Quality Related Safety Evaluation of a South African Traditional Formulation (PHELA®) as Novel Anti-Biofilm Candidate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Procurement of Plant Material
2.3. Extraction of the Plant Material
2.4. UPLC-MS Analysis of PHELA® Extract
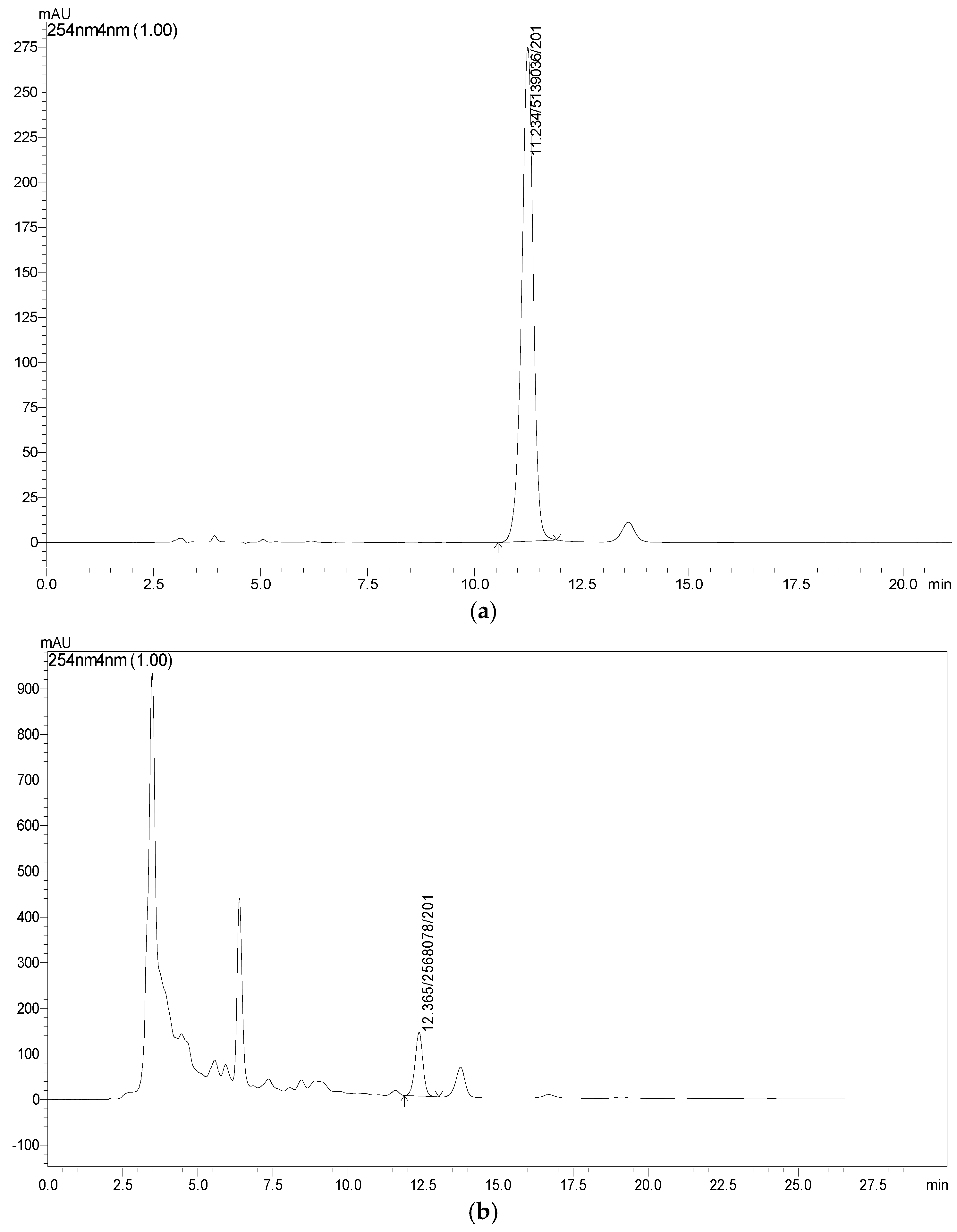
2.5. RP-HPLC Analysis of PHELA® Extract
2.6. Safety Assessment of PHELA® Extract
2.6.1. MTT Cytotoxicity Assay
2.6.2. CYP450 Enzyme Inhibition Study
2.7. Antibacterial and Anti-Biofilm Activity of PHELA® Extract
2.7.1. MIC Determination by Broth Dilution Method Using the 96-Well Plate
2.7.2. Determination of MBC
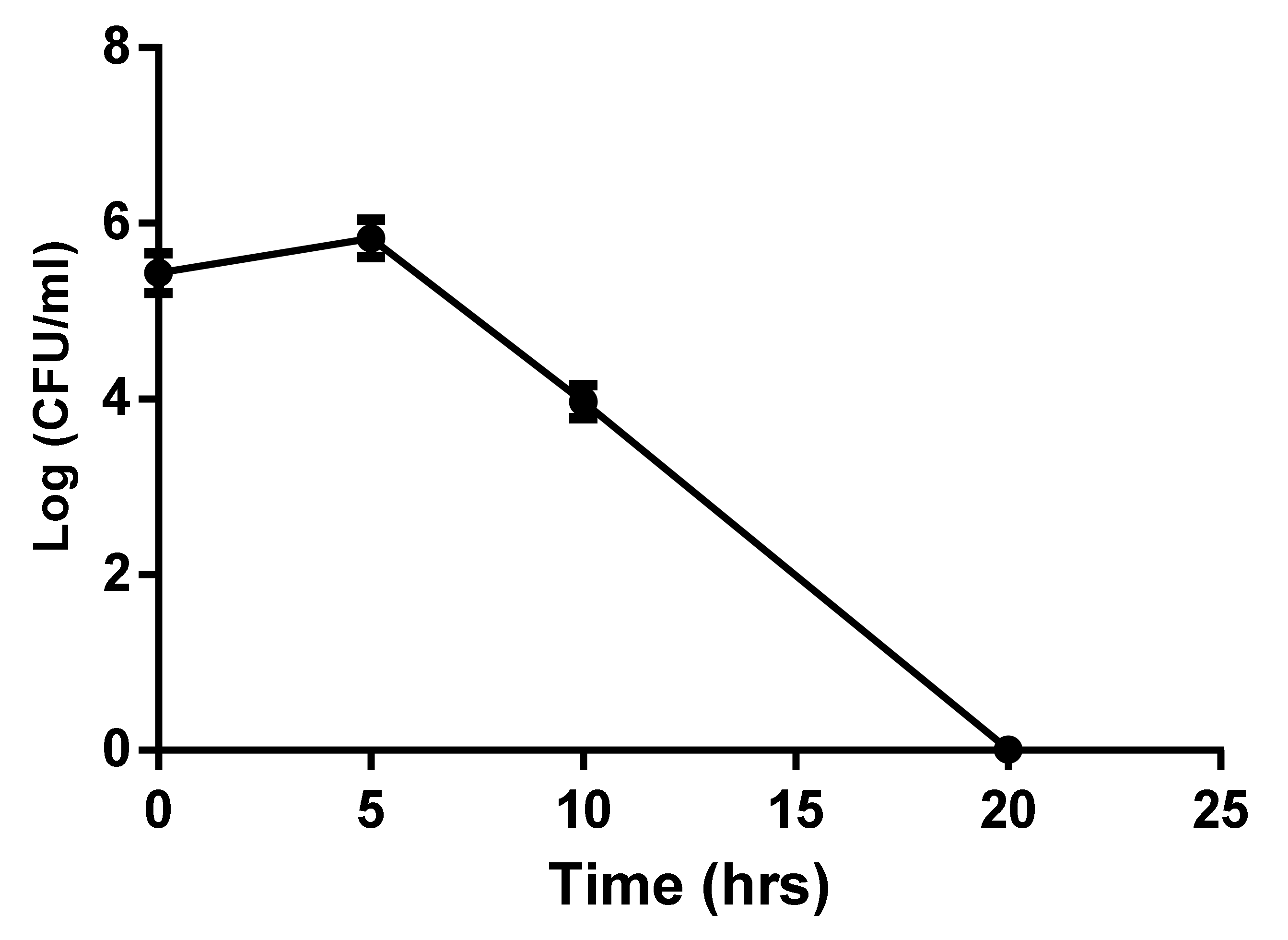
2.7.3. Growth Curves Analysis
2.7.4. Inhibition of Biofilm Formation
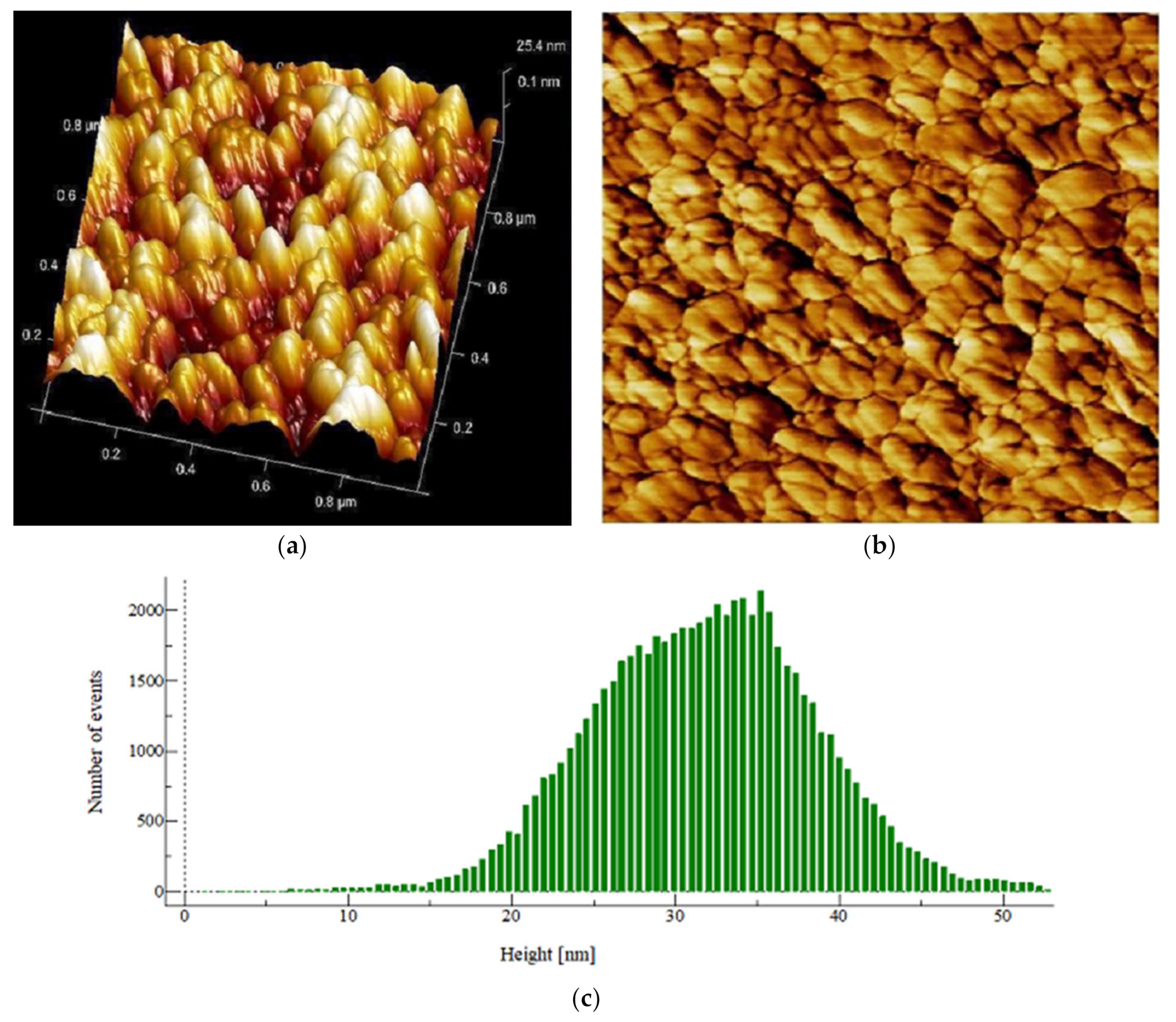
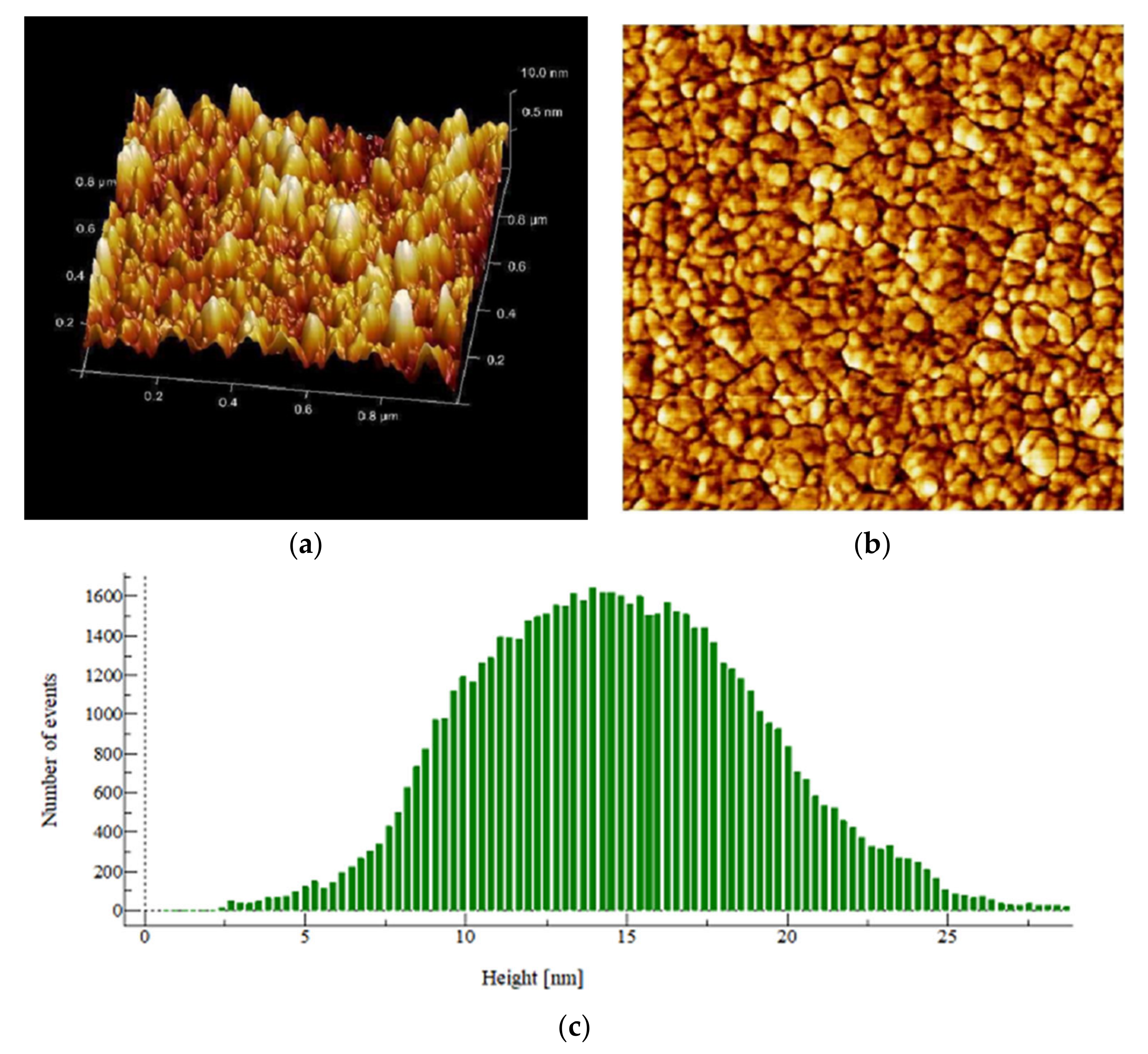
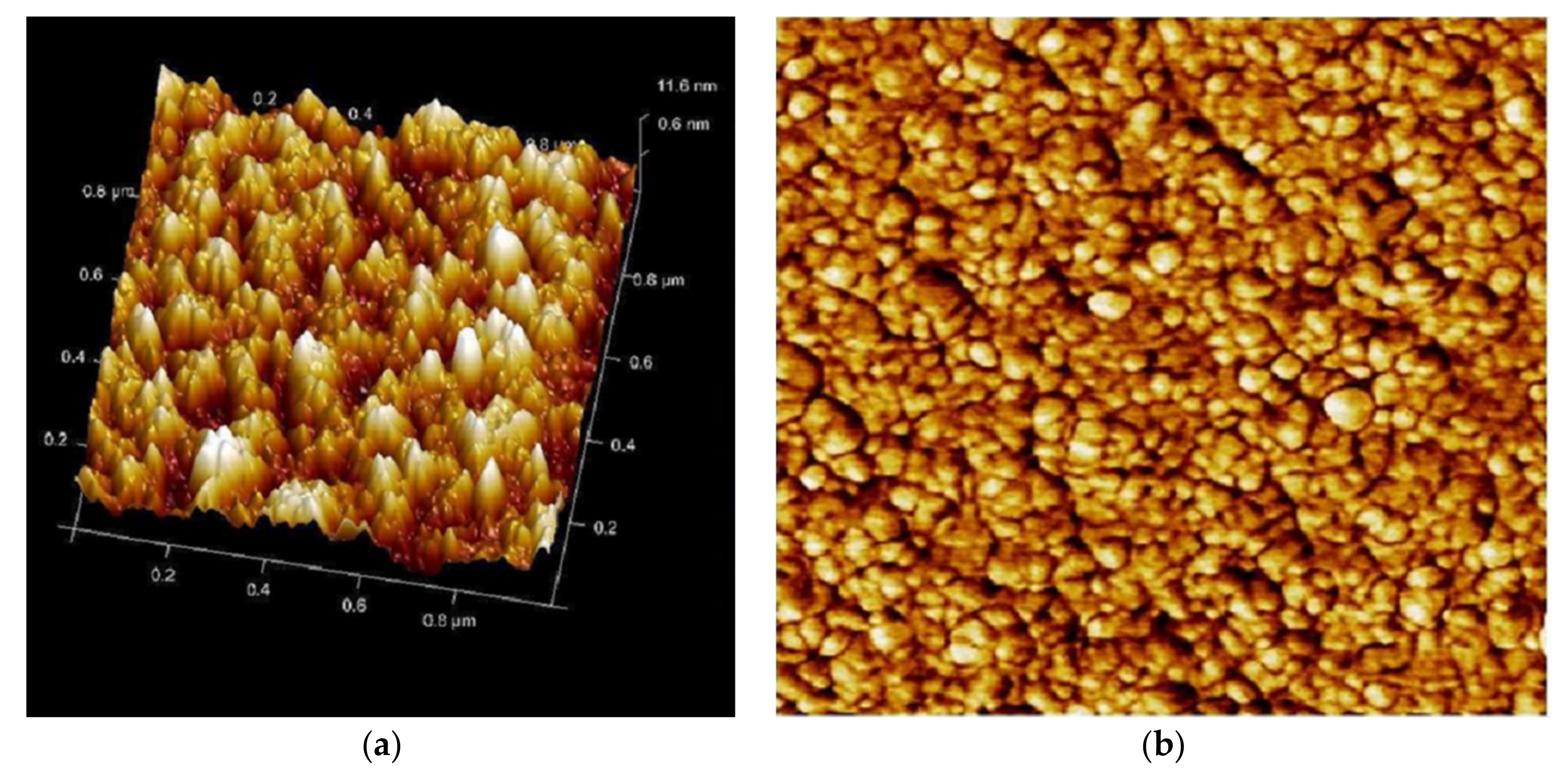
2.7.5. AFM Assisted Characterization of the Effects of PHELA® Extract on S. aureus Biofilm
3. Statistical Analysis
4. Results
4.1. UPLC-MS Analysis of PHELA® Extract
4.2. RP-HPLC Analysis of PHELA® Extract
4.3. Safety Assessment of PHELA® Extract
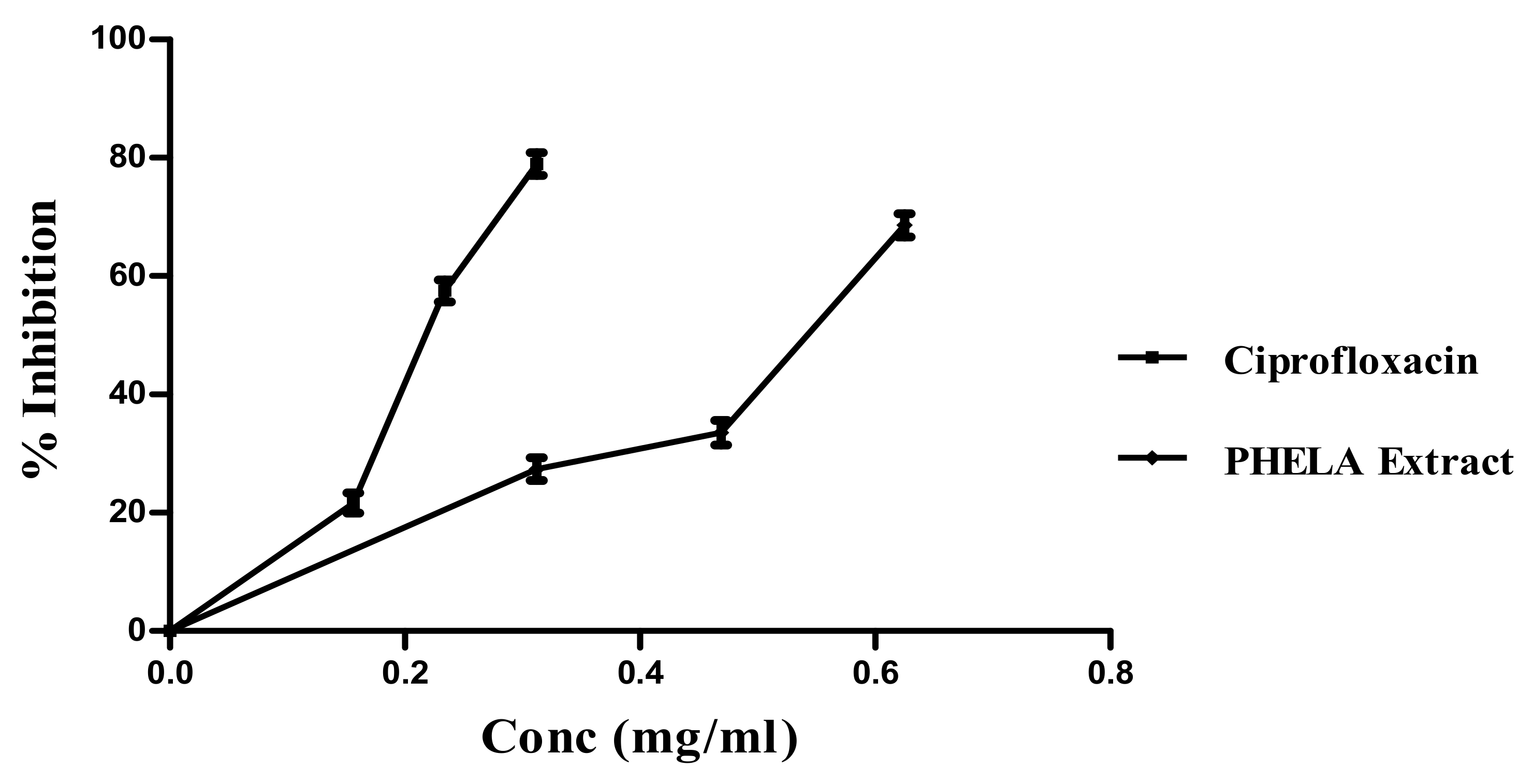
4.4. Antibacterial and Anti-Biofilm Activity of PHELA® Extract
Determination of MIC and MBC
4.5. Growth Curve Analysis
4.6. Effect of PHELA® on Formation of Biofilm
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EPS | Extracellular polymeric substances |
| MHB | Muller Hinton Broth |
| ATCC | American Type Culture Collection |
| PHAE | Hydro-alcoholic extract of PHELA® |
| RP-HPLC | Reverse Phase- High Performance Liquid Chromatography |
| PDA | Photodiode Array |
| CYP | Cytochrome |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| AFM | Atomic Force Microscopy |
| Sq | Root Mean Square |
| Spk | Reduced peak height |
| Svk | Reduced valley depth |
| Sa | Roughness Average |
| Ssk | Surface skewness |
| Ska | Coefficient of kurtosis |
| Sdr | Surface Area Ratio |
| Sz | % Maximum height |
| r2 | correlation coefficient |
| Rt | Retention time |
References
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial Biofilms: A Common Cause of Persistent Infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [Green Version]
- Mah, T.-F.C.; O’Toole, G.A. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 2001, 9, 34–39. [Google Scholar] [CrossRef]
- Hugo, W.B.; Denyer, S.P.; Hodges, N.A.; Gorman, S.P. Hugo and Russell’s Pharmaceutical Microbiology, 7th ed.; Blackwell Science: Malden, MA, USA, 2004. [Google Scholar]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Neu, T.R.; Lawrence, J.R. Innovative techniques, sensors, and approaches for imaging biofilms at different scales. Trends Microbiol. 2015, 23, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T.; Jensen, P.Ø.; Burmølle, M.; Hentzer, M.; Haagensen, J.A.J.; Hougen, H.P.; Calum, H.; Madsen, K.G.; Moser, C.; Molin, S.; et al. Pseudomonas aeruginosa tolerance to tobramycin, hydrogen peroxide and polymorphonuclear leukocytes is quorum-sensing dependent. Microbiology 2005, 151 Pt 2, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Essawi, T.; Srour, M. Screening of some Palestinian medicinal plants for antibacterial activity. J. Ethnopharmacol. 2000, 70, 343–349. [Google Scholar] [CrossRef]
- Guarrera, P.M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and Latium). Fitoterapia 2005, 76, 1–25. [Google Scholar] [CrossRef]
- Mukherjee, P.K. Evaluation of Herbal Drugs for Antimicrobial and Parasiticidal Effects. In Quality Control and Evaluation of Herbal Drugs; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 573–598. [Google Scholar]
- Sen, T.; Karmakar, S.; Sarkar, R. Evaluation of Natural Products against Biofilm-Mediated Bacterial Resistance. In Evidence-Based Validation of Herbal Medicine; Mukherjee, P.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 321–338. [Google Scholar]
- Colalto, C. Herbal interactions on absorption of drugs: Mechanisms of action and clinical risk assessment. Pharmacol. Res. 2010, 62, 207–227. [Google Scholar] [CrossRef]
- Ma, H. The Formulation, Manufacture and Evaluation of Capsules Containing Freeze-Dried Aqueous Extracts of Leonotis Leonorus or Mentha Longifolia. 2006. Available online: http://etd.uwc.ac.za/xmlui/bitstream/handle/11394/1925/Ma_MPHARM_2006.pdf?isAllowed=y&sequence=1 (accessed on 8 May 2021).
- Taylor, L. The Healing Power of Rainforest Herbs: A Guide to Understanding and Using Herbal Medicinals; Square One Publishers: Garden City Park, NY, USA, 2005; pp. 243–253. [Google Scholar]
- Lekhooa, M.R.; Walubo, A.; du Plessis, J.B.; Matsabisa, M.G. The development and use of a drug-induced immunosuppressed rat-model to screen Phela for mechanism of immune stimulation. J. Ethnopharmacol. 2017, 206, 8–18. [Google Scholar] [CrossRef]
- Das, B.; Kar, A.; Matsabisa, M.; Mukherjee, P. Anti-Cholinesterase Potential of Standardized Extract of PHELA a Traditional South African Medicine Formulation. J. Herb. Med. 2002, 22, 100348. [Google Scholar] [CrossRef]
- Lekhooa, M.; Walubo, A.; Du Plessis, J.J.; Matsabisa, M.C.; Molefe, D. Evaluation of traditional medicines I: Identification of Phela using different chromatographic techniques. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 27–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekhooa, M.; Walubo, A.; Du Plessis, J.J.; Matsabisa, M.G. Evaluation of traditional medicines II: The use of metabolite peak-kinetics to monitor PHELA in rat plasma. Afr. J. Tradit. Complement. Altern. Med. 2012, 9, 73–80. [Google Scholar]
- Husein, A.; Ali-shtayeh, M.S.; Jondi, W.; Zatar, N.; Al-Nuri, M.A. High Performance Liquid Chromatographic Method for Quantitative Determination of Emodin in Rumex Cyprius Marb, Spectrophotometric Studies. Int. J. Sci. Basic Appl. Res. 2014, 18, 217–222. [Google Scholar]
- Kar, A.; Mukherjee, P.K.; Saha, S.; Banerjee, S.; Goswami, D.; Matsabisa, M.G.; Charoensub, R.; Duangyodd, T. Metabolite profiling and evaluation of CYP450 interaction potential of ‘Trimada’—An Ayurvedic formulation. J. Ethnopharmacol. 2021, 266, 113457. [Google Scholar] [CrossRef] [PubMed]
- Bahadur, S.; Mukherjee, P.K.; Milan Ahmmed, S.K.; Kar, A.; Harwansh, R.K.; Pandit, S. Metabolism-mediated interaction potential of standardized extract of Tinospora cordifolia through rat and human liver microsomes. Indian J. Pharmacol. 2016, 48, 576–581. [Google Scholar]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, R.; Chaudhary, S.K.; Sharma, A.; Yadav, K.K.; Nema, N.K.; Sekhoacha, M.; Karmakar, S.; Braga, F.C.; Matsabisa, M.G.; Mukherjee, P.K.; et al. Anti-biofilm activity of Marula—A study with the standardized bark extract. J. Ethnopharmacol. 2014, 154, 170–175. [Google Scholar] [CrossRef]
- Pompilio, A.; Scocchi, M.; Pomponio, S.; Guida, F.; Di Primio, A.; Fiscarelli, E.; Gennaro, R.; Di Bonaventura, G. Antibacterial and anti-biofilm effects of cathelicidin peptides against pathogens isolated from cystic fibrosis patients. Peptides 2011, 32, 1807–1814. [Google Scholar] [CrossRef]
- Azeredo, J.; Azevedo, N.F.; Briandet, R.; Cerca, N.; Coenye, T.; Costa, A.R.; Desvaux, M.; Di Bonaventura, G.; Hébraud, M.; Jaglic, Z.; et al. Critical review on biofilm methods. Crit. Rev. Microbiol. 2017, 43, 313–351. [Google Scholar] [CrossRef] [Green Version]
- Wright, C.J.; Shah, M.K.; Powell, L.C.; Armstrong, I. Application of AFM from microbial cell to biofilm. Scanning 2010, 32, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.J.; Lee, N.R.; Jo, W.; Jung, W.K.; Lim, J.S. Effects of substrates on biofilm formation observed by atomic force microscopy. Ultramicroscopy 2009, 109, 874–880. [Google Scholar] [CrossRef]
- Gordon, V.D.; Davis-Fields, M.; Kovach, K.; Rodesney, C.A. Biofilms and mechanics: A review of experimental techniques and findings. J. Phys. D Appl. Phys. 2017, 50, 223002. [Google Scholar] [CrossRef]
- Chatterjee, S.; Biswas, N.; Datta, A.; Dey, R.; Maiti, P.K. Atomic force microscopy in biofilm study. Microscopy 2014, 63, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Kunti, A.K.; Sharma, S.K.; Gupta, M. A Comparative Study on Structural Growth of Copper Oxide Deposited by dc-MS and HiPIMS. ECS J. Solid State Sci. Technol. 2016, 5, 650–655. [Google Scholar] [CrossRef]
- Halabalaki, M.; Urbain, A.; Paschali, A.; Mitakou, S.; Tillequin, F.; Skaltsounis, A.-L. Quercetin and Kaempferol 3-O-[alpha-l-Rhamnopyranosyl-(1 → 2)-alpha-l-arabinopyranoside]-7-O-alpha-l-rhamnopyranosides from Anthyllis hermanniae: Structure Determination and Conformational Studies. J. Nat. Prod. 2011, 74, 1939–1945. [Google Scholar] [CrossRef]
- Calvano, C.D.; Cataldi, T.R.I.; Kögel, J.F.; Monopoli, A.; Palmisano, F.; Sundermeyer, J. Structural Characterization of Neutral Saccharides by Negative Ion MALDI Mass Spectrometry Using a Superbasic Proton Sponge as Deprotonating Matrix. J. Am. Soc. Mass Spectrom. 2017, 28, 1666–1675. [Google Scholar] [CrossRef]
- Okada, H.; Fukushi, E.; Yamamori, A.; Kawazoe, N.; Onodera, S.; Kawabata, J.; Shiomi, N. Novel fructopyranose oligosaccharides isolated from fermented beverage of plant extract. Carbohydr. Res. 2010, 345, 414–418. [Google Scholar] [CrossRef]
- Tayler, N.M.; Boya, C.A.; Herrera, L.; Moy, J.; Ng, M.; Pineda, L.; Almanza, A.; Rosero, S.; Coronado, L.M.; Correa, R.; et al. Analysis of the antiparasitic and anticancer activity of the coconut palm (Cocos nucifera L. ARECACEAE) from the natural reserve of Punta Patiño, Darién. PLoS ONE 2019, 14, e0214193. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Kiss, A.K. Determination of the phenolic profile and antioxidant properties of Salvia viridis L. Shoots: A comparison of aqueous and hydroethanolic extracts. Molecules 2018, 23, 1468. [Google Scholar] [CrossRef] [Green Version]
- Horiuchi, K.; Shiota, S.; Kuroda, T.; Hatano, T.; Yoshida, T.; Tsuchiya, T. Potentiation of antimicrobial activity of aminoglycosides by carnosol from Salvia officinalis. Biol. Pharm. Bull. 2007, 30, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Sun, X.; Kong, F.; Xia, L.; Deng, X.; Wang, D.; Wang, J. Specific NDM-1 Inhibitor of Isoliquiritin Enhances the Activity of Meropenem against NDM-1-positive Enterobacteriaceae in vitro. Int. J. Environ. Res. Public Health 2020, 17, 2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerca, N.; Martins, S.; Pier, G.B.; Oliveira, R.; Azeredo, J. The relationship between inhibition of bacterial adhesion to a solid surface by sub-MICs of antibiotics and subsequent development of a biofilm. Res. Microbiol. 2005, 156, 650–655. [Google Scholar] [CrossRef] [Green Version]
- Bavington, C.; Page, C. Stopping Bacterial Adhesion: A Novel Approach to Treating Infections. Respiration 2005, 72, 335–344. [Google Scholar] [CrossRef]
- Lieberman, D. Pseudomonal infections in patients with COPD: Epidemiology and management. Am. J. Respir. Med. Drugs Devices Interv. 2003, 2, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K. Evidence-Based Validation of Herbal Medicine; Elsevier: Amsterdam, The Netherlands, 2015; p. 537. [Google Scholar] [CrossRef]
- Man, M.; Qiang, Y.; Bin, E.; Peter, M. Benefits of Hesperidin for Cutaneous Functions. Evid.-Based Complement. Altern. Med. 2019, 2019, 2676307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pendota, S.C.; Aderogba, M.A.; Van Staden, J. In vitro antimicrobial activity of extracts and an isolated compound from Boscia albitrunca leaves. S. Afr. J. Bot. 2015, 96, 91–93. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Bi, S.; Chen, H.; Chen, T.; Yang, R.; Li, M.; Fu, Y.; Jia, A.Q. Anti-Biofilm and Antivirulence Activities of Metabolites from Plectosphaerella cucumerina against Pseudomonas aeruginosa. Front. Microbiol. 2017, 8, 769. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Gu, S.; Shi, Y.; Cui, X.; Wen, S.; Ge, J. The effect of emodin on Staphylococcus aureus strains in planktonic form and biofilm formation in vitro. Arch. Microbiol. 2017, 199, 1267–1275. [Google Scholar] [CrossRef]
- Kunti, A.K.; Sekhar, K.C.; Pereira, M.; Gomes, M.J.M.; Sharma, S.K. Oxygen partial pressure induced effects on the microstructure and the luminescence properties of pulsed laser deposited TiO2 thin films. AIP Adv. 2017, 7, 015021. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Rai, S.; Bhattacharya, S.; Wahile, A.; Saha, B.P. Marker analysis of polyherbal formulation, Triphala—A well known Indian Traditional Medicine. Indian J. Tradit. Knowl. 2008, 7, 379–383. [Google Scholar]
- Waxman, D. P450 Gene Induction by Structurally Diverse Xenochemicals: Central Role of Nuclear Receptors CAR, PXR, and PPAR. Arch. Biochem. Biophys. 1999, 369, 11–23. [Google Scholar]
- McKinnon, R.; Burgess, W.; Hall, P.; Roberts-Thomson, S.; González, J.F.; McManus, M. Characterisation of CYP3A gene subfamily expression in human gastrointestinal tissues. Gut 1995, 36, 259–267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, T.; Yamazaki, H.; Mimura, M.; Inui, Y.; Guengerich, F. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: Studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 1994, 270, 414–423. [Google Scholar]
- Pelkonen, O.; Mäenpää, J.; Taavitsainen, P.; Rautio, A.; Raunio, H. Inhibition and induction of human cytochrome P450 (CYP) enzymes. Xenobiotica 1999, 28, 1203–1253. [Google Scholar] [CrossRef]
- Guengerich, F. Cytochrome P-450 3A4: Regulation and role in drug metabolism. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 1–17. [Google Scholar] [CrossRef]
- Imaoka, S.; Yamada, T.; Hiroi, T.; Hayashi, K.; Sakaki, T.; Yabusaki, Y.; Funae, Y. Multiple forms of human P450 expressed in Saccharomyces cerevisiae. Systematic characterization and comparison with those of the rat. Biochem. Pharmacol. 1996, 51, 1041–1050. [Google Scholar] [CrossRef]
- Pelkonen, O.; Raunio, H. Metabolic Activation of Toxins: Tissue-Specific Expression and Metabolism in Target Organs. Environ. Health Perspect. 1997, 105 (Suppl. 4), 767–774. [Google Scholar] [PubMed]
- Danielson, P. The Cytochrome P450 Superfamily: Biochemistry, Evolution and Drug Metabolism in Humans. Curr. Drug Metab. 2003, 3, 561–597. [Google Scholar] [CrossRef]

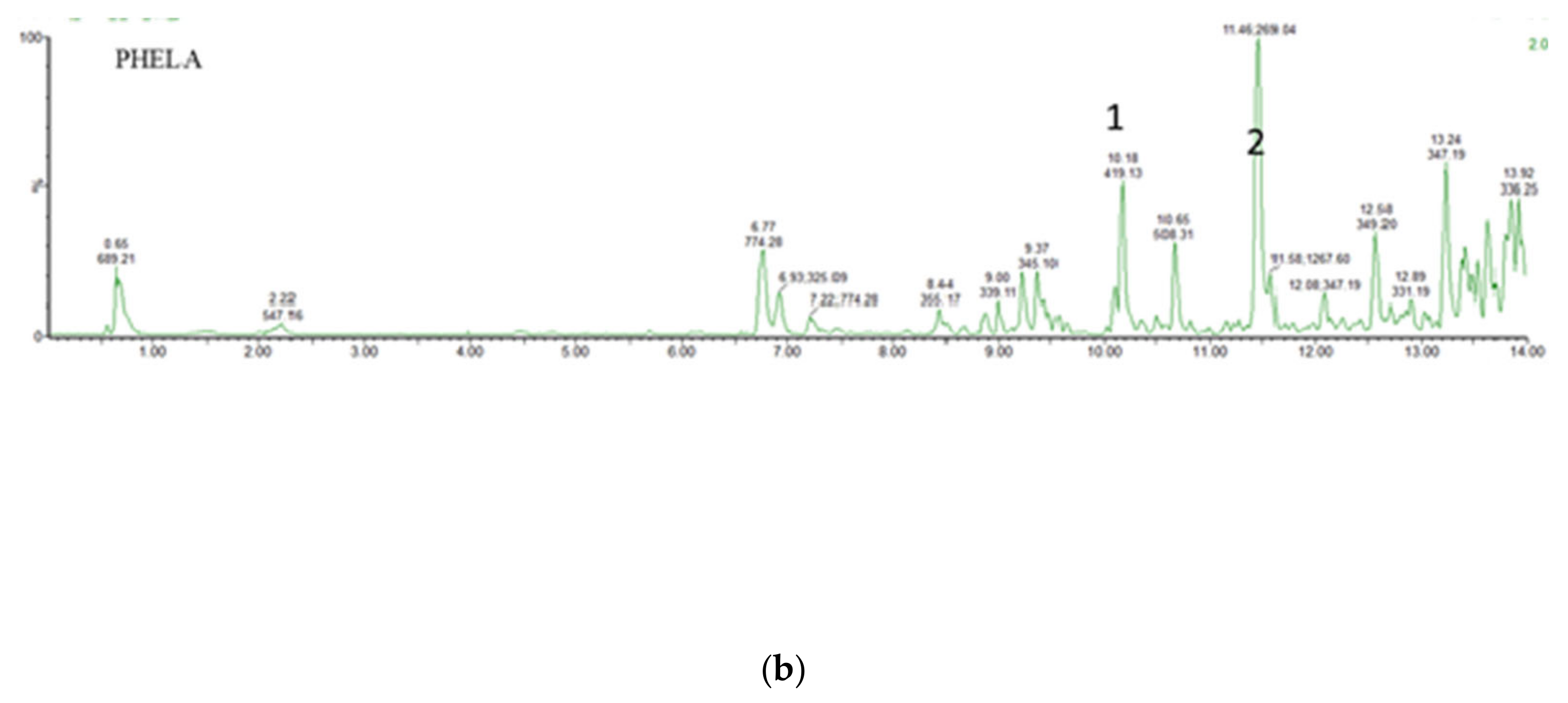


| Rt (min) | Compound Name | Chemical Formula | Acquired [M-H]− m/z Ratio | Theoretical [M-H]− m/z Ratio | Calculated Accurate Mass (Da) | Mass Error (ppm) | References |
|---|---|---|---|---|---|---|---|
| 0.6 | 6-0-α-D-glucopyranosyl-D-fructose | C12H22O11 | 341.1089 | 341.1084 | 342.1162 | 1.5 | [32] |
| 6.7 | Quercetin-3-O-alpha-l-rhamnopyranosyl(1-2)-beta-D-glucopyranoside-7-O-alpha-l-rhamnopyranoside | C34H44O19 | 755.2406 | 755.2399 | 756.2476 | 0.9 | [31,33] |
| 6.8 | Hesperidin methyl chalcone | C29H36O15 | 623.1974 | 623.1976 | 624.2054 | −0.3 | [34] |
| 7.9 | Martynoside | C31H40O15 | 651.2285 | 651.2289 | 652.2366 | −0.6 | [35] |
| 13.4 | Carnosol | C20H26O4 | 331.1906 | 331.1909 | 330.1831 | −0.9 | [36] |
| Rt(min) | Compound name | Chemical formula | Acquired [M+H]+ m/z | Theoretical [M+H]+ m/z | Calculated accurate mass (Da) | Mass error (ppm) | References |
| 10.18 | Isoliquiritin | C21H22O9 | 419.1349 | 419.1342 | 418.1263 | 1.7 | [37] |
| 11.4 | Emodin | C15H9O5 | 269.0448 | 269.0450 | 268.0371 | −0.7 | [38] |
| Name of the Plant | CYP3A4 (mg/mL) ± SEM | CYP2D6 (mg/mL) ± SEM |
|---|---|---|
| PHELA® formulation | 0.2317 ± 2.14 | 0.2817 ± 3.65 |
| Positive control | Ketoconazole | Fluoxetine |
| 0.0643 ± 1.12 | 0.0739 ± 1.69 |
| Gram-Positive Bacteria | MIC (mg/mL) | MBC (mg/mL) |
|---|---|---|
| S. aureus | 0.3125 ± 0.019 | <1.00 |
| B. substilis | 0.356 ± 0.007 | >1.00 |
| Gram-negative bacteria | ||
| P. auregenosa | 0.625 ± 0.017 | >1.25 |
| E. coli | 0.512 ± 0.011 | >1.5 |
| Statistical Parameters | Control | Standard | PHELA® Extract |
|---|---|---|---|
| Root Mean Square (Sq) | 9.165 nm | 4.344 nm | 3.523 nm |
| Reduced peak height (Spk) | 36 nm | 14.12 nm | 17.51 nm |
| Reduced valley depth (Svk) | 28.56 nm | 14.75 nm | 10.42 nm |
| Roughness Average (Sa) | 7.30 nm | 3.52 nm | 2.81 nm |
| Surface skewness (Ssk) | 0.19 | 0.14 | 0.4 |
| Coefficient of kurtosis (Ska) | 0.07 | 0.19 | 0.27 |
| Surface Area Ratio (Sdr) % | 5.98 | 1.75 | 1.90 |
| Maximum height (Sz) | 64.56 nm | 28.87 nm | 27.93 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.; Kar, A.; Bhowmik, R.; Karmakar, S.; Tripathy, S.; Matsabisa, M.G.; Mukherjee, P.K. Quality Related Safety Evaluation of a South African Traditional Formulation (PHELA®) as Novel Anti-Biofilm Candidate. Molecules 2022, 27, 1219. https://doi.org/10.3390/molecules27041219
Das B, Kar A, Bhowmik R, Karmakar S, Tripathy S, Matsabisa MG, Mukherjee PK. Quality Related Safety Evaluation of a South African Traditional Formulation (PHELA®) as Novel Anti-Biofilm Candidate. Molecules. 2022; 27(4):1219. https://doi.org/10.3390/molecules27041219
Chicago/Turabian StyleDas, Bhaskar, Amit Kar, Rudranil Bhowmik, Sanmoy Karmakar, Satyajit Tripathy, Motlalepula G. Matsabisa, and Pulok Kumar Mukherjee. 2022. "Quality Related Safety Evaluation of a South African Traditional Formulation (PHELA®) as Novel Anti-Biofilm Candidate" Molecules 27, no. 4: 1219. https://doi.org/10.3390/molecules27041219
APA StyleDas, B., Kar, A., Bhowmik, R., Karmakar, S., Tripathy, S., Matsabisa, M. G., & Mukherjee, P. K. (2022). Quality Related Safety Evaluation of a South African Traditional Formulation (PHELA®) as Novel Anti-Biofilm Candidate. Molecules, 27(4), 1219. https://doi.org/10.3390/molecules27041219






