Effects of BPZ and BPC on Oxidative Stress of Zebrafish under Different pH Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effects of a Mixed Solution of Bisphenol C (BPC) and Bisphenol Z (BPZ) on the Expression of Oxidative Stress-Related Genes and Estrogen Receptor Genes in Zebrafish
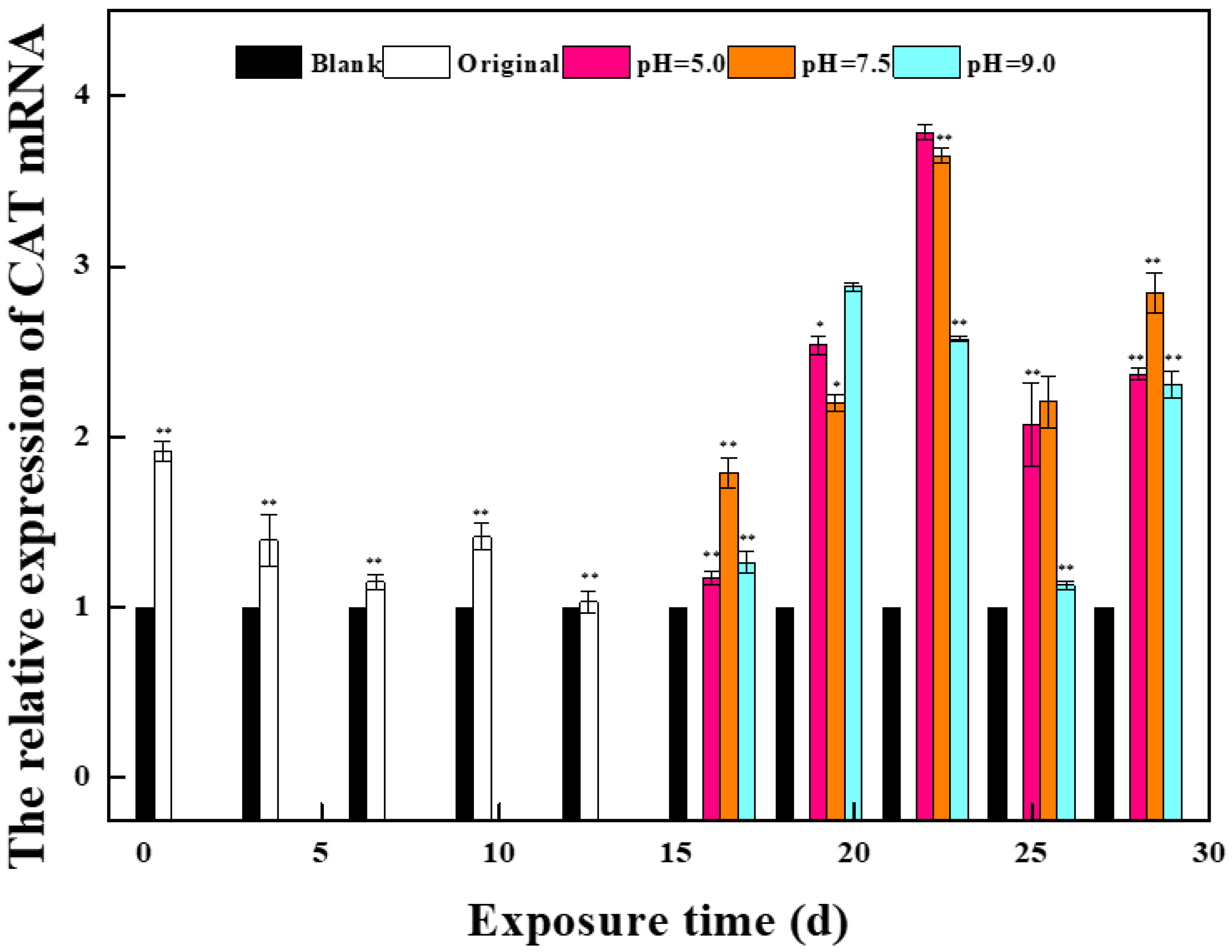
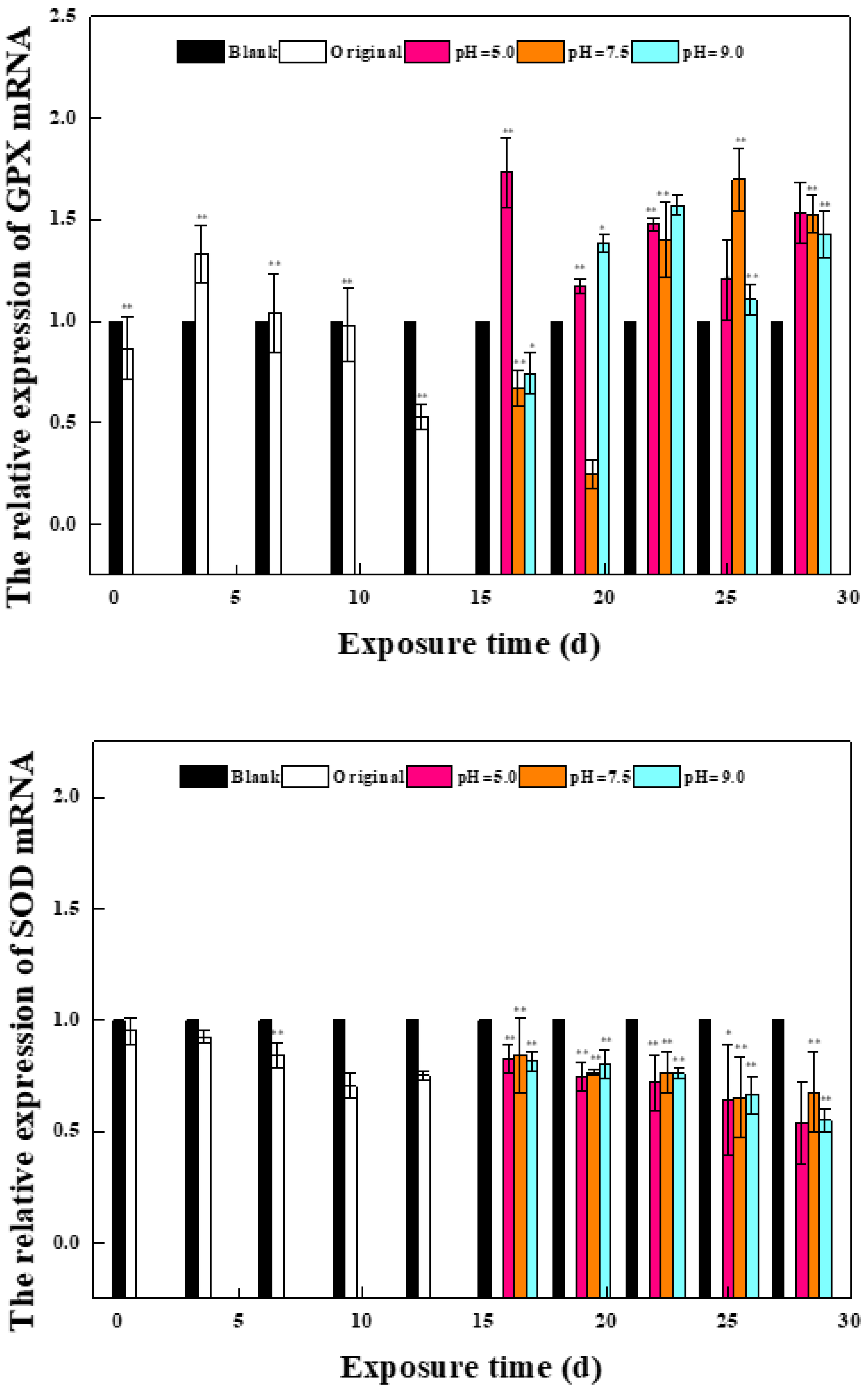
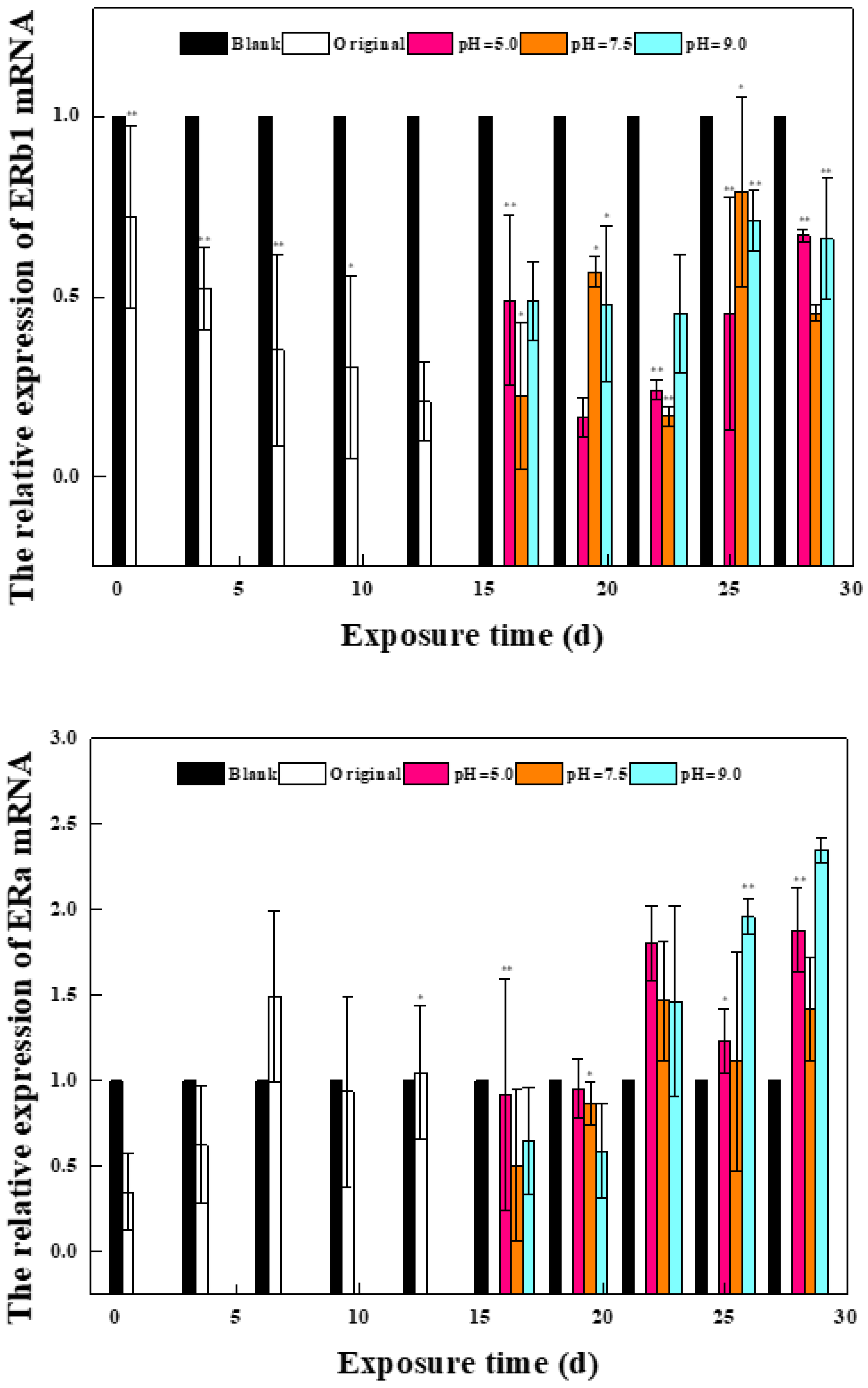
2.1.1. Effects of Phase I Mixed Solution on the Expression of Oxidative Stress Gene and Estrogen Receptor Gene in Zebrafish
2.1.2. Effects of a Mixed Solution of the Second Stage on the Expression of Oxidative Stress Gene and Estrogen Receptor Gene in Zebrafish
2.2. Effects of a Mixed Solution of BPC and BPZ on the Expression of Oxidative Stress-Related Genes and Estrogen Receptor Genes in Zebrafish
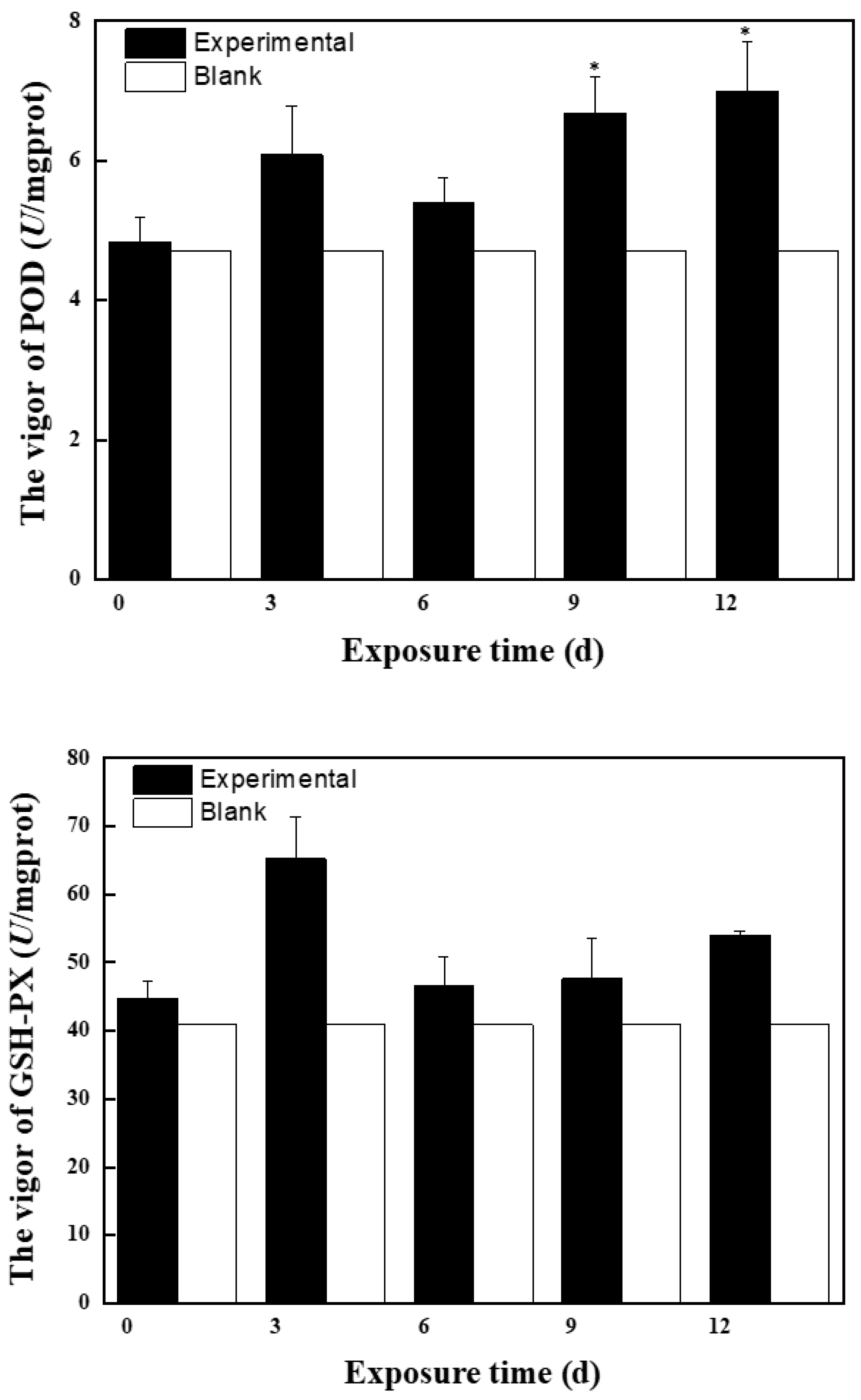
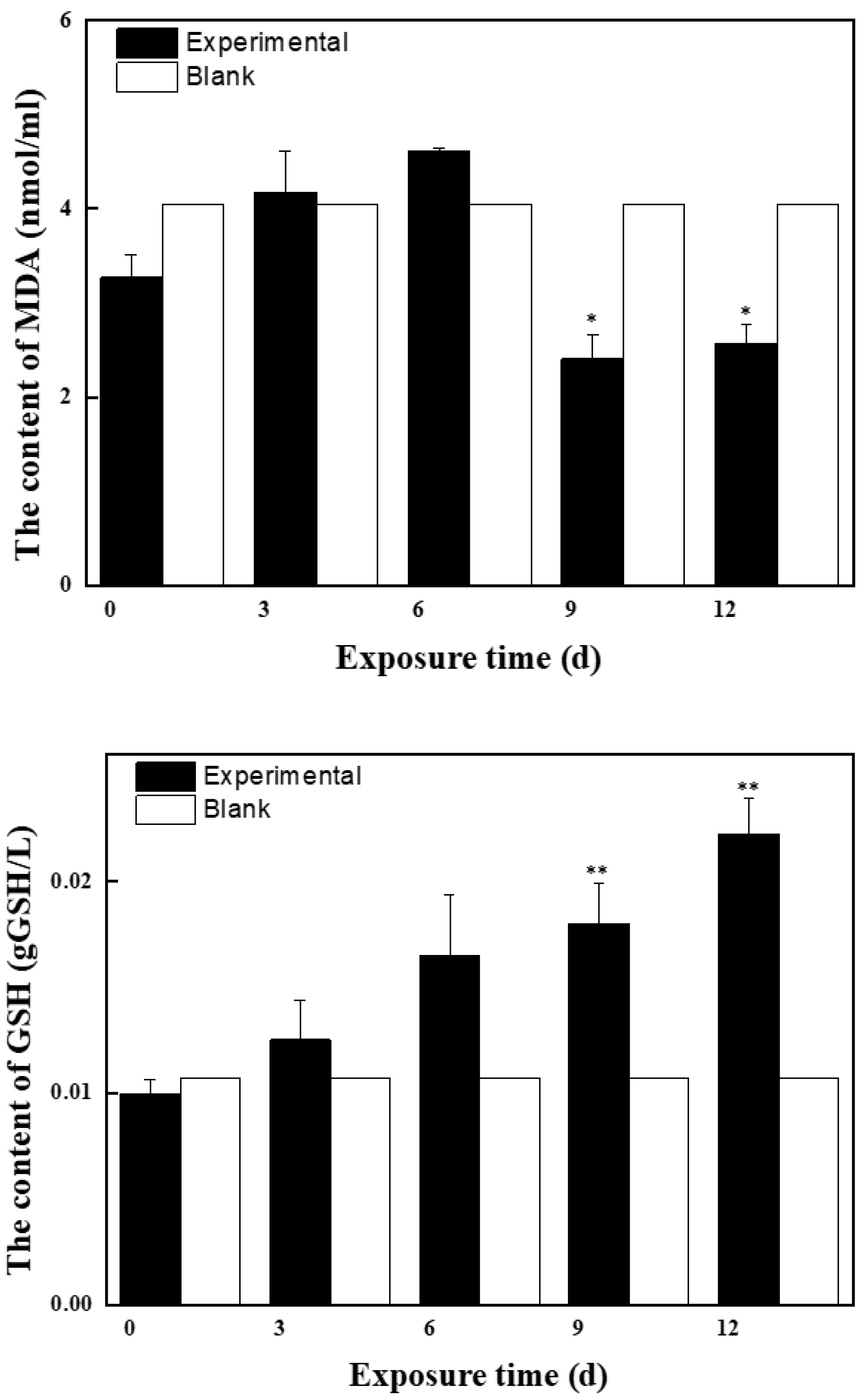
2.2.1. Effects of Mixed Solution at the First Stage on Antioxidant Enzyme Activity and MDA Content in Zebrafish
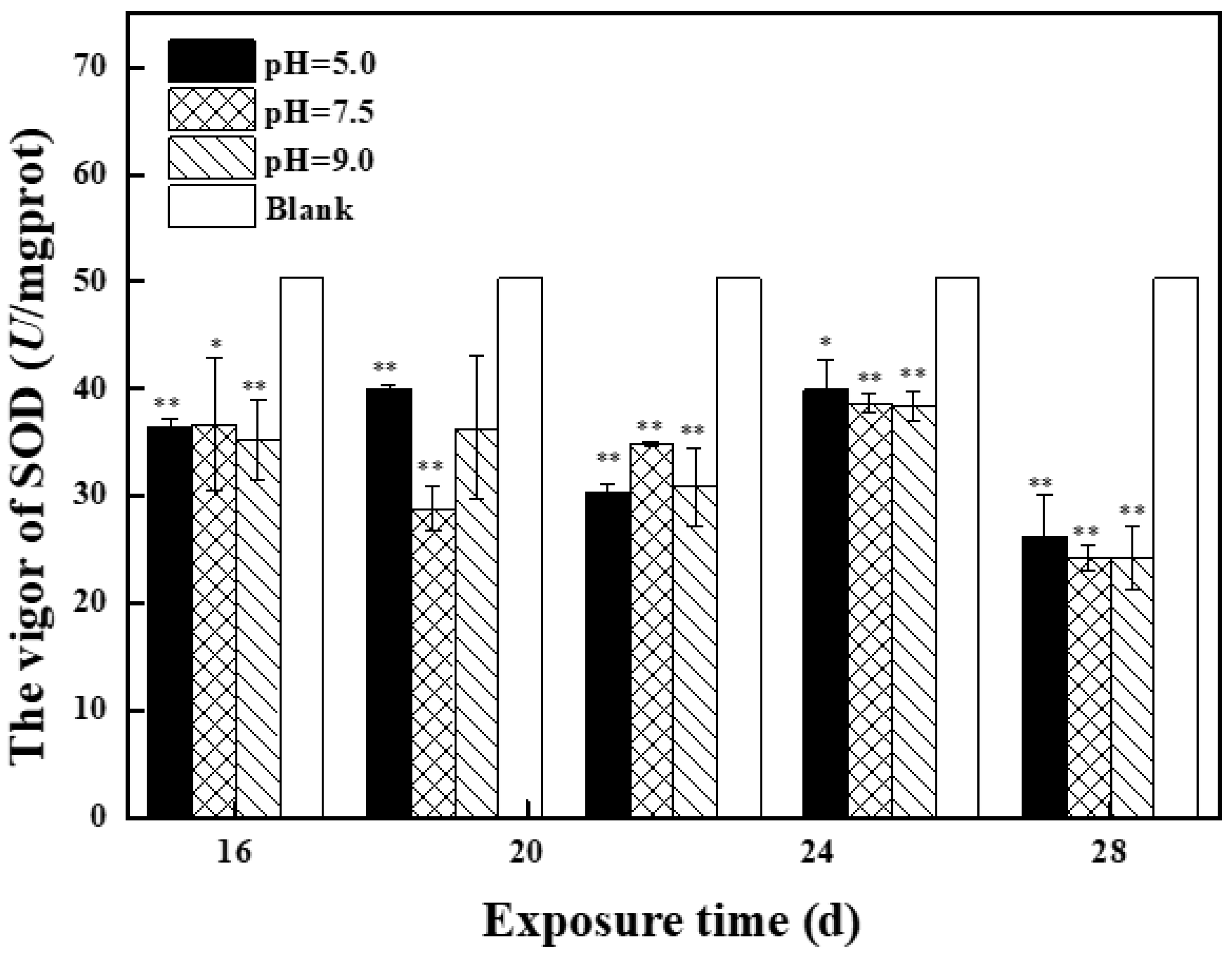
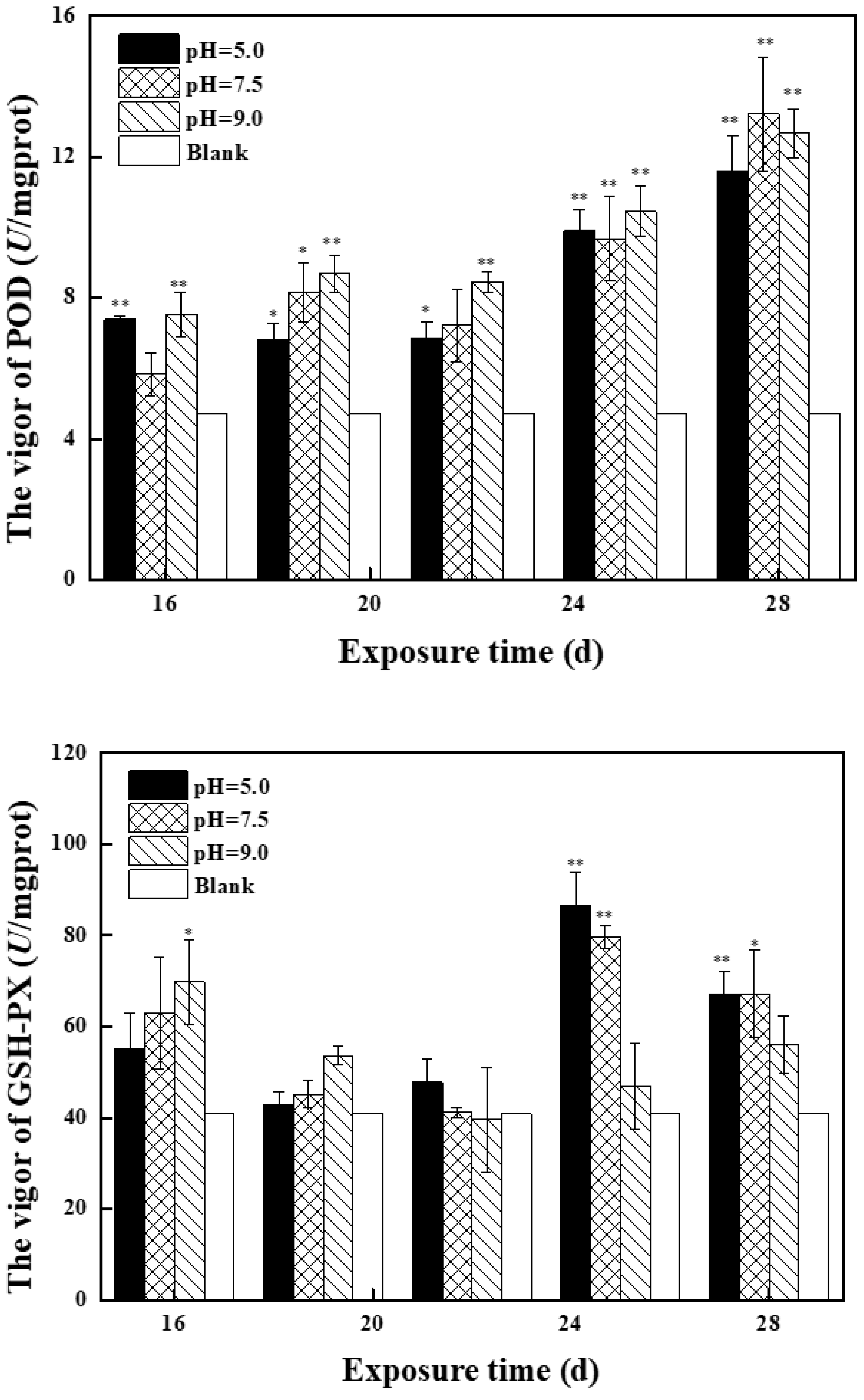
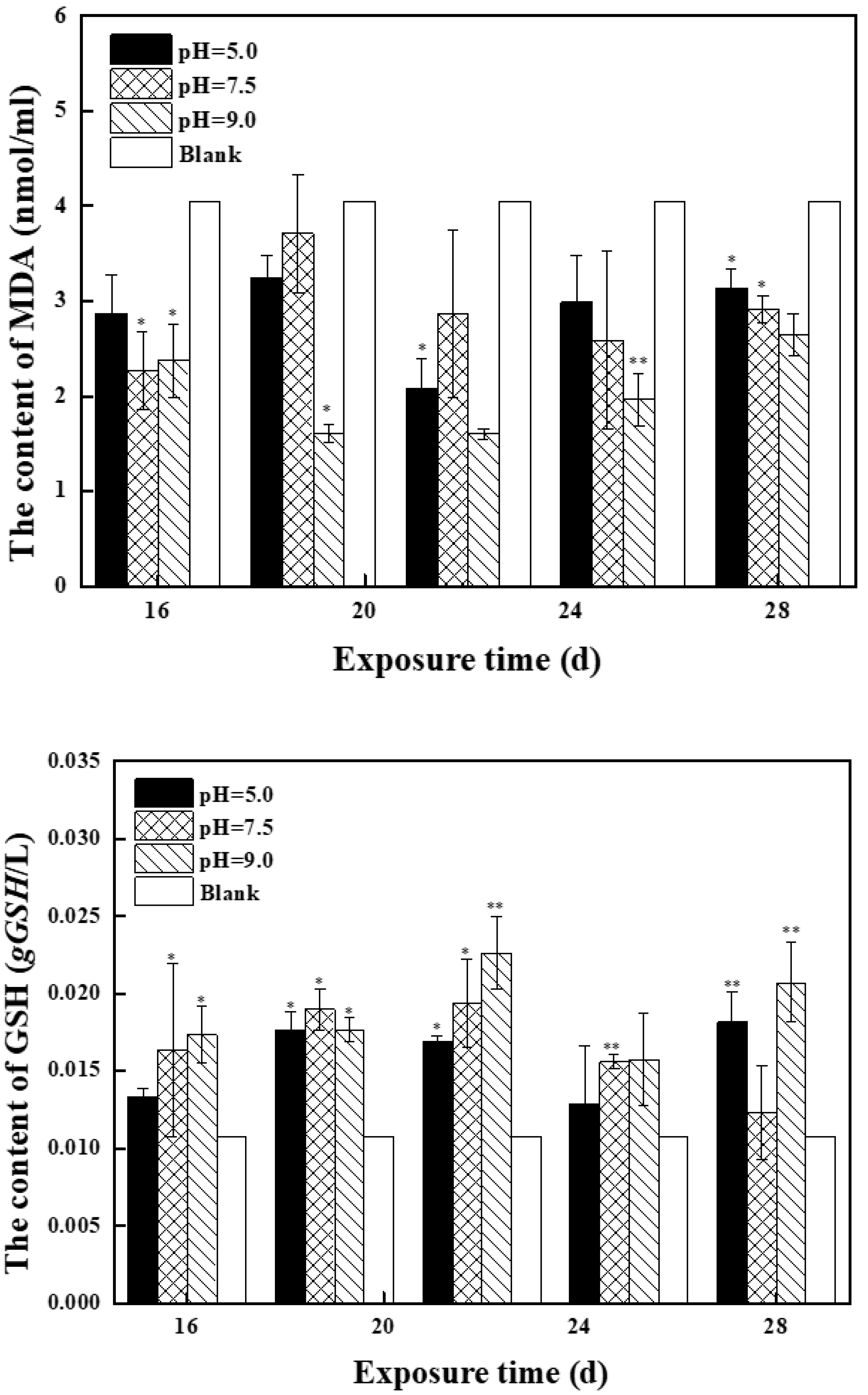
2.2.2. Effects of Mixed Solution at the Second Stage on Antioxidant Enzyme Activity and MDA Content in Zebrafish
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Experimental Instruments
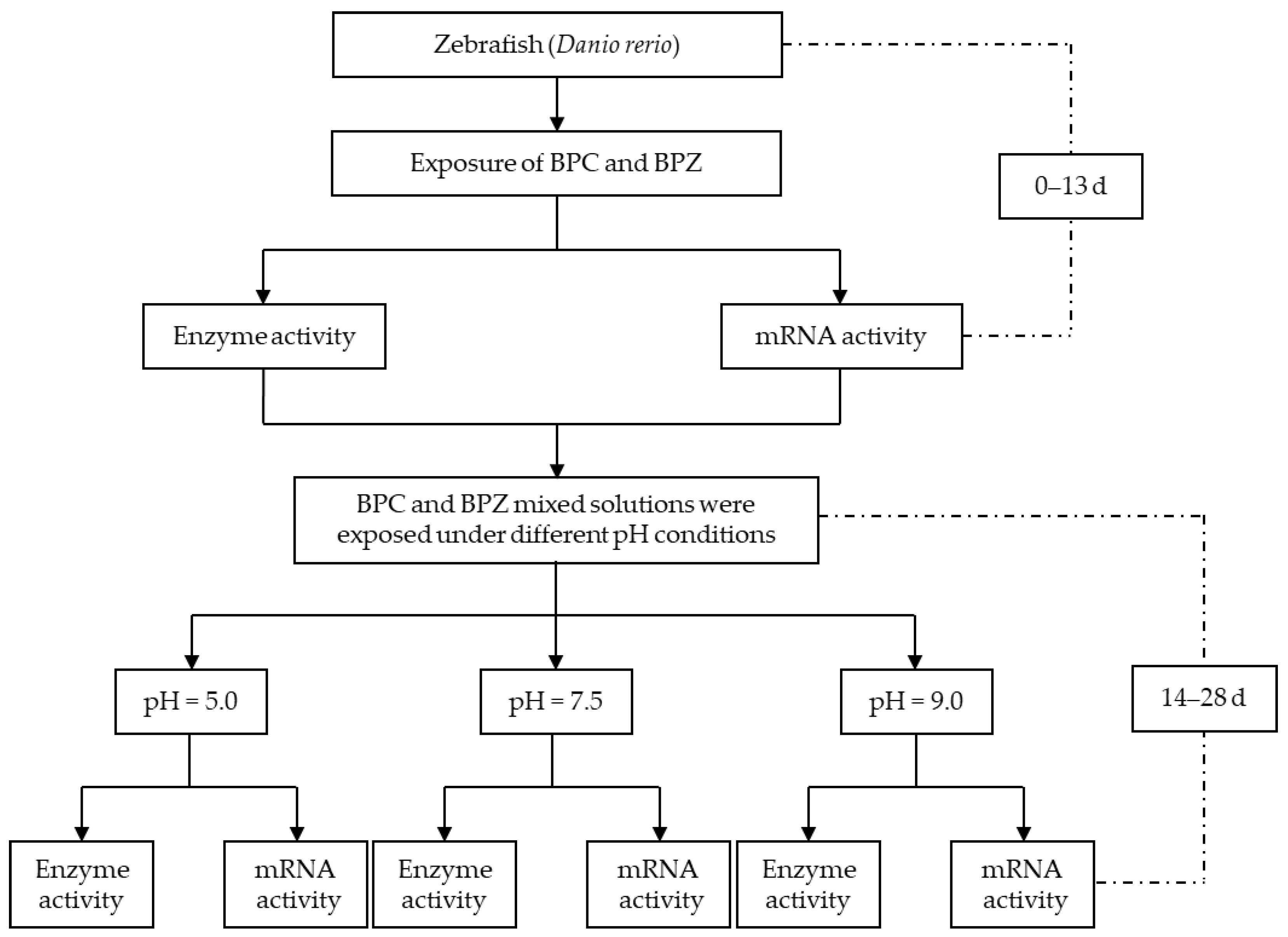
3.3. Experiment Design
3.3.1. Zebrafish Farming Method
3.3.2. Method of Exposure
3.3.3. Determination of Physiological Indicators in Zebrafish
3.3.4. Expression of Oxidative Stress Gene and Estrogen Receptor Gene in Zebrafish
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Mustieles, V.; D’Cruz, S.C.; Couderq, S.; Rodríguez-Carrillo, A.; David, A. Bisphenol A and its analogues: A comprehensive review to identify and prioritize effect biomarkers for human biomonitoring. Environ. Int. 2020, 144, 105811. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.G.; Tang, Y.; Liu, Y.X.; Yuan, Y.; Zhao, B.Q.; Chao, X.J.; Zhu, B.Z. Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a nongenomic mechanism. Toxicol. Appl. Pharmacol. 2012, 259, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; LehmLer, H.J.; Sun, Y.; Xu, G.; Bao, W. Association of bisphenol A and its substitutes, bisphenol F and bisphenol S, with obesity in United States children and adolescents. Diabetes Metab. J. 2019, 43, 59. [Google Scholar] [CrossRef] [PubMed]
- Braver-Sewradj, S.P.D.; Spronsen, R.V.; Hessel, E.V.S. Substitution of bisphenol A: A review of the carcinogenicity, reproductive toxicity, and endocrine disruption potential of alternative substances. Crit. Rev. Toxicol. 2020, 50, 128–147. [Google Scholar] [CrossRef] [PubMed]
- Mok, S.; Jeong, Y.; Park, M.; Kim, S.; Moon, H.B. Exposure to phthalates and bisphenol analogues among childbearing-aged women in Korea: Influencing factors and potential health risks. Chemosphere 2021, 264, 128425. [Google Scholar] [CrossRef] [PubMed]
- Hc-Wydro, K.; Poe, K.; Broniatowski, M. The comparative analysis of the effect of environmental toxicants: Bisphenol A, S and F on model plant, fungi and bacteria membranes. The studies on multicomponent systems. J. Mol. Liq. 2019, 289, 111136. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerova, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Bambino, K.; Chu, J. Zebrafish in Toxicology and Environmental Health. Curr. Top. Dev. Biol. 2017, 124, 331–367. [Google Scholar] [PubMed] [Green Version]
- Nadia, T.; Tai, H.; Da-Woon, J.; Williams, D.R. Fishing for nature’s hits: Establishment of the zebrafish as a model for screening antidiabetic natural products. Evid.-Based Complementary Altern. Med. 2015, 2015, 287847. [Google Scholar]
- Stegeman, J.J.; Goldstone, J.V.; Hahn, M.E. Perspectives on zebrafish as a model in environmental toxicology. Fish Physiol. 2010, 29, 367–439. [Google Scholar]
- Zhang, F.; Han, L.; Wang, J.; Shu, M.; Liu, K.; Zhang, Y.; Hsiao, C.D.; Tian, Q.; He, Q. Clozapine induced developmental and cardiac toxicity on zebrafish embryos by elevating oxidative stress. Cardiovasc. Toxicol. 2021, 21, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Kannan, K.; Tan, H.; Zheng, Z.G.; Widelka, M. Bisphenol analogues other than BPA: Environmental occurrence, human exposure, and toxicity—A review. Environ. Sci. Technol. 2016, 50, 5438–5453. [Google Scholar] [CrossRef] [PubMed]
- Mcdonough, C.M.; Xu, H.S.; Guo, T.L. Toxicity of bisphenol analogues on the reproductive, nervous, and immune systems, and their relationships to gut microbiome and metabolism: Insights from a multi-species comparison. Crit. Rev. Toxicol. 2021, 51, 283–300. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Pirzada, M.; Jahan, S.; Ullah, H.; Khan, M.J. Bisphenol A analogues bisphenol B, bisphenol F, and bisphenol S induce oxidative stress, disrupt daily sperm production, and damage DNA in rat spermatozoa: A comparative in vitro and in vivo study. Toxicol. Ind. Health 2019, 35, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Park, J.C.; Lee, M.C.; Yoon, D.S.; Han, J.; Kim, M.; Hwang, U.K.; Jung, J.H.; Lee, J.S. Effects of bisphenol A and its analogs bisphenol F and S on life parameters, antioxidant system, and response of defensome in the marine rotifer Brachionus koreanus. Aquat. Toxicol. 2018, 199, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Xu, H.; Shen, Y.; Qiu, W.; Yang, M. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environ. Toxicol. Chem. 2011, 30, 2335–2341. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fei, Y.; Wang, M.; Xue, Y.; Chen, H.; Liu, Y. Study on the joint toxicity of BPZ, BPS, BPC and BPF to zebrafish. Molecules 2021, 26, 4180. [Google Scholar] [CrossRef] [PubMed]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Accession NO. | Primer Sequence (5′-3′) | Product Length (bp) |
|---|---|---|---|
| β-actin | AF025305.1 | F: CGAGCTGTCTTCCCATCCA R: TCACCAACGTAGCTGTCTTTCTG | 86 |
| rp17 | NM_213213644.2 | F: CAGAGGTATCAATGGTGTCAGCCC R: TTCGGAGCATGTTGATGGAGGC | 119 |
| CAT | AF170069.1 | F: CTCCTGATGTGGCCCGATAC R: TCAGATGCCCGGCCATATTC | 126 |
| SOD | BX055516 | F: GTCCGCACTTCAACCCTCA R: TCCTCATTGCCACCCTTCC | 217 |
| GPX | AW232474 | F: AGATGTCATTCCTGCACACG R: AAGGAGAAGCTTCCTCAGCC | 94 |
| ERα | AF268283 | F: CCC ACA GGA CAA GAG GAA GA R: CCT GGT CAT GCA GAG ACA GA | 250 |
| ERβ1 | AJ414566 | F: GGG GAG AGT TCA ACC ACG GAG R: GCT TTC GGA CAC AGG AGG ACG | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Y.; Fei, Y.; Wang, M.; Xue, Y.; Liu, Y. Effects of BPZ and BPC on Oxidative Stress of Zebrafish under Different pH Conditions. Molecules 2022, 27, 1568. https://doi.org/10.3390/molecules27051568
Han Y, Fei Y, Wang M, Xue Y, Liu Y. Effects of BPZ and BPC on Oxidative Stress of Zebrafish under Different pH Conditions. Molecules. 2022; 27(5):1568. https://doi.org/10.3390/molecules27051568
Chicago/Turabian StyleHan, Ying, Yumeng Fei, Mingxin Wang, Yingang Xue, and Yuxuan Liu. 2022. "Effects of BPZ and BPC on Oxidative Stress of Zebrafish under Different pH Conditions" Molecules 27, no. 5: 1568. https://doi.org/10.3390/molecules27051568
APA StyleHan, Y., Fei, Y., Wang, M., Xue, Y., & Liu, Y. (2022). Effects of BPZ and BPC on Oxidative Stress of Zebrafish under Different pH Conditions. Molecules, 27(5), 1568. https://doi.org/10.3390/molecules27051568





