Pharmacological Activities of Soursop (Annona muricata Lin.)
Abstract
1. Introduction
2. Botanical Description
3. Traditional Uses
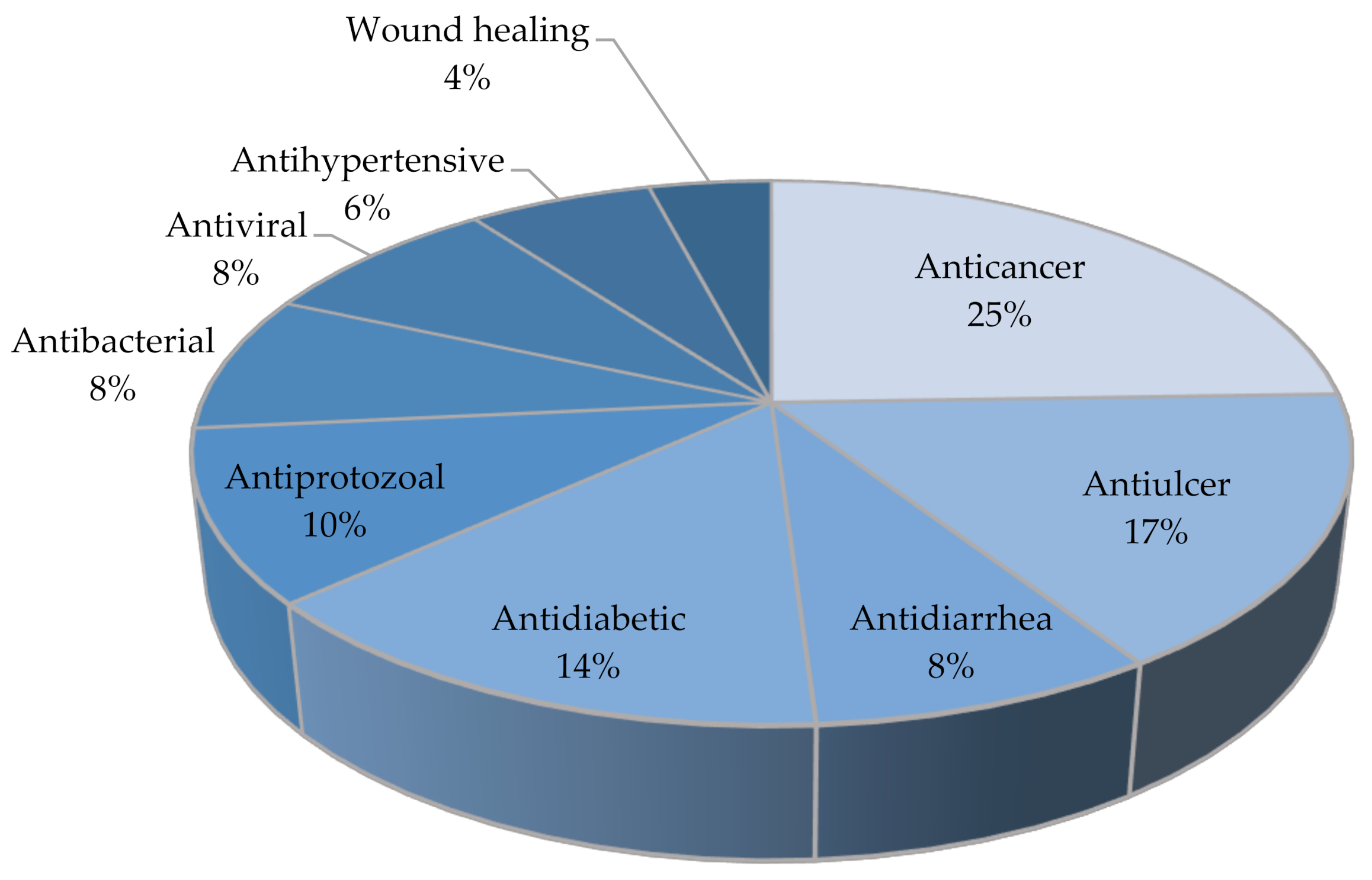
| Pharmacological Activity | Plant Parts | Mechanisms | Ref. |
|---|---|---|---|
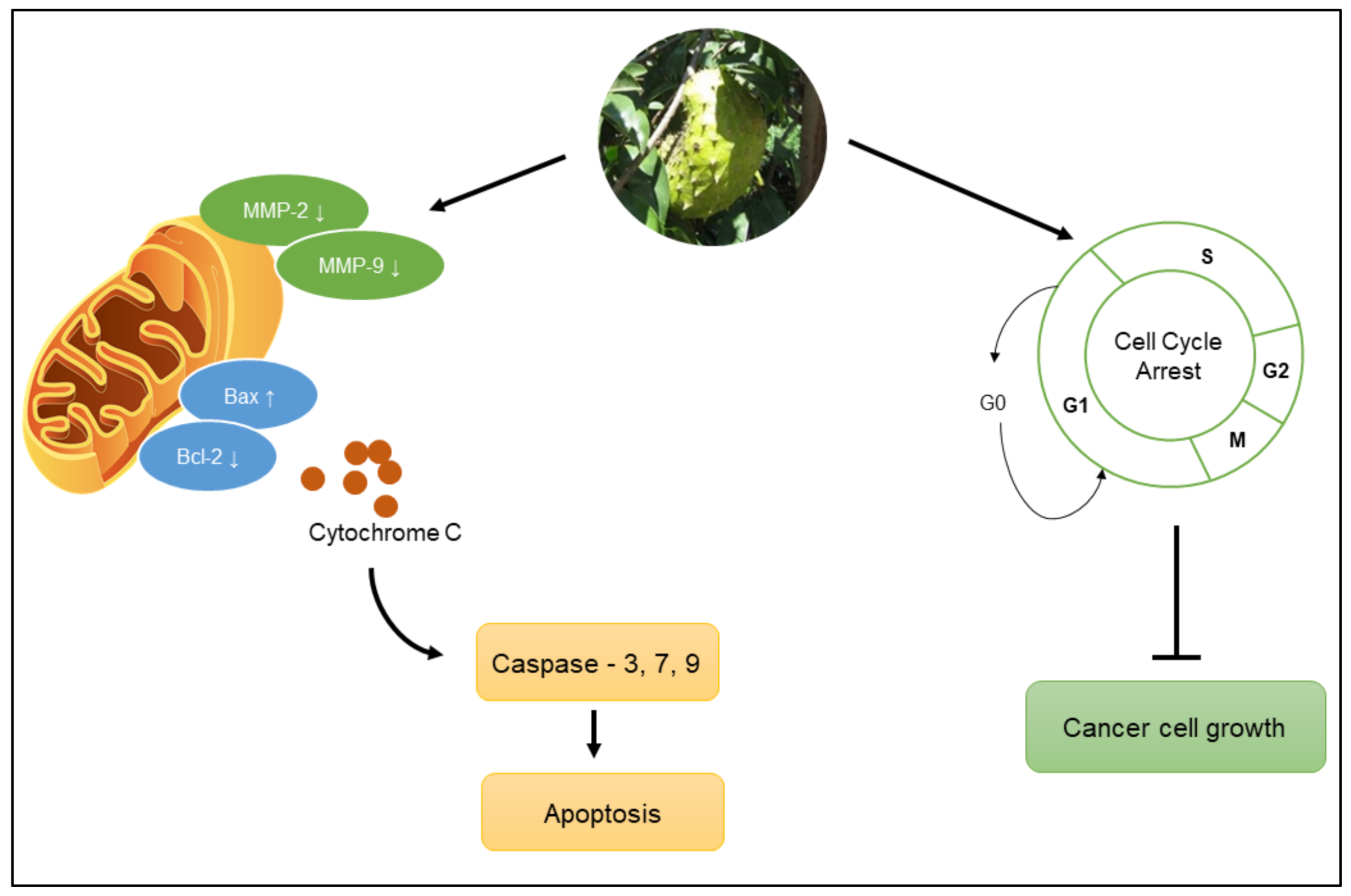
| Anticancer | Fruit, stem, seed, and twigs | Inhibits MMP-2 and MMP-9, which play an important role in cancer progression, in HT1080 fibrosarcoma cells. | [17] |
| Leaf, twigs, and root | Disrupts MMP function, reactive oxygen species (ROS) generation, and G0/G1 cell cycle arrest in HL-60 leukemia cells. | [10] | |
| Leaf | Increases Bax expression and decreases Bcl-2 expression, cell cycle arrest at G0/G1 phase in A-549 lung cancer cells. | [18] | |
| Induces apoptosis by enhancing caspase-3 expression in COLO-205 colorectal cancer cells. | [19] | ||
| Induces apoptosis by enhancing the expression of caspase-3 in MDA-MB-231 breast cancer cells. | [20] | ||
| Inhibits the proliferation of PC-3 human prostate cancer cells. | [21] | ||
| Disrupts MMP function, causes leakage of cytochrome C from mitochondria, and activates caspase-3, caspase-7, and caspase-9 expression in HT-29 colon cancer cells. | [22] | ||
| Apoptosis mechanism mediated by a decrease in Bcl-2 expression and an increase in caspase-3 and caspase-9 expression in MCF7 breast cancer cells. | [23] | ||
| Seeds | Increases caspase-3 cleavage and DNA fragmentation in endometrial cancer cells. | [24] | |
| Antiulcer | Leaf | Activates prostaglandin synthesis and supresses aggressive factors of gastric mucosa. | [25] |
| Protects against ROS scavenging and gastric wall damage.Upregulates Hsp70 and downregulates Bax expression. | [26] | ||
| Downregulates Bax and malondialdehyde (MDA) expression.Upregulates CAT, SOD, GSH, NO, PGE2, glycogen, and Hsp70 expression. | [27] | ||
| Antidiarrhea | Fruit | Inhibits intestinal motility and secretions. | [28] |
| Antiprotozoal | Leaf | Antiprotozoal activity against Toxoplasma gondii. | [29] |
| Antiprotozoal activity against Leishmania spp. and Trypanosoma cruzii. | [30] | ||
