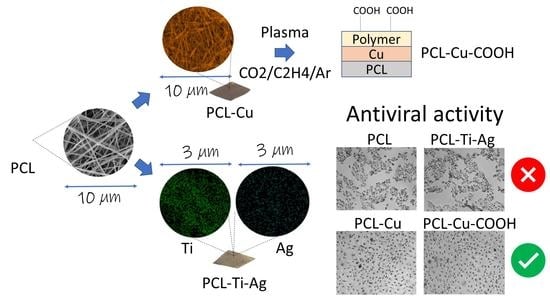
Biodegradable Nanohybrid Materials as Candidates for Self-Sanitizing Filters Aimed at Protection from SARS-CoV-2 in Public Areas
Abstract
:1. Introduction
2. Results
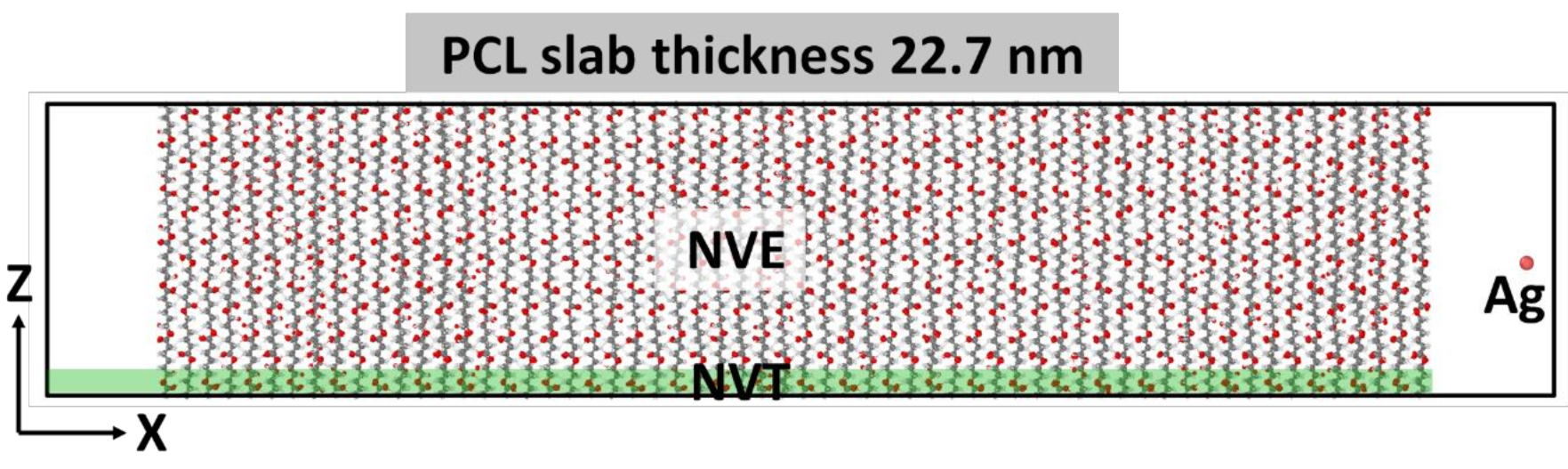
2.1. Simulation of Ag+ Implantation into PCL Matrix
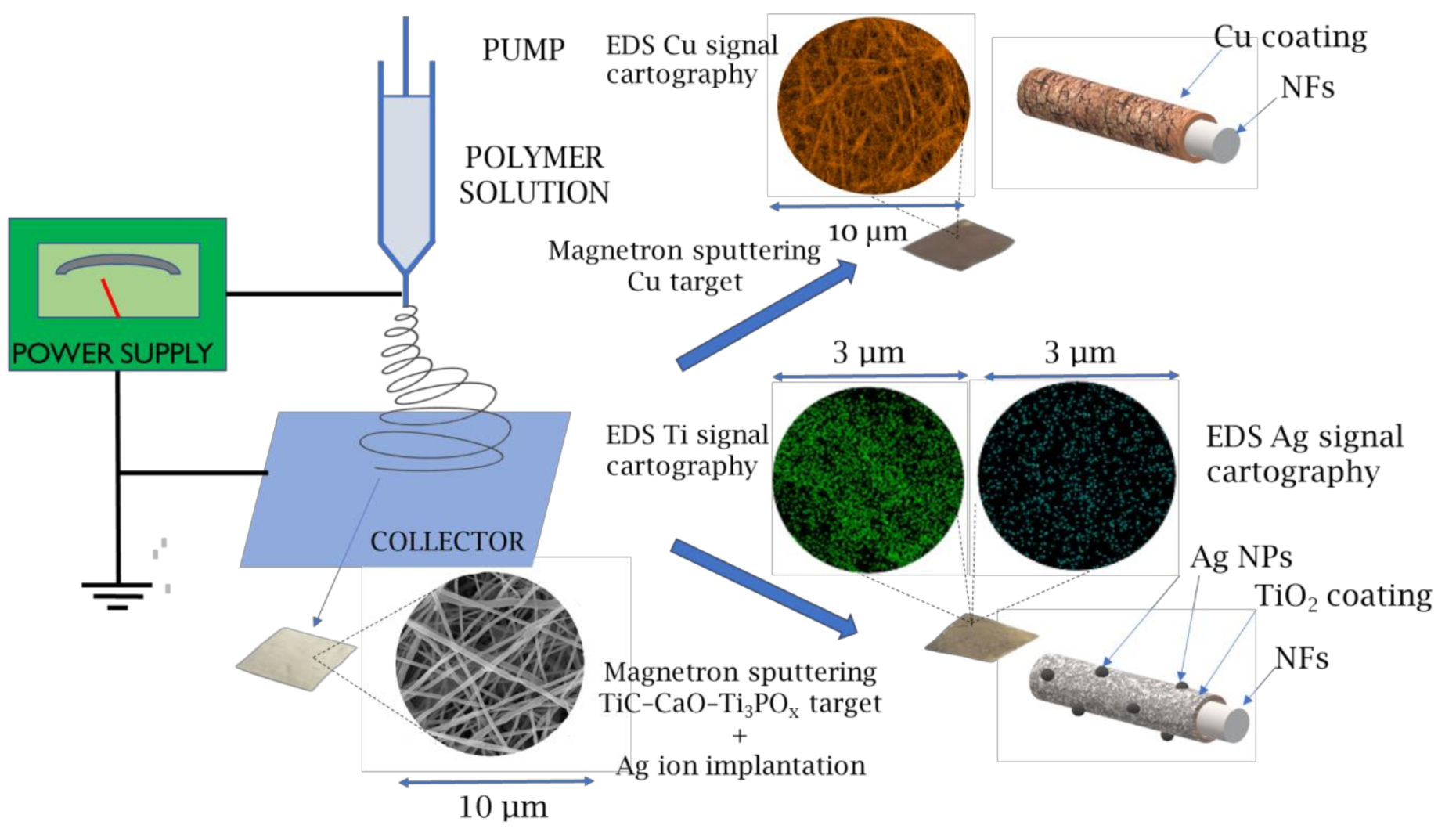
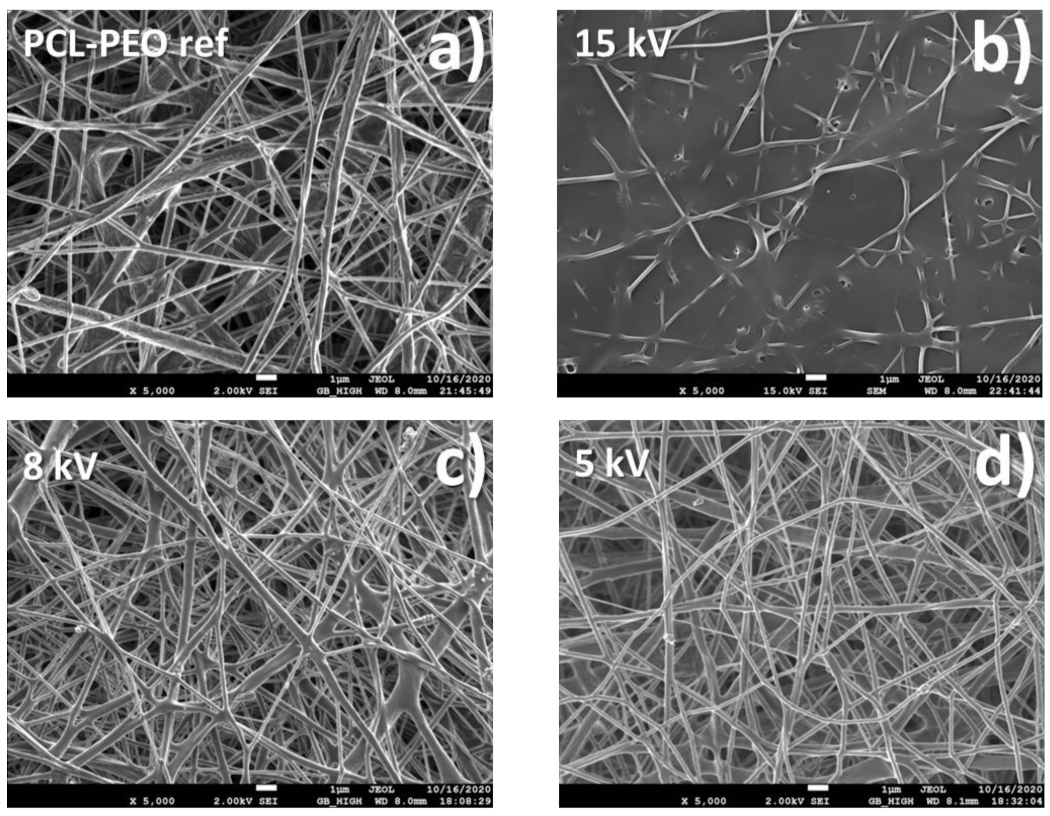
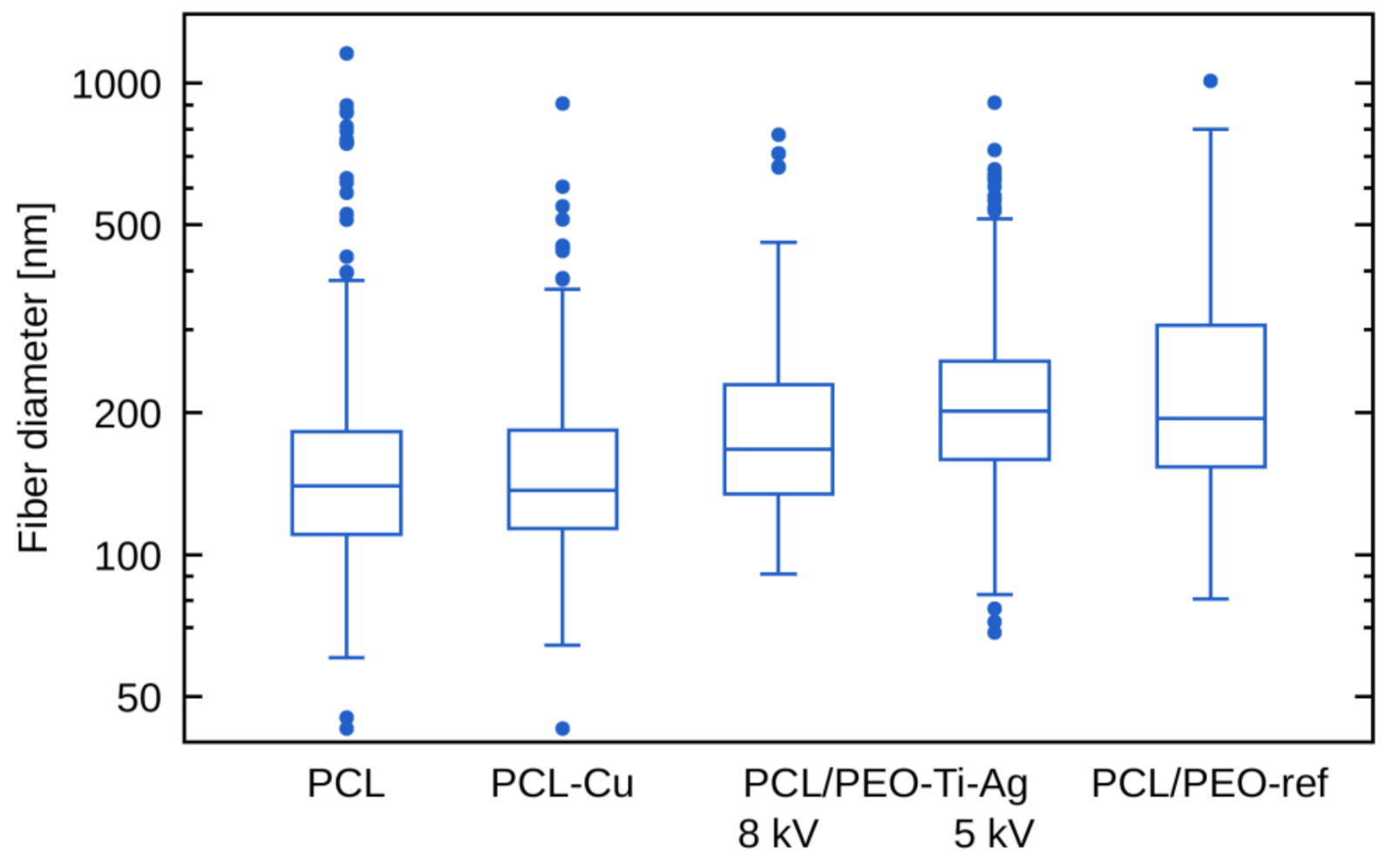
2.2. Morphology of Biodegradable Nanohybrid Materials
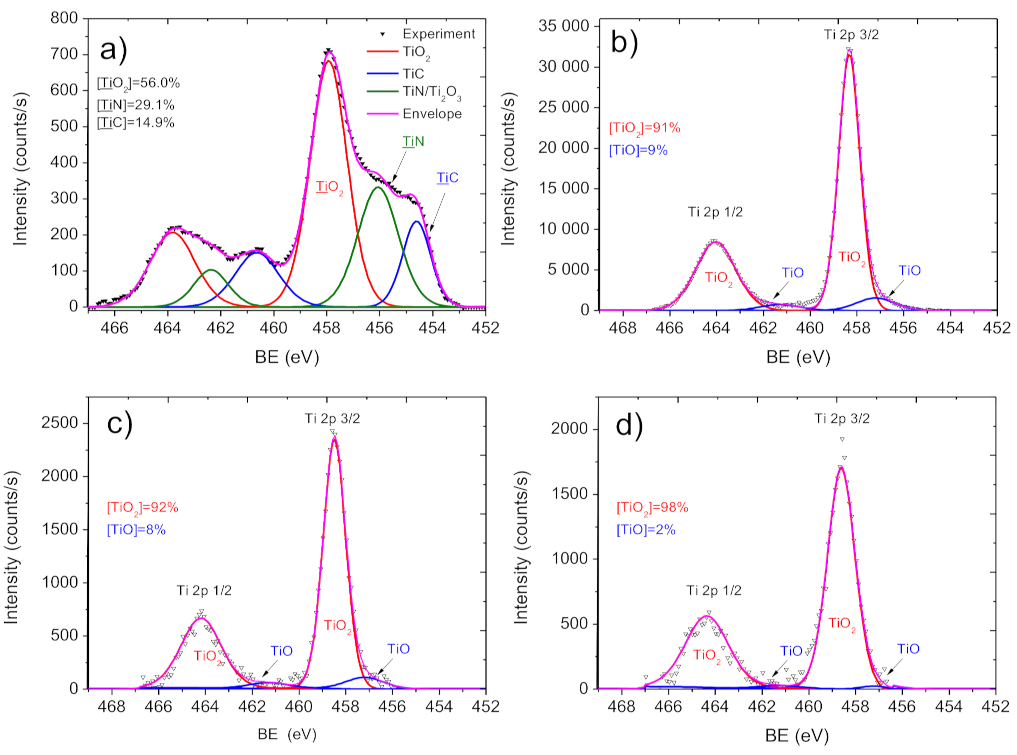
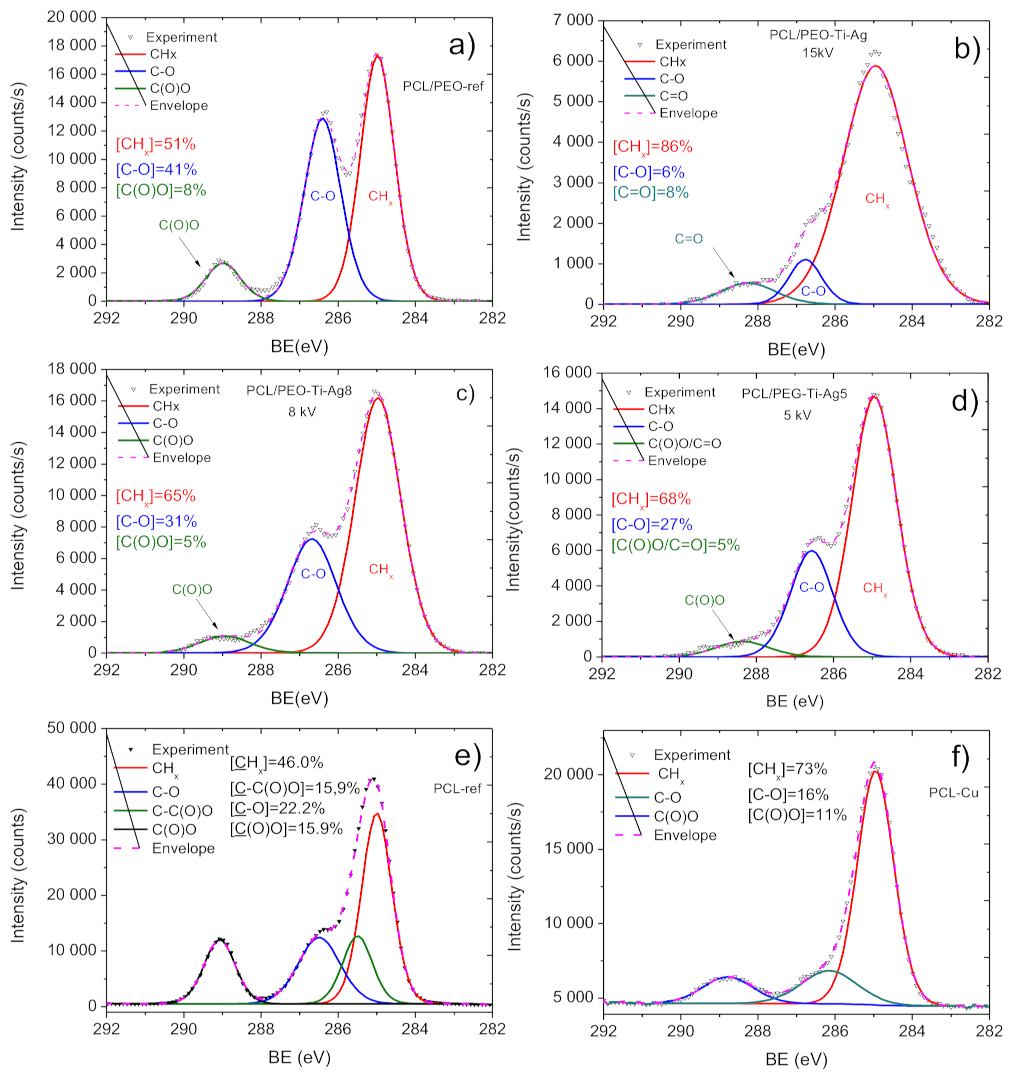
2.3. Chemical Characterization of Biodegradable Nanohybrid Materials
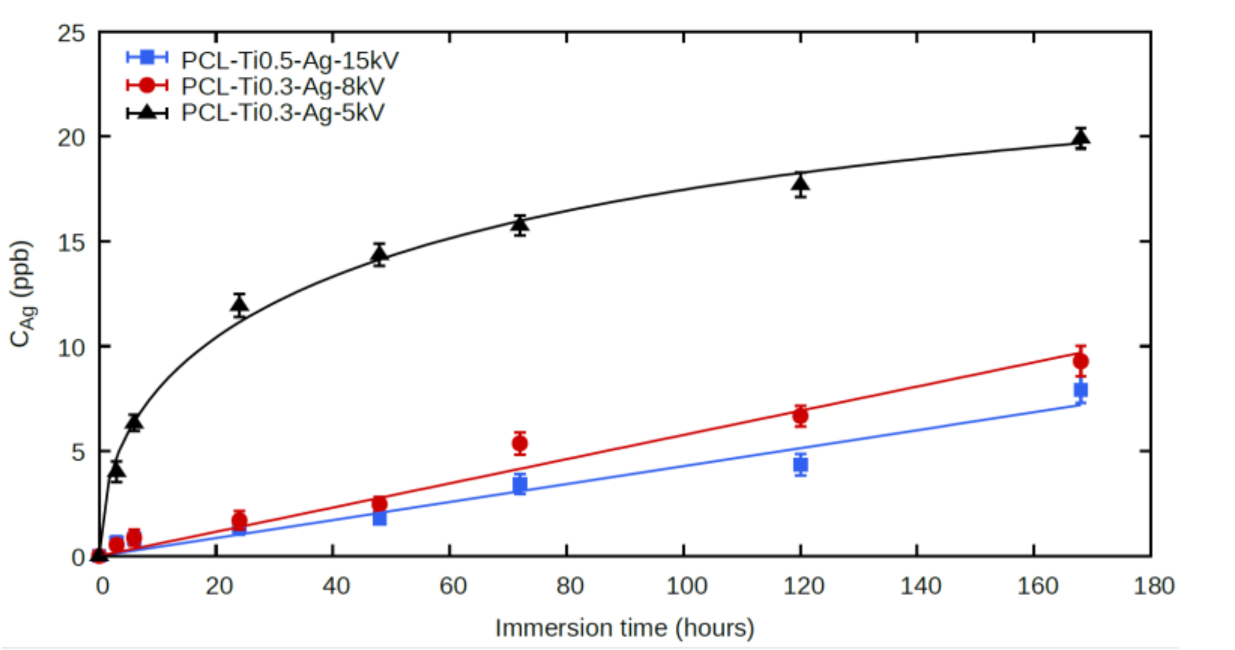
2.4. Ag+ Ion Release
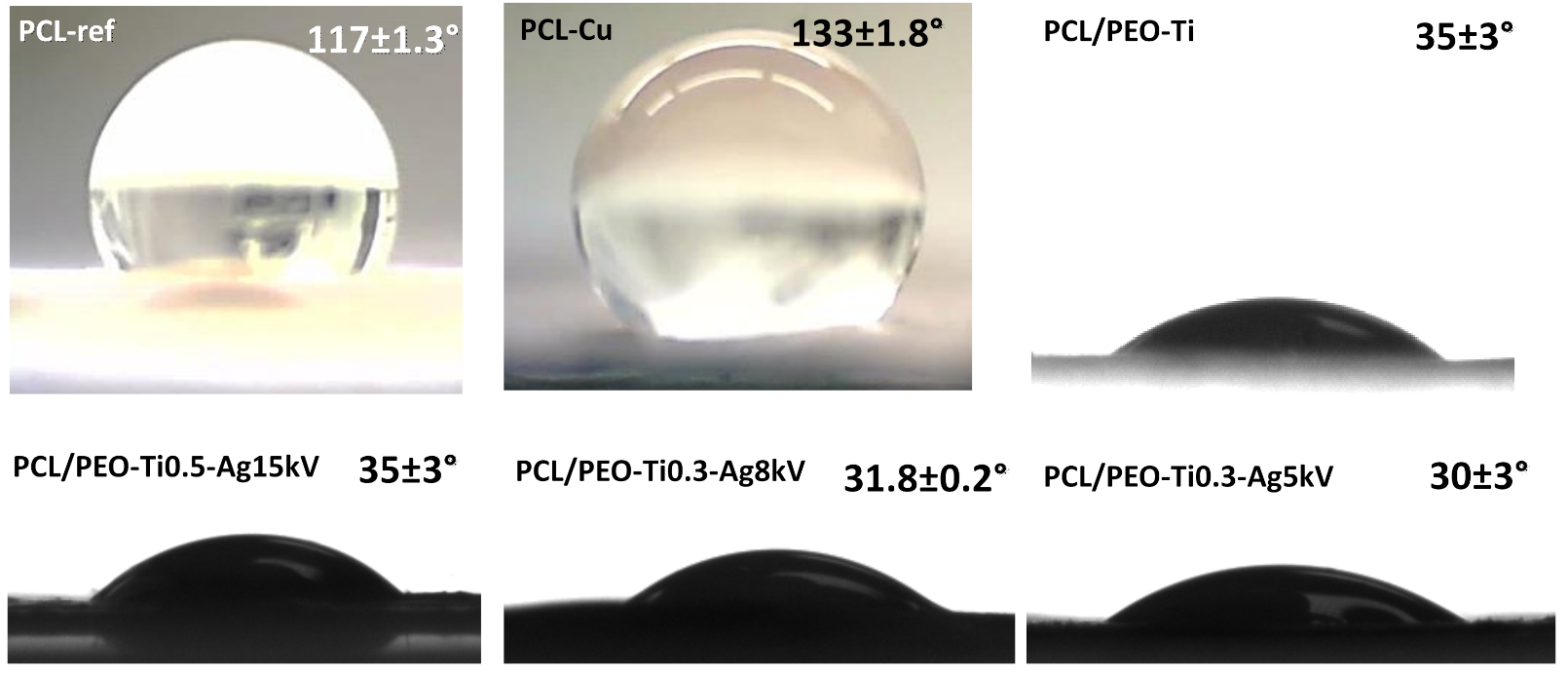
2.5. Wettability
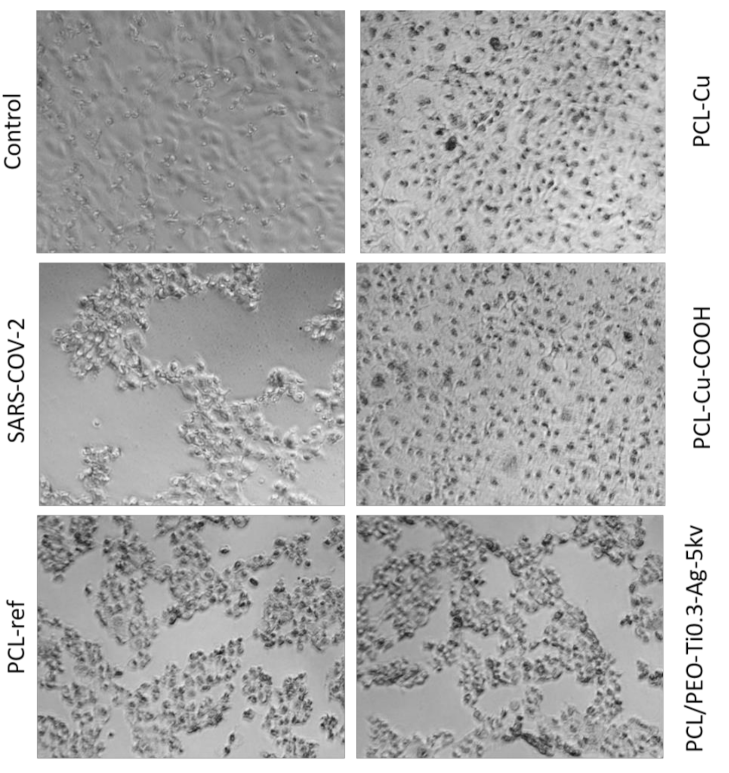
2.6. Antiviral Tests
3. Discussion
| Type of Material | Polymer | Concentration of Ag | Virus | Ref |
|---|---|---|---|---|
| nanofibers | Polyvinyl alcohol (PVA) Thermoplastic polyurethane (TPU) | 2, 4wt% | SARS-CoV-2 | [40] |
| nanofibers | Polyamide6 | 4.29 wt% | PDCoV | [45] |
| hydrogel | Carbopol 974P | 25 ppm/100g 50 ppm/100g | Herpes simplex virus (HSV)-1,2 | [41] |
| Textile | cotton | 1,46 wt% | Influenza A Feline calicivirus | [42] |
| microfibers | poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) | 2 wt% | feline calicivirus (FCV) murine norovirus 27 (MNV) | [43] |
4. Materials and Methods
4.1. Electrospinning of PCL and PCL/PEO Nanofibers
4.2. Magnetron Sputtering of Cu
4.3. Deposition of TiO2 Coating and Ag Ion Implantation
4.4. Plasma COOH Coating
4.5. Chemistry and Morphology Analysis
4.6. Modeling of Ag Atom Irradiation of PCL Surface
4.7. Ion Release and Wettability Measurements
4.8. Antiviral Tests
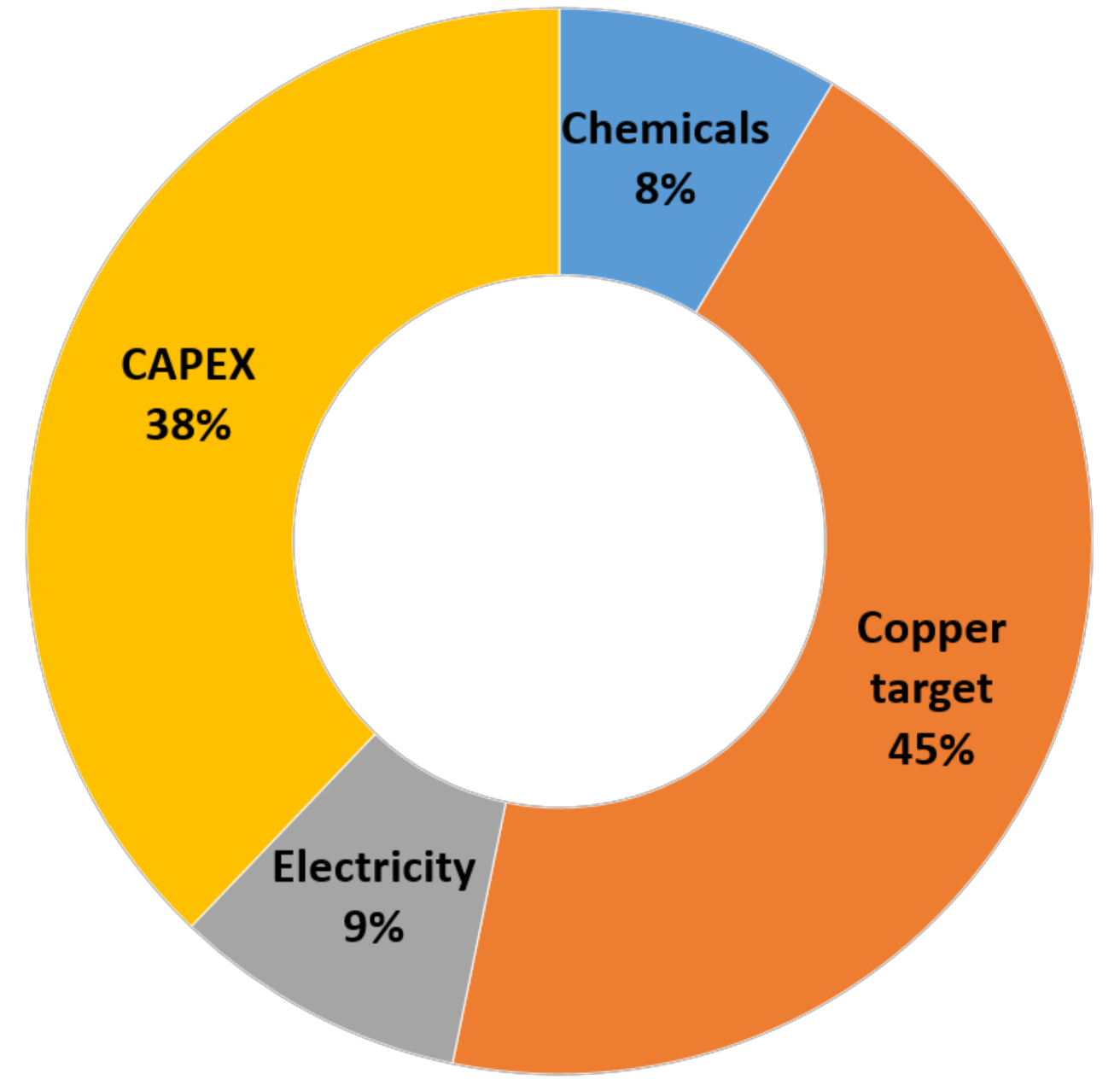
4.9. Techno-Economic Assessment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Syed, A.; Elgorban, A.M.; Marraiki, N.; Kim, D. Biological characteristics and biomarkers of novel SARS-CoV-2 facilitated rapid development and implementation of diagnostic tools and surveillance measures. Biosens. Bioelectron. 2021, 177, 112969. [Google Scholar] [CrossRef]
- Kumar, M.; Kumari, N.; Thakur, N.; Bhatia, S.K.; Saratale, G.D.; Ghodake, G.; Mistry, B.M.; Alavilli, H.; Kishor, D.S.; Du, X.; et al. A Comprehensive Overview on the Production of Vaccines in Plant-Based Expression Systems and the Scope of Plant Biotechnology to Combat against SARS-CoV-2 Virus Pandemics. Plants 2021, 10, 1213. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Jimenez, J.L.; Prather, K.A.; Tufekci, Z.; Fisman, D.; Schooley, R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet 2021, 397, 1603–1605. [Google Scholar] [CrossRef]
- Essa, W.; Yasin, S.; Saeed, I.; Ali, G. Nanofiber-Based Face Masks and Respirators as COVID-19 Protection: A Review. Membranes 2021, 11, 250. [Google Scholar] [CrossRef] [PubMed]
- Omori, Y.; Gu, T.; Bao, L.; Otani, Y.; Seto, T. Performance of nanofiber/microfiber hybrid air filter prepared by wet paper processing. Aerosol Sci. Technol. 2019, 53, 1149–1157. [Google Scholar] [CrossRef]
- Skaria, S.D.; Smaldone, G.C. Respiratory Source Control Using Surgical Masks with Nanofiber Media. Ann. Occup. Hyg. 2014, 58, 771–781. [Google Scholar] [PubMed] [Green Version]
- Hashmi, M.; Ullah, S.; Kim, I.S. Copper oxide (CuO) loaded polyacrylonitrile (PAN) nanofiber membranes for antimicrobial breath mask applications. Curr. Res. Biotechnol. 2019, 1, 1–10. [Google Scholar] [CrossRef]
- Huang, X.; Jiao, T.; Liu, Q.; Zhang, L.; Zhou, J.; Li, B.; Peng, Q. Hierarchical electrospun nanofibers treated by solvent vapor annealing as air filtration mat for high-efficiency PM2.5 capture. Sci. China Mater. 2019, 62, 423–436. [Google Scholar] [CrossRef] [Green Version]
- Tian, X.; Xin, B.; Gao, W.; Jin, S.; Chen, Z. Preparation and characterization of polyvinylidene fluoride/polysulfone-amide composite nanofiber mats. J. Text. Inst. 2019, 110, 815–821. [Google Scholar] [CrossRef]
- Ullah, S.; Ullah, A.; Lee, J.; Jeong, Y.; Hashmi, M.; Zhu, C.; Joo, K.I.; Cha, H.J.; Kim, I.S. Reusability Comparison of Melt-Blown vs Nanofiber Face Mask Filters for Use in the Coronavirus Pandemic. ACS Appl. Nano Mater. 2020, 3, 7231–7241. [Google Scholar] [CrossRef]
- Lee, S.; Cho, A.R.; Park, D.; Kim, J.K.; Han, K.S.; Yoon, I.-J.; Lee, M.H.; Nah, J. Reusable Polybenzimidazole Nanofiber Membrane Filter for Highly Breathable PM 2.5 Dust Proof Mask. ACS Appl. Mater. Interfaces 2019, 11, 2750–2757. [Google Scholar] [CrossRef]
- Liwanag, V. A Reusable Nanofiber Mask Ensuring High Breathability. Available online: https://emag.medicalexpo.com/a-reusable-nanofiber-mask-ensuring-high-breathability/ (accessed on 29 December 2021).
- Zhou, L.; Ayeh, S.K.; Chidambaram, V.; Karakousis, P.C. Modes of transmission of SARS-CoV-2 and evidence for preventive behavioral interventions. BMC Infect. Dis. 2021, 21, 496. [Google Scholar] [CrossRef]
- Babaahmadi, V.; Amid, H.; Naeimirad, M.; Ramakrishna, S. Biodegradable and multifunctional surgical face masks: A brief review on demands during COVID-19 pandemic, recent developments, and future perspectives. Sci. Total Environ. 2021, 798, 149233. [Google Scholar] [CrossRef] [PubMed]
- Jarach, N.; Dodiuk, H.; Kenig, S. Polymers in the medical antiviral front-line. Polymers 2020, 12, 1727. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, Y.; Liu, W.; Wang, L.; Choi, Y.W.; Fulton, M.; Fuchs, S.; Shariati, K.; Qiao, M.; Bernat, V.; et al. A Broad-Spectrum Antimicrobial and Antiviral Membrane Inactivates SARS-CoV-2 in Minutes. Adv. Funct. Mater. 2021, 31, 2103477. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.Y.; Cheeseman, S.; Frias-De-Diego, A.; Hong, H.; Yang, J.; Jung, W.; Yin, H.; Murdoch, B.J.; Scholle, F.; Crook, N.; et al. A Liquid Metal Mediated Metallic Coating for Antimicrobial and Antiviral Fabrics. Adv. Mater. 2021, 33, 2170352. [Google Scholar] [CrossRef]
- Pemmada, R.; Zhu, X.; Dash, M.; Zhou, Y.; Ramakrishna, S.; Peng, X.; Thomas, V.; Jain, S.; Nanda, H.S. Science-based strategies of antiviral coatings with viricidal properties for the COVID-19 like pandemics. Materials 2020, 13, 4041. [Google Scholar] [CrossRef]
- Mantlo, E.K.; Paessler, S.; Seregin, A.; Mitchell, A. Luminore coppertouch surface coating effectively inactivates SARS-CoV-2, Ebola virus, and Marburg virus in vitro. Antimicrob. Agents Chemother. 2021, 65. [Google Scholar] [CrossRef] [PubMed]
- Kuzderová, G.; Rendošová, M.; Gyepes, R.; Sovová, S.; Sabolová, D.; Vilková, M.; Olejníková, P.; Bačová, I.; Stokič, S.; Kello, M.; et al. Antimicrobial and Anticancer Application of Silver(I) Dipeptide Complexes. Molecules 2021, 26, 6335. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Alkhudhair, S.K.; Alharbi, R.I.; Al-Askar, A.A.; Aljowaie, R.M.; Al-Shehri, S. The antimicrobial activities of silver nanoparticles from aqueous extract of grape seeds against pathogenic bacteria and fungi. Molecules 2021, 26, 6081. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.C.; Cote, D.L. Antimicrobial Copper Cold Spray Coatings and SARS-CoV-2 Surface Inactivation. MRS Adv. 2020, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Tremiliosi, G.C.; Simoes, L.G.P.; Minozzi, D.T.; Santos, R.I.; Vilela, D.C.B.; Durigon, E.L.; Machado, R.R.G.; Medina, D.S.; Ribeiro, L.K.; Rosa, I.L.V.; et al. Ag nanoparticles-based antimicrobial polycotton fabrics to prevent the transmission and spread of SARS-CoV-2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Zulfiqar, H.; Zafar, A.; Rasheed, M.N.; Ali, Z.; Mehmood, K.; Mazher, A.; Hasan, M.; Mahmood, N. Synthesis of silver nanoparticles using Fagonia cretica and their antimicrobial activities. Nanoscale Adv. 2019, 1, 1707–1713. [Google Scholar] [CrossRef] [Green Version]
- Homaeigohar, S.; Liu, Q.; Kordbacheh, D. Biomedical applications of antiviral nanohybrid materials relating to the COVID-19 pandemic and other viral crises. Polymers 2021, 13, 2833. [Google Scholar] [CrossRef]
- Misra, N.; Bhatt, S.; Arefi-Khonsari, F.; Kumar, V. State of the art in nonthermal plasma processing for biomedical applications: Can it help fight viral pandemics like COVID-19? Plasma Process. Polym. 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Tučeková, Z.K.; Vacek, L.; Krumpolec, R.; Kelar, J.; Zemánek, M.; Černák, M.; Růžička, F. Multi-hollow surface dielectric barrier discharge for bacterial biofilm decontamination. Molecules 2021, 26, 910. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, S.; Lan, W.; Hossen, M.A.; Qin, W.; Lee, K. Electrospun antibacterial and antiviral poly(ε-caprolactone)/zein/Ag bead-on-string membranes and its application in air filtration. Mater. Today Adv. 2021, 12, 100173. [Google Scholar] [CrossRef]
- Park, K.; Kang, S.; Park, J.-W.; Hwang, J. Fabrication of silver nanowire coated fibrous air filter medium via a two-step process of electrospinning and electrospray for anti-bioaerosol treatment. J. Hazard. Mater. 2021, 411, 125043. [Google Scholar] [CrossRef]
- Chatani, Y.; Okita, Y.; Tadokoro, H.; Yamashita, Y. Structural Studies of Polyesters. III. Crystal Structure of Poly-ε-caprolactone. Polym. J. 1970, 1, 555–562. [Google Scholar] [CrossRef] [Green Version]
- Manakhov, A.M.; Sitnikova, N.A.; Tsygankova, A.R.; Alekseev, A.Y.; Adamenko, L.S.; Permyakova, E.; Baidyshev, V.S.; Popov, Z.I.; Blahová, L.; Eliáš, M.; et al. Electrospun Biodegradable Nanofibers Coated Homogenously by Cu Magnetron Sputtering Exhibit Fast Ion Release. Computational and Experimental Study. Membranes 2021, 11, 965. [Google Scholar] [CrossRef]
- Kupka, V.; Dvořáková, E.; Manakhov, A.; Michlíček, M.; Petruš, J.; Vojtová, L.; Zajíčková, L. Well-Blended PCL/PEO Electrospun Nanofibers with Functional Properties Enhanced by Plasma Processing. Polymers 2020, 12, 1403. [Google Scholar] [CrossRef]
- Solovieva, A.; Miroshnichenko, S.; Kovalskii, A.; Permyakova, E.; Popov, Z.; Dvořáková, E.; Kiryukhantsev-Korneev, P.; Obrosov, A.; Polčak, J.; Zajíčková, L.; et al. Immobilization of Platelet-Rich Plasma onto COOH Plasma-Coated PCL Nanofibers Boost Viability and Proliferation of Human Mesenchymal Stem Cells. Polymers 2017, 9, 736. [Google Scholar] [CrossRef] [Green Version]
- Manakhov, A.; Permyakova, E.S.; Ershov, S.; Sheveyko, A.; Kovalskii, A.; Polčák, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Zajíčková, L.; Shtansky, D.V. Bioactive TiCaPCON-coated PCL nanofibers as a promising material for bone tissue engineering. Appl. Surf. Sci. 2019, 479, 796–802. [Google Scholar] [CrossRef]
- Ponomarev, V.A.; Sheveyko, A.N.; Permyakova, E.S.; Lee, J.; Voevodin, A.A.; Berman, D.; Manakhov, A.M.; Michlíček, M.; Slukin, P.V.; Firstova, V.V.; et al. TiCaPCON-Supported Pt- and Fe-Based Nanoparticles and Related Antibacterial Activity. ACS Appl. Mater. Interfaces 2019, 11, 28699–28719. [Google Scholar] [CrossRef]
- Permyakova, E.S.; Manakhov, A.M.; Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Gudz, K.Y.; Kovalskii, A.M.; Polčak, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Dyatlov, I.A.; et al. Different concepts for creating antibacterial yet biocompatible surfaces: Adding bactericidal element, grafting therapeutic agent through COOH plasma polymer and their combination. Appl. Surf. Sci. 2021, 556, 149751. [Google Scholar] [CrossRef]
- Konopatsky, A.; Firestein, K.L.; Leybo, D.V.; Popov, Z.I.; Larionov, K.; Steinman, A.E.; Kovalskii, A.M.; Matveev, A.; Manakhov, A.; Sorokin, P.B.; et al. BN Nanoparticle/Ag Hybrids with Enhanced Catalytic Activity: Theory and Experiments. Catal. Sci. Technol. 2018, 8, 1652–1662. [Google Scholar] [CrossRef] [Green Version]
- Permyakova, E.S.; Kiryukhantsev-Korneev, P.V.; Gudz, K.Y.; Konopatsky, A.S.; Polčak, J.; Zhitnyak, I.Y.; Gloushankova, N.A.; Shtansky, D.V.; Manakhov, A.M. Comparison of Different Approaches to Surface Functionalization of Biodegradable Polycaprolactone Scaffolds. Nanomaterials 2019, 9, 1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshabanah, L.A.; Hagar, M.; Al-Mutabagani, L.A.; Abozaid, G.M.; Abdallah, S.M.; Shehata, N.; Ahmed, H.; Hassanin, A.H. Hybrid nanofibrous membranes as a promising functional layer for personal protection equipment: Manufacturing and antiviral/antibacterial assessments. Polymers 2021, 13, 1776. [Google Scholar] [CrossRef]
- Szymańska, E.; Orłowski, P.; Winnicka, K.; Tomaszewska, E.; Bąska, P.; Celichowski, G.; Grobełny, J.; Basa, A.; Krzyżowska, M. Multifunctional tannic acid/silver nanoparticle-based mucoadhesive hydrogel for improved local treatment of HSV infection: In vitro and in vivo studies. Int. J. Mol. Sci. 2018, 19, 387. [Google Scholar] [CrossRef] [Green Version]
- Seino, S.; Imoto, Y.; Kosaka, T.; Nishida, T.; Nakagawa, T.; Yamamoto, T.A. Antiviral Activity of Silver Nanoparticles Immobilized onto Textile Fabrics Synthesized by Radiochemical Process. MRS Adv. 2016, 1, 705–710. [Google Scholar] [CrossRef]
- Castro-Mayorga, J.L.; Randazzo, W.; Fabra, M.J.; Lagaron, J.M.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, G.; Aznar, R. Evaluation of Natural Compounds of Plant Origin for Inactivation of Enteric Viruses. Food Environ. Virol. 2015, 7, 183–187. [Google Scholar] [CrossRef]
- Ju, Y.; Han, T.; Yin, J.; Li, Q.; Chen, Z.; Wei, Z.; Zhang, Y.; Dong, L. Bumpy structured nanofibrous membrane as a highly efficient air filter with antibacterial and antiviral property. Sci. Total Environ. 2021, 777, 145768. [Google Scholar] [CrossRef] [PubMed]
- Sukhorukova, I.V.; Sheveyko, A.N.; Manakhov, A.; Zhitnyak, I.Y.; Gloushankova, N.A.; Denisenko, E.A.; Filippovich, S.Y.; Ignatov, S.G.; Shtansky, D.V. Synergistic and long-lasting antibacterial effect of antibiotic-loaded TiCaPCON-Ag films against pathogenic bacteria and fungi. Mater. Sci. Eng. C 2018, 90, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, A.; Landová, M.; Medalová, J.; Michlíček, M.; Polčák, J.; Nečas, D.; Zajíčková, L. Cyclopropylamine plasma polymers for increased cell adhesion and growth. Plasma Process. Polym. 2017, 14, 1600123. [Google Scholar] [CrossRef]
- Manakhov, A.; Moreno-Couranjou, M.; Choquet, P.; Boscher, N.D.; Pireaux, J.-J.J. Diene functionalisation of atmospheric plasma copolymer thin films. Surf. Coatings Technol. 2011, 205, S466–S469. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Lloyd, A.; Cornil, D.; van Duin, A.C.T.; van Duin, D.; Smith, R.; Kenny, S.D.; Cornil, J.; Beljonne, D. Development of a ReaxFF potential for Ag/Zn/O and application to Ag deposition on ZnO. Surf. Sci. 2016, 645, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Keyaerts, E.; Vijgen, L.; Maes, P.; Neyts, J.; Ranst, M. Van Growth kinetics of SARS-coronavirus in Vero E6 cells. Biochem. Biophys. Res. Commun. 2005, 329, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Gendrot, M.; Andreani, J.; Jardot, P.; Hutter, S.; Delandre, O.; Boxberger, M.; Mosnier, J.; Le Bideau, M.; Duflot, I.; Fonta, I.; et al. In Vitro Antiviral Activity of Doxycycline against SARS-CoV-2. Molecules 2020, 25, 5064. [Google Scholar] [CrossRef]



| Sample | C (at.%) | O (at.%) | Ti (at.%) | Ag (at.%) | Cu (at.%) |
|---|---|---|---|---|---|
| PCL/PEO-ref | 75.0 | 25.0 | 0.0 | 0.0 | 0.0 |
| PCL/PEO-Ti0.5-Ag15kV | 31.9 | 49.5 | 18.0 | 0.6 | 0.0 |
| PCL/PEO-Ti0.3-Ag-8kV | 77.2 | 21.1 | 1.3 | 0.4 | 0.0 |
| PCL/PEO-Ti0.3-Ag-5kV | 70.3 | 27.9 | 1.1 | 0.7 | 0.0 |
| PCL-Cu | 50.6 | 29.0 | 0.0 | 0.0 | 20.4 |
| PCL-Cu-COOH | 73.1 | 26.5 | 0.0 | 0.0 | 0.4 |
| Sample | Virus Titer (LgCPE50) |
|---|---|
| PCL-Cu | 1 |
| PCL-Cu-COOH | 1 |
| PCL/PEO-Ti0.3-Ag-5kV | 1,7 |
| Dimer | E, eV (DFT) | E, eV (ReaxFF) | ΔE, eV | R, Ǻ (DFT) | R, Ǻ (ReaxFF) |
|---|---|---|---|---|---|
| Ag-Ag | −2.17 | −1.56 | −0.61 | 2.56 | 2.64 |
| Ag-C | −5.60 | −4.63 | −0.53 | 1.95 | 2.02 |
| Ag-H | −6.77 | −4.71 | −0.41 | 1.62 | 1.48 |
| Ag-O | −7.09 | −5.63 | −0.29 | 1.95 | 2.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manakhov, A.M.; Permyakova, E.S.; Sitnikova, N.A.; Tsygankova, A.R.; Alekseev, A.Y.; Solomatina, M.V.; Baidyshev, V.S.; Popov, Z.I.; Blahová, L.; Eliáš, M.; et al. Biodegradable Nanohybrid Materials as Candidates for Self-Sanitizing Filters Aimed at Protection from SARS-CoV-2 in Public Areas. Molecules 2022, 27, 1333. https://doi.org/10.3390/molecules27041333
Manakhov AM, Permyakova ES, Sitnikova NA, Tsygankova AR, Alekseev AY, Solomatina MV, Baidyshev VS, Popov ZI, Blahová L, Eliáš M, et al. Biodegradable Nanohybrid Materials as Candidates for Self-Sanitizing Filters Aimed at Protection from SARS-CoV-2 in Public Areas. Molecules. 2022; 27(4):1333. https://doi.org/10.3390/molecules27041333
Chicago/Turabian StyleManakhov, Anton M., Elizaveta S. Permyakova, Natalya A. Sitnikova, Alphiya R. Tsygankova, Alexander Y. Alekseev, Maria V. Solomatina, Victor S. Baidyshev, Zakhar I. Popov, Lucie Blahová, Marek Eliáš, and et al. 2022. "Biodegradable Nanohybrid Materials as Candidates for Self-Sanitizing Filters Aimed at Protection from SARS-CoV-2 in Public Areas" Molecules 27, no. 4: 1333. https://doi.org/10.3390/molecules27041333
APA StyleManakhov, A. M., Permyakova, E. S., Sitnikova, N. A., Tsygankova, A. R., Alekseev, A. Y., Solomatina, M. V., Baidyshev, V. S., Popov, Z. I., Blahová, L., Eliáš, M., Zajíčková, L., Kovalskii, A. M., Sheveyko, A. N., Kiryukhantsev-Korneev, P. V., Shtansky, D. V., Nečas, D., & Solovieva, A. O. (2022). Biodegradable Nanohybrid Materials as Candidates for Self-Sanitizing Filters Aimed at Protection from SARS-CoV-2 in Public Areas. Molecules, 27(4), 1333. https://doi.org/10.3390/molecules27041333