ± 0.35 B | 1.61 ± 0.14 B | 1.23 ± 0.04 B | NS | |
| Carotenoid | 10.65 ± 1.50 cA | 5.36 ± 0.29 dA | 10.88 ± 0.88 cbA | 17.48 ± 0.83 aA | 16.93 ± 0.29 aA | 13.53 ± 0.83 bA | ** | |
| p-Value | *** | *** | *** | *** | *** | *** | ||
| 0.02 | Chl a | 0.688 ± 0.05 cB | 2.82 ± 0.52 b | 1.92 ± 0.37 cb | 5.30 ± 0.55 aA | 2.92 ± 0.41 b | 2.92 ± 0.558 bA | *** |
| Chl b | 1.91 ± 0.06 aA | 1.69 ± 0.14 ab | 1.36 ± 0.226 ab | 1.58 ± 0.44 abB | 1.77 ± 0.36 ab | 0.93 ± 0.197 bB | * | |
| Carotenoid | 0.68 ± 0.05 cB | 2.82 ± 0.526 b | 1.92 ± 0.37 cb | 5.31 ± 0.55 aA | 2.92 ± 0.41 b | 2.92 ± 0.55 bA | *** | |
| p-Value | ** | NS | NS | ** | NS | ** | ||
| 0.04 | Chl a | 0.353 ± 0.03 c | 2.88 ± 0.75 a | 2.17 ± 0.44 ab | 1.41 ± 0.35 bc | 2.20 ± 0.20 ab | 1.98 ± 0.17 ab | *** |
| Chl b | 0.40 ± 0.07 c | 1.49 ± 0.56 cb | 1.98 ± 0.38 ab | 3.11 ± 0.84 a | 2.37 ± 0.15 ab | 1.56 ± 0.25 bc | *** | |
| Carotenoid | 0.35 ± 0.03 c | 2.88 ± 0.75 a | 2.17 ± 0.44 ab | 1.41 ± 0.35 bc | 2.20 ± 0.20 ab | 1.98 ± 0.17 ab | *** | |
| p-Value | NS | NS | NS | NS | NS | NS | ||
| 0.06 | Chl a | 0.04 ± 0.12 bB | 4.93 ± 2.87 a | 1.67 ± 0.169 ab | 2.60 ± 0.85 ab | 1.81 ± 0.33 abB | 1.70 ± 0.16 ab | *** |
| Chl b | 1.51 ± 0.52 cbA | 1.13 ± 0.94 c | 1.81 ± 0.197 cb | 4.48 ± 1.08 a | 3.50 ± 0.11 abA | 2.19 ± 0.36 bc | ** | |
| Carotenoid | 0.04 ± 0.12 bB | 4.93 ± 2.87 a | 1.67 ± 0.16 ab | 2.61 ± 0.85 ab | 1.81 ± 0.33 abB | 1.70 ± 0.16 ab | *** | |
| p-Value | ** | NS | NS | NS | *** | NS | ||
| 0.08 | Chl a | 0.44 ± 0.27 c | 1.81 ± 0.35 b | 1.47 ± 0.132 bB | 2.99 ± 0.47 a | 2.15 ± 0.25 abB | 1.88 ± 0.12 b | ** |
| Chl b | 1.04 ± 0.38 c | 2.26 ± 0.13 b | 2.63 ± 0.01 abA | 3.49 ± 0.50 a | 3.15 ± 0.05 abA | 2.16 ± 0.49 b | ** | |
| Carotenoid | 0.44 ± 0.27 c | 1.81 ± 0.35 b | 1.47 ± 0.13 bB | 2.99 ± 0.47 a | 2.15 ± 0.25 abB | 1.88 ± 0.12 b | ** | |
| p-Value | NS | NS | ** | NS | * | NS | *** | |
| Vitamin | Concentration (µg/L) | Carbohydrate (mg/g) | Proteins (mg/g) |
|---|---|---|---|
| Control | 0 | 149.97 ± 0.88 | 50.676 ± 0.864 |
| Thiamine | 0.02 | 165.30 ± 0.57 c | 55.088 ± 1.452 b |
| 0.04 | 147.64 ± 1.76 d | 48.552 ± 0.589 c | |
| 0.06 | 195.30 ± 0.57 b | 52.637 ± 2.536 cb | |
| 0.08 | 250.30 ± 0.57 a | 64.402 ± 0.712 a | |
| p-Value | *** | *** | |
| Riboflavin | 0.02 | 194.64 ± 0.33 c | 49.533 ± 0.993 b |
| 0.04 | 171.30 ± 0.57 d | 46.755 ± 1.337 b | |
| 0.06 | 207.97 ± 1.45 b | 63.912 ± 0.993 a | |
| 0.08 | 215.97 ± 1.20 a | 47.572 ± 1.609 b | |
| p-Value | *** | *** | |
| Asorbic acid | 0.02 | 206.30 ± 1.15 c | 63.748 ± 0.432 a |
| 0.04 | 244.64 ± 0.33 b | 48.552 ± 0.589 d | |
| 0.06 | 206.64 ± 0.88 c | 50.350 ± 0.283 c | |
| 0.08 | 284.64 ± 0.33 a | 53.781 ± 0.748 b | |
| p-Value | *** | *** | |
| Pyridoxal | 0.02 | 244.64 ± 0.33 c | 45.774 ± 1.452 b |
| 0.04 | 224.64 ± 0.33 d | 43.650 ± 0.589 b | |
| 0.06 | 345.30 ± 0.57 b | 56.069 ± 1.884 a | |
| 0.08 | 348.97 ± 1.85 a | 45.284 ± 2.305 b | |
| p-Value | *** | *** |
| Cancer Cell | HePG-2 | HCT-116 | Hela | PC3 | ||||
|---|---|---|---|---|---|---|---|---|
| Conc., µg/mL | Chl | Chl + Thia | Chl | Chl + Thia | Chl | Chl + Thia | Chl | Chl + Thia |
| 100 | 71.2 ± 4 | 83.8 ± 2 | 68.9 ± 6 | 89.3 ± 5 | 80.1 ± 9 | 88.4 ± 4 | 64 ± 4 | 84.8 ± 6 |
| 200 | 83.8 ± 5 | 89.5 ± 5 | 76.5 ± 8 | 93.8 ± 4 | 90.2± | 90.7 ± 9 | 80.3 ± 5 | 91.3 ± 4 |
| 400 | 90.9 ± 9 | 92.6 ± 6 | 87.7 ± 4 | 97.7 ± 7 | 95.5 ± 3 | 95 ± 7 | 88.8 ± 4 | 95.9 ± 3 |
| 600 | 93.6 ± 3 | 96.4 ± 4 | 95.1 ± 3 | 98.2 ± 8 | 97.4 ± 8 | 97.8 ± 4 | 92.7 ± 3 | 96.9 ± 7 |
| 800 | 95.7 ± 6 | 97.9 ± 9 | 97.6 ± 5 | 99 ± 4 | 98.1 ± 4 | 98.8 ± 8 | 94.4 ± 3 | 98.2 ± 4 |
| Constituent A | Constituent C | ||
|---|---|---|---|
| Stock solution | g/L | Trace elements | mg/L |
| NaNO3 | 1.5 | H3BO3 | 2.8 |
| K2HPO4·3H2O | 0.04 | MnCl2·4H2O | 1.81 |
| MgSO4·7H2O | 0.075 | ZnSO4·7H2O | 0.222 |
| CaCl2·2H2O | 0.036 | Na·MoO4·2H2O | 0.39 |
| Na2CO3 | 0.02 | CuSO4·H2O | 0.079 |
| Constituent B | Co(NO3)2·6H2O | 0.0494 | |
| EDTA (disodium magnesium salt) | 0.001 g/L |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamouda, R.A.; Abd El Latif, A.; Elkaw, E.M.; Alotaibi, A.S.; Alenzi, A.M.; Hamza, H.A. Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella vulgaris and Its Blend with Different Vitamins. Molecules 2022, 27, 1602. https://doi.org/10.3390/molecules27051602
Hamouda RA, Abd El Latif A, Elkaw EM, Alotaibi AS, Alenzi AM, Hamza HA. Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella vulgaris and Its Blend with Different Vitamins. Molecules. 2022; 27(5):1602. https://doi.org/10.3390/molecules27051602
Chicago/Turabian StyleHamouda, Ragaa A., Amera Abd El Latif, Ebtihal M. Elkaw, Amenah S. Alotaibi, Asma Massad Alenzi, and Hanafy A. Hamza. 2022. "Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella vulgaris and Its Blend with Different Vitamins" Molecules 27, no. 5: 1602. https://doi.org/10.3390/molecules27051602
APA StyleHamouda, R. A., Abd El Latif, A., Elkaw, E. M., Alotaibi, A. S., Alenzi, A. M., & Hamza, H. A. (2022). Assessment of Antioxidant and Anticancer Activities of Microgreen Alga Chlorella vulgaris and Its Blend with Different Vitamins. Molecules, 27(5), 1602. https://doi.org/10.3390/molecules27051602