| Antiprotozoal activity against Plasmodium falciparum. | [29] | ||
| Seeds | Antiprotozoal activity against Leishmania spp. | [31] | |
| Bark and roots | Antiprotozoal activity against Plasmodium falciparum. | [32] | |
| Antidiabetic | Fruit | Inhibits α-amylase and α-glucosidase enzymes. | [33] |
| Leaf | Decreases lipid peroxidation and indirectly affects insulin production and endogenous antioxidants in streptozotocin-induced mice. | [12] | |
| Antibacterial | Leaf | Attacks the bacterial membrane. | [34] |
| Antihypertensive | Fruit and leaves | Inhibits angiotensin-I-converting enzyme and blocks calcium ion channels | [33,35] |
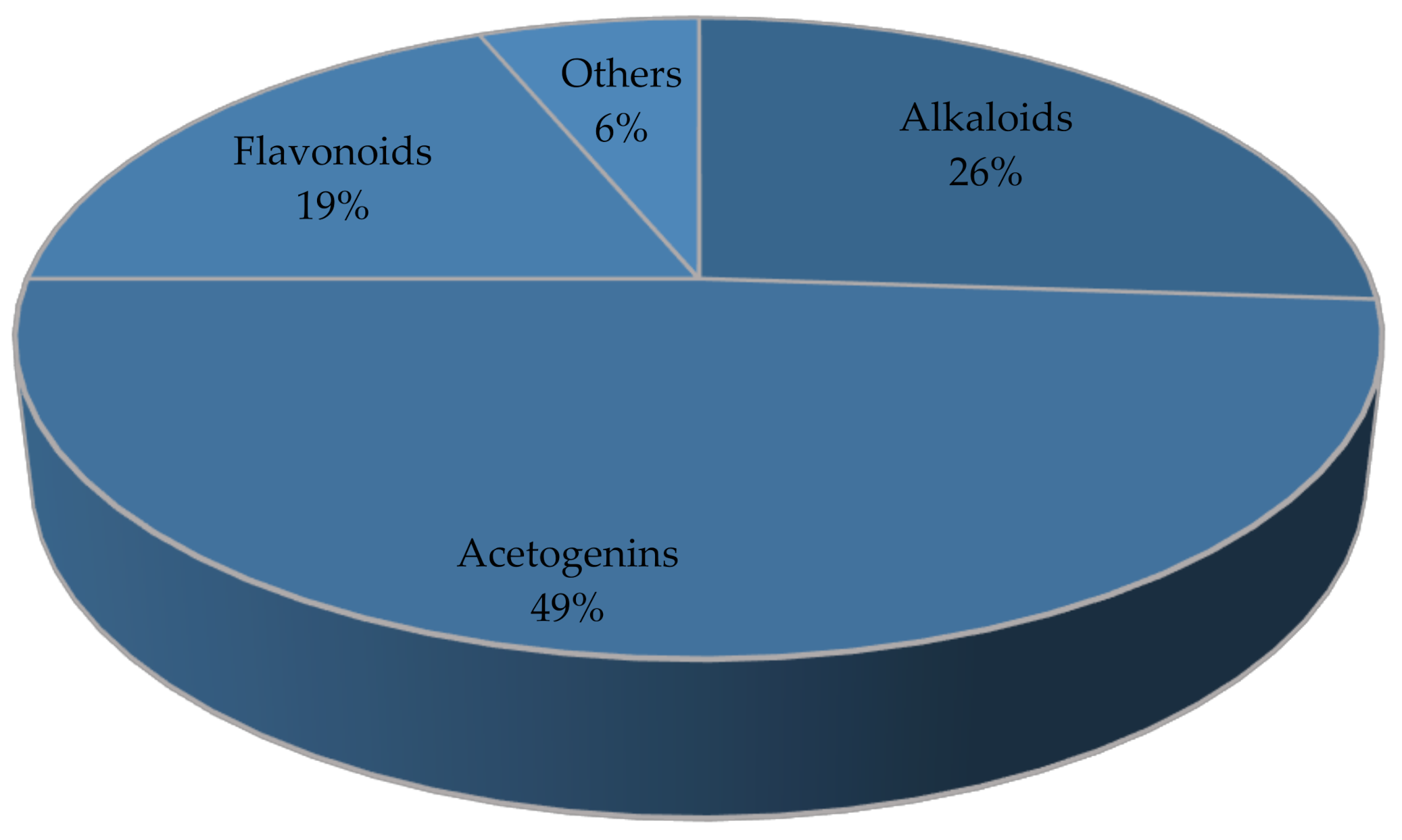
4. Phytochemical Properties
| No. | Compound | Part of Plant | Type | Refs. |
|---|---|---|---|---|
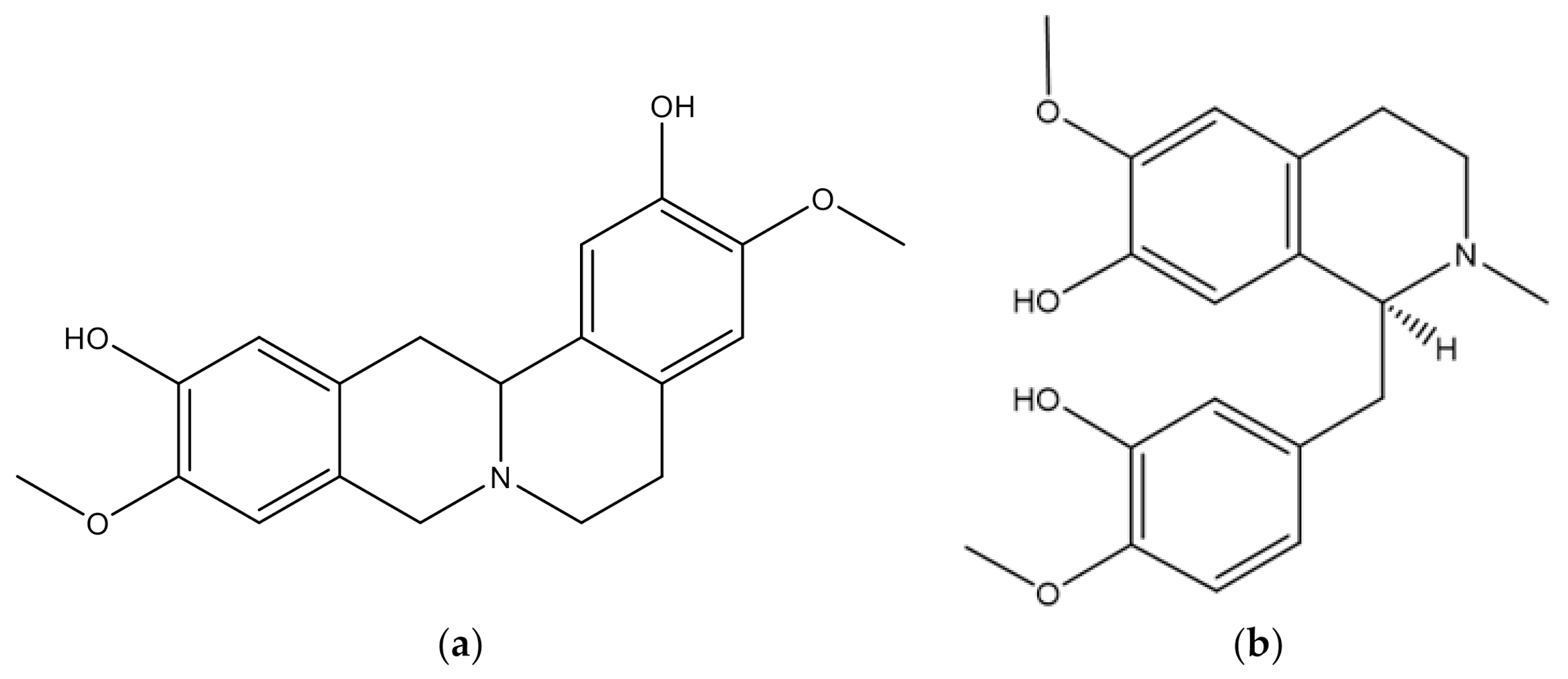
| 1 | Anomuricine | Leaf, root, stem, bark | Alkaloid | [44] |
| 2 | Anomurine | Leaf, root, stem, bark | Alkaloid | [44] |
| 3 | Annonaine | Fruit, leaf | Alkaloid | [45,46] |
| 4 | Annonamine | Leaf | Alkaloid | [47] |
| 5 | Asimilobine | Fruit | Alkaloid | [45,46,48] |
| 6 | Atherospermine | Stem | Alkaloid | [44] |
| 7 | Atherosperminine | Root, bark | Alkaloid | [44] |
| 8 | Casuarine | Leaf | Alkaloid | [40] |
| 9 | Coclaurine | Root, bark | Alkaloid | [44,45] |
| 10 | Coreximine | Leaf, root, stem, bark | Alkaloid | [44] |
| 11 | DMDP (2,5- Dihydroxymethyl-3,4, dihydroxypyrrolidine) | Leaf | Alkaloid | [40] |
| 12 | deoxymannojirimycin | Leaf | Alkaloid | [40] |
| 13 | deoxynojirmycin | Leaf | Alkaloid | [40] |
| 14 | (R)-O,O-dimethylcoclaurine | Leaf | Alkaloid | [47] |
| 15 | Isoboldine | Leaf | Alkaloid | [45] |
| 16 | Isolaureline | Leaf | Alkaloid | [48] |
| 17 | Liriodenine | Leaf | Alkaloid | [45] |
| 18 | (R)-4’O-methylcocaurine | Leaf | Alkaloid | [47] |
| 19 | N-methylcoclaurine | Leaf | Alkaloid | [45] |
| 20 | (S)-narcorydine | Leaf | Alkaloid | [47] |
| 21 | Nornuciferine | Fruit | Alkaloid | [46] |
| 22 | Remerine | Leaf | Alkaloid | [45] |
| 23 | Reticuline | Leaf, root, stem, bark | Alkaloid | [44] |
| 24 | Stepharine | Leaf | Alkaloid | [44] |
| 25 | Swainsonine | Leaf | Alkaloid | [40] |
| 26 | Xylopine | Leaf | Alkaloid | [48] |
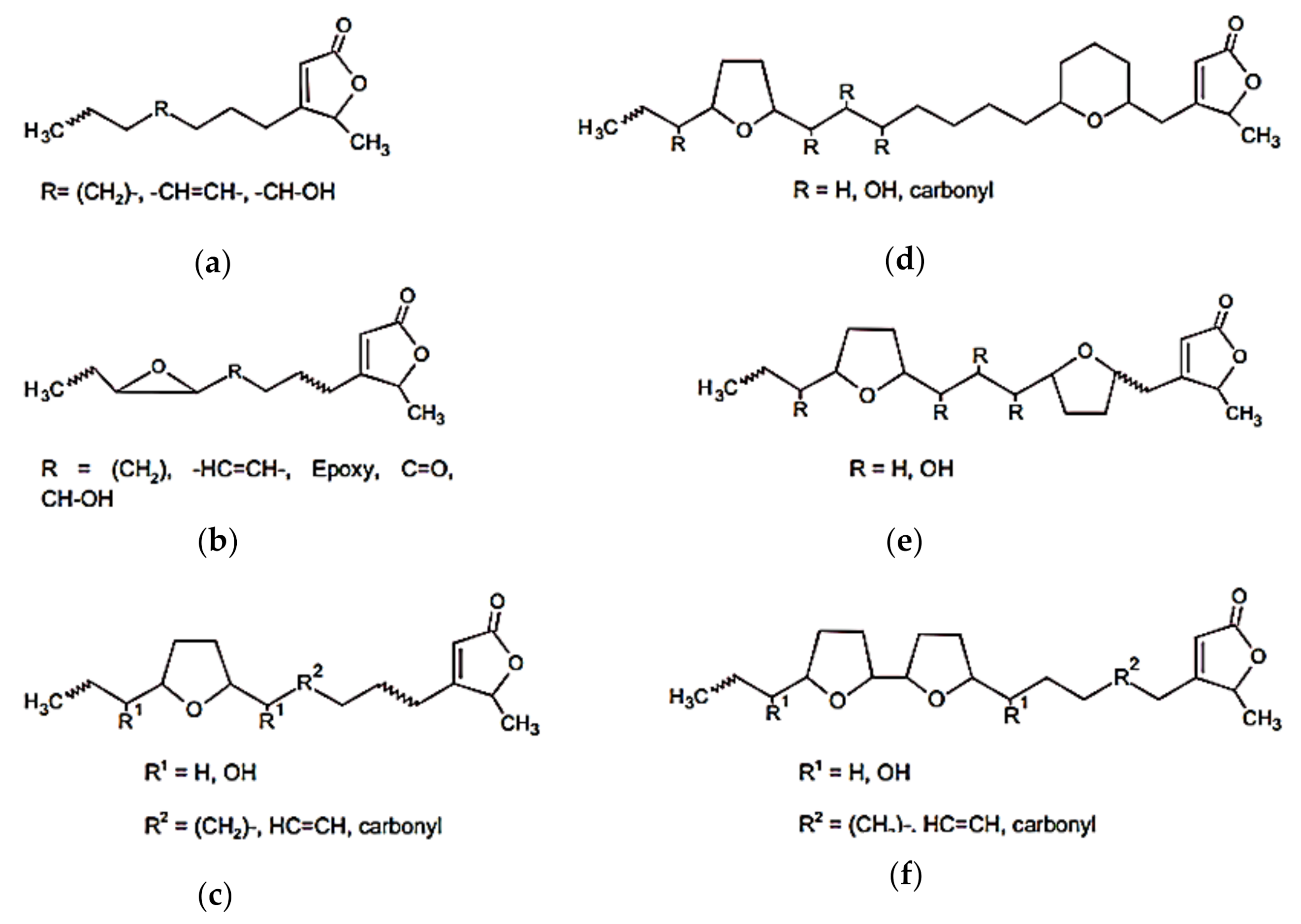
| 27 | 15-acetylguanacone | Fruit | Acetogenin | [49] |
| 28 | Annocatalin | Leaf | Acetogenin | [50] |
| 29 | Annocatacin A | Seed | Acetogenin | [51] |
| 30 | Annocatacin B | Leaf | Acetogenin | [51] |
| 31 | Annomontacin | Seed | Acetogenin | [50] |
| 32 | Annomuricin | Leaf | Acetogenin | [52] |
| 33 | Annomuricin A | Leaf | Acetogenin | [53] |
| 34 | Annomuricin B | Leaf | Acetogenin | [53] |
| 35 | Annomutacin | Leaf | Acetogenin | [54] |
| 36 | Annonacin | Leaf, seed | Acetogenin | [50,54] |
| 37 | Annonacin A | Leaf | Acetogenin | [54] |
| 38 | Annonacinone | Leaf | Acetogenin | [50] |
| 39 | Annoreticuin-9-one | Seed | Acetogenin | [55] |
| 40 | Annoreticuin, cis | Pulp | Acetogenin | [55] |
| 41 | Bullatacin | Seed | Acetogenin | [38] |
| 42 | Cohibin A | Root | Acetogenin | [56] |
| 43 | Cohibin B | Seed | Acetogenin | [56] |
| 44 | Cohibin C | Seed | Acetogenin | [57] |
| 45 | Cohibin D | Seed | Acetogenin | [57] |
| 46 | Corepoxylone | Seed | Acetogenin | [58] |
| 47 | Corossolin | Seed, leaf | Acetogenin | [59,60] |
| 48 | Corossolone | Leaf | Acetogenin | [50] |
| 49 | Epomurinins A, B | Pulp | Acetogenin | [61] |
| 50 | Epomurisenins A, B | Pulp | Acetogenin | [61] |
| 51 | Gigantecin | Seed, leaf | Acetogenin | [60] |
| 52 | 2,4 Cis or trans Gigantetrocinone | Seed | Acetogenin | [62] |
| 53 | Gigantetronenin | Leaf, seed | Acetogenin | [63] |
| 54 | Goniothalamicin | Seed, leaf | Acetogenin | [64] |
| 55 | Javoricin | Seed | Acetogenin | [64] |
| 56 | Longifolicin | Seed | Acetogenin | [59] |
| 57 | Montanacin | Leaf | Acetogenin | [60] |
| 58 | Montanacin D | Leaf, pulp | Acetogenin | [60] |
| 59 | Montanacin E | Leaf, pulp | Acetogenin | [60] |
| 60 | Montanacin H | Leaf | Acetogenin | [60] |
| 61 | Montecristin | Pulp | Acetogenin | [60] |
| 62 | Muricatenol | Seed | Acetogenin | [65] |
| 62 | Muricatetrocin A | Seed | Acetogenin | [59] |
| 63 | Muricatetrocin B | Seed | Acetogenin | [59] |
| 64 | Muricatocin A | Leaf | Acetogenin | [66] |
| 65 | Muricatocin B | Leaf | Acetogenin | [66] |
| 66 | Muricatocin C | Leaf | Acetogenin | [63] |
| 67 | Muricin | Seed | Acetogenin | [50] |
| 68 | Muricenin | Pulp | Acetogenin | [67] |
| 69 | Murisolin | Seed | Acetogenin | [50] |
| 70 | Sabadelin | Pulp | Acetogenin | [55] |
| 72 | Solamin | Leaf | Acetogenin | [50] |
| 73 | Xylomaticin | Seed | Acetogenin | [50] |
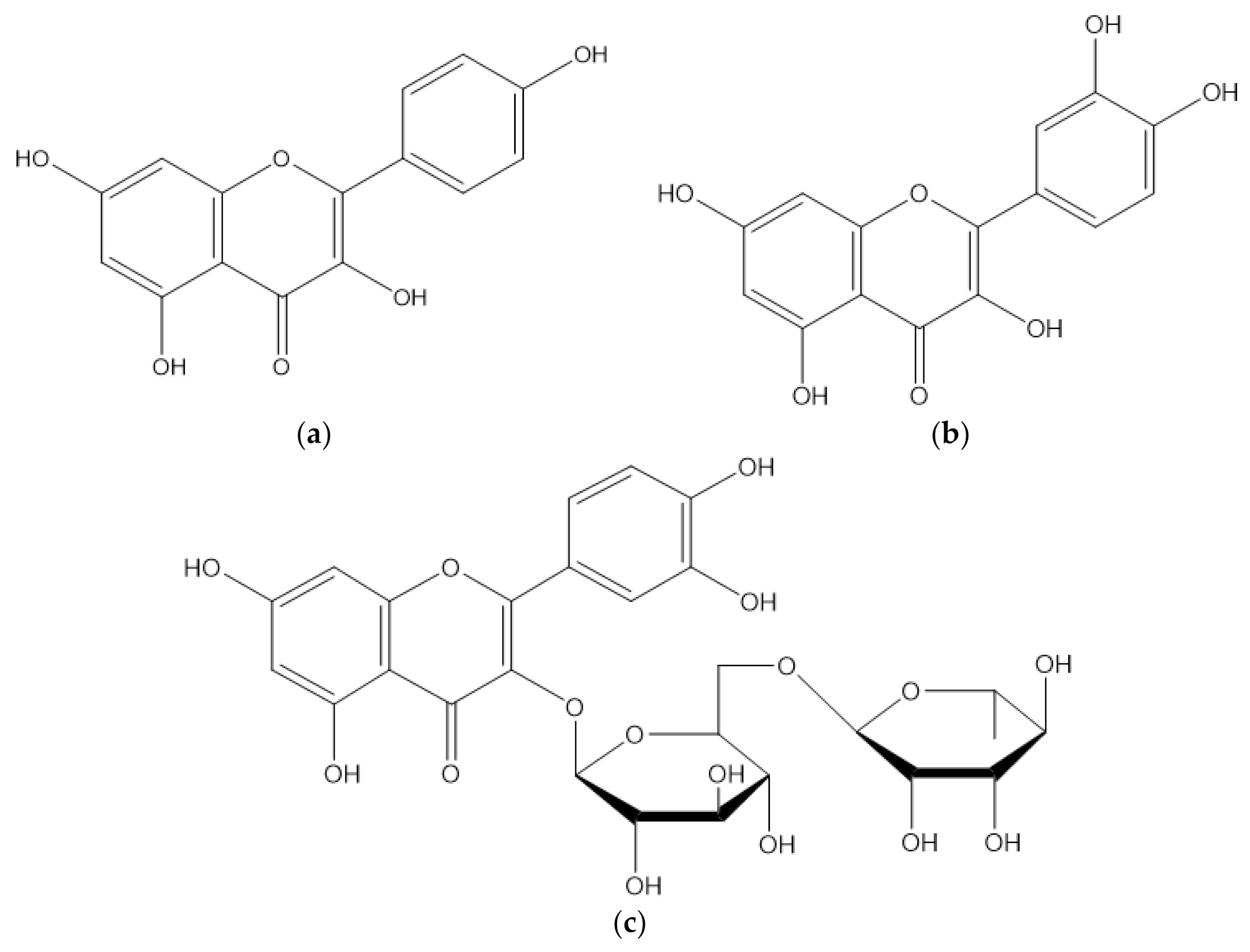
| 74 | Apigenin-6-C-glucoside | Leaf | Flavonoid | [68] |
| 75 | Argentinine | Leaf | Flavonoid | [41] |
| 76 | Catechin | Leaf | Flavonoid | [41] |
| 77 | Coumaric acid | Pulp | Flavonoid | [69] |
| 78 | Daidzein | Leaf | Flavonoid | [68] |
| 79 | Dihydrokaempferol-hexoside | Pulp | Flavonoid | [69] |
| 80 | Epicatechin | Leaf | Flavonoid | [41] |
| 81 | Gallocatechin | Leaf | Flavonoid | [68] |
| 82 | Genistein | Leaf | Flavonoid | [68] |
| 83 | Glycitein | Leaf | Flavonoid | [68] |
| 84 | Homoorientin | Leaf | Flavonoid | [68] |
| 85 | Isoferulic acid | Leaf | Flavonoid | [68] |
| 86 | Kaempferol | Leaf, | Flavonoid | [41] |
| 87 | Quercetin | Leaf | Flavonoid | [41] |
| 88 | Quercetin-3-O-glucoside | Leaf | Flavonoid | [41] |
| 89 | Robinetin | Leaf | Flavonoid | [68] |
| 90 | Tangeretin | Leaf | Flavonoid | [68] |
| 91 | Rutin | Leaf | Flavonoid | [21] |
| 92 | Gallic acid | Leaf | Tannin | [41,68] |
| 93 | Vitamin C | Pulp, leaf | Vitamin | [36] |
| 94 | Vitamin E | Leaf, seed, pulp | Vitamin | [36] |
| 95 | Annoionol A | Leaf | Megastigmane | [70] |
| 96 | Annoionol B | Leaf | Megastigmane | [70] |
| 97 | Annoionol C | Leaf | Megastigmane | [70] |
| 98 | Annoionoside | Leaf | Megastigmane | [70] |
| 99 | N-p-coumaroyl tyramine | Leaf | Amide | [54] |
| 100 | Annomuricatin A | Seed | Cyclopeptides | [71] |
| 101 | Annomuricatin B | Seed | Cyclopeptides | [72] |
| 102 | Annomuricatin C | Seed | Cyclopeptides | [3] |
5. Pharmacological Activities
5.1. Anticancer
| Plant Parts | Cancer Cell Line | IC50 | Refs. |
|---|---|---|---|
| Leaf, twig, and root | HL-60 human leukemia cell line | 6–49 µg/mL | [10] |
| Leaf | A-549 lung cancer cell line | 5.09 µg/mL | [18] |
| Leaf | MCF7 breast cancer cell line | Ethanolic extract: 5.3 µg/mL; ethyl acetate fraction: 2.86 µg/mL; n-hexane fraction: 3.08 µg/mL; water fraction: 48.31 µg/mL | [23] |
| Leaf | PC-3 human prostate cancer cell line | 63 µg/mL | [21] |
| Annomuricin E from leaves | HT-29 colon cancer cell line | 1.62 µg/mL | [22] |
| Annoniacin from seeds | ECC-1 and HEC-1 human endometrial cancer cell lines | 4.62–4.75 µg/mL | [24] |
5.2. Antiulcer
5.3. Antidiarrhea
5.4. Antidiabetic
5.5. Antiprotozoal
5.6. Antibacterial
5.7. Antiviral
5.8. Antihypertensive
5.9. Wound Healing
6. Toxicology
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- De Souza, E.B.R.; Da Silva, R.R.; Afonso, S.; Scarminio, I.S. Enhanced Extraction Yields and Mobile Phase Separations by Solvent Mixtures for the Analysis of Metabolites in Annona muricata L. Leaves. J. Sep. Sci. 2009, 32, 4176–4185. [Google Scholar] [CrossRef] [PubMed]
- Wélé, A.; Zhang, Y.; Caux, C.; Brouard, J.P.; Pousset, J.L.; Bodo, B. Annomuricatin C, a Novel Cyclohexapeptide from the Seeds of Annona muricata. Comptes Rendus Chim. 2004, 7, 981–988. [Google Scholar] [CrossRef]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A Comprehensive Review on Its Traditional Medicinal Uses, Phytochemicals, Pharmacological Activities, Mechanisms of Action and Toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef]
- Gavamukulya, Y.; Abou-Elella, F.; Wamunyokoli, F.; AEl-Shemy, H. Phytochemical Screening, Anti-Oxidant Activity and in Vitro Anticancer Potential of Ethanolic and Water Leaves Extracts of Annona muricata (Graviola). Asian Pac. J. Trop. Med. 2014, 7, S355–S363. [Google Scholar] [CrossRef]
- Pinto, A.D.Q.; Cordeiro, M.C.R.; de Andrade, S.R.M.; Ferreira, F.R.; Filgueiras, H.D.C.; Alves, R.E.; Kinpara, D.I. Annona Species; International Centre for Underutilised Crops: Southampton, UK, 2005. [Google Scholar]
- Gavamukulya, Y.; Wamunyokoli, F.; El-Shemy, H.A. Annona muricata: Is the Natural Therapy to Most Disease Conditions Including Cancer Growing in Our Backyard? A Systematic Review of Its Research History and Future Prospects. Asian Pac. J. Trop. Med. 2017, 10, 835–848. [Google Scholar] [CrossRef]
- Nugraha, A.S.; Damayanti, Y.D.; Wangchuk, P.; Keller, P.A. Anti-Infective and Anti-Cancer Properties of the Annona Species: Their Ethnomedicinal Uses, Alkaloid Diversity, and Pharmacological Activities. Molecules 2019, 24, 4419. [Google Scholar] [CrossRef]
- Badrie, N.; Schauss, A.G. Soursop (Annona muricata L.): Composition, Nutritional Value, Medicinal Uses, and Toxicology, 1st ed.; Elsevier Inc.: London, UK, 2010. [Google Scholar] [CrossRef]
- Pieme, A.A.; Kumar, G.G.; Dongmo, S.S.; Moukette, M.M.; Boyoum, F.F.; Ngogang, Y.Y.; Saxena, K.K. Antiproliferative Activity and Induction of Apoptosis by Annona muricata (Annonaceae) Extract on Human Cancer Cells. BMC Complement. Altern. Med. 2014, 14, 1–10. [Google Scholar] [CrossRef]
- Ssenyange, C.W.; Namulindwa, A.; Oyik, B.; Ssebuliba, J. Plants Used to Manage Type Ii Diabetes Mellitus in Selected Districts of Central Uganda. Afr. Health Sci. 2015, 15, 496–502. [Google Scholar] [CrossRef]
- Adewole, S.O.; Ojewole, J.A.O. Protective Effects of Annona muricata Linn. (Annonaceae) Leaf Aqueous Extract on Serum Lipid Profiles and Oxidative Stress in Hepatocytes of Streptozotocin-Treated Diabetic Rats. Afr. J. Tradit. Complement. Altern. Med. 2009, 6. [Google Scholar] [CrossRef]
- Mishra, S.; Ahmad, S.; Kumar, N.; Sharma, B.K. Annona muricata (the Cancer Killer): A Review. Glob. J. Pharm. Res. 2013, 2, 1613–1618. [Google Scholar]
- De Sousa, O.V.; Vieira, G.D.V.; de Pinho, J.D.J.R.; Yamamoto, C.H.; Alves, M.S. Antinociceptive and Anti-Inflammatory Activities of the Ethanol Extract of Annona muricata L. Leaves in Animal Models. Int. J. Mol. Sci. 2010, 11, 2067–2078. [Google Scholar] [CrossRef]
- Shaw, D.; Graeme, L.; Pierre, D.; Elizabeth, W.; Kelvin, C. Pharmacovigilance of Herbal Medicine. J. Ethnopharmacol. 2012, 140, 513–518. [Google Scholar] [CrossRef]
- Hajdu, Z.; Hohmann, J. An Ethnopharmacological Survey of the Traditional Medicine Utilized in the Community of Porvenir, Bajo Paraguá Indian Reservation, Bolivia. J. Ethnopharmacol. 2012, 139, 838–857. [Google Scholar] [CrossRef]
- Drishya, G.; Nambiar, J.; Shaji, S.K.; Vanuopadath, M.; Achuthan, A.; Kumar, A.; Alias, A.; Sherif, A.; Joseph, C.; Divya, P.; et al. RECK and TIMP-2 Mediate Inhibition of MMP-2 and MMP-9 by Annona muricata. J. Biosci. 2020, 45, 1. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Kadir, H.A.; Paydar, M.; Rouhollahi, E.; Karimian, H. Annona muricata Leaves Induced Apoptosis in A549 Cells through Mitochondrial-Mediated Pathway and Involvement of NF-κB. BMC Complement. Altern. Med. 2014, 14, 1–13. [Google Scholar] [CrossRef]
- Abdullah, M.; Syam, A.F.; Meilany, S.; Laksono, B.; Prabu, O.G.; Bekti, H.S.; Indrawati, L.; Makmun, D. The Value of Caspase-3 after the Application of Annona muricata Leaf Extract in COLO-205 Colorectal Cancer Cell Line. Gastroenterol. Res. Pract. 2017, 2017, 165. [Google Scholar] [CrossRef]
- Kim, J.Y.; Dao, T.T.P.; Song, K.; Park, S.B.; Jang, H.; Park, M.K.; Gan, S.U.; Kim, Y.S. Annona muricata Leaf Extract Triggered Intrinsic Apoptotic Pathway to Attenuate Cancerous Features of Triple Negative Breast Cancer MDA-MB-231 Cells. Evid.-Based Complement. Altern. Med. 2018, 2018, 916. [Google Scholar] [CrossRef]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic Interactions among Flavonoids and Acetogenins in Graviola (Annona muricata) Leaves Confer Protection against Prostate Cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef]
- Indrawati, L.; Ascobat, P.; Bela, B.; Abdullah, M.; Surono, I.S. The Effect of an Annona muricata Leaf Extract on Nutritional Status and Cytotoxicity in Colorectal Cancer: A Randomized Controlled Trial. Asia Pac. J. Clin. Nutr. 2017, 26, 606–612. [Google Scholar] [CrossRef]
- Hadisaputri, Y.E.; Habibah, U.; Abdullah, F.F.; Halimah, E.; Mutakin, M.; Abdulah, R. Antiproliferation Activity and Apoptotic Mechanism of Soursop (Annona muricata L.) Leaves Extract and Fractions on MCF7 Breast Cancer Cells. Breast Cancer Targets Ther. 2021, 13, 447–457. [Google Scholar] [CrossRef]
- Yap, C.; Subramaniam, K.; Khor, S.; Chung, I. Annonacin Exerts Antitumor Activity through Induction of Apoptosis and Extracellular Signal-Regulated Kinase Inhibition. Pharmacogn. Res. 2017, 9, 378. [Google Scholar] [CrossRef]
- Bento, E.B.; de Brito Júnior, F.E.; de Oliveira, D.R.; Fernandes, C.N.; de Araújo Delmondes, G.; Cesário, F.R.A.S.; de Sousa Rodrigues, C.K.; dos Santos Sales, V.; de Figueiredo, F.R.S.D.N.; Lemos, I.C.S.; et al. Antiulcerogenic Activity of the Hydroalcoholic Extract of Leaves of Annona muricata Linnaeus in Mice. Saudi J. Biol. Sci. 2016, 25, 609–621. [Google Scholar] [CrossRef]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Karimian, H.; Fadaeinasab, M.; Abdulla, M.A.; Kadir, H.A. Gastroprotective Activity of Annona muricata Leaves against Ethanol-Induced Gastric Injury in Rats via Hsp70/Bax Involvement. Drug Des. Devel. Ther. 2014, 8, 2099–2111. [Google Scholar] [CrossRef]
- Harsha, C.; Banik, K.; Bordoloi, D.; Kunnumakkara, A.B. Antiulcer Properties of Fruits and Vegetables: A Mechanism Based Perspective. Food Chem. Toxicol. 2017, 108, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Afroz, N.; Ahsanul Hoq, M.; Jahan, S.; Mainul Islam, M.; Ahmed, F.; Shahid-Ud-Daula, A.F.M.; Hasanuzzaman, M. Methanol Soluble Fraction of Fruits of Annona muricata Possesses Significant Antidiarrheal Activities. Heliyon 2020, 6, e03112. [Google Scholar] [CrossRef]
- Leesombun, A.; Boonmasawai, S.; Nishikawa, Y. Ethanol Extracts from Thai Plants Have Anti-Plasmodium and Anti-Toxoplasma Activities In Vitro. Acta Parasitol. 2019, 64, 257–261. [Google Scholar] [CrossRef]
- Osorio, E.; Arango, G.J.; Jiménez, N.; Alzate, F.; Ruiz, G.; Gutiérrez, D.; Paco, M.A.; Giménez, A.; Robledo, S. Antiprotozoal and Cytotoxic Activities in Vitro of Colombian Annonaceae. J. Ethnopharmacol. 2007, 111, 630–635. [Google Scholar] [CrossRef]
- Vila-Nova, N.S.; de Morais, S.M.; Falcão, M.J.C.; Machado, L.K.A.; Beviláqua, C.M.L.; Costa, I.R.S.; Brasil, N.V.G.P.D.S.; de Andrade Júnior, H.F. Leishmanicidal Activity and Cytotoxicity of Compounds from Two Annonacea Species Cultivated in Northeastern Brazil. Rev. Soc. Bras. Med. Trop. 2011, 44. [Google Scholar] [CrossRef]
- Yamthe, L.; Fokou, P.; Mbouna, C.; Keumoe, R.; Ndjakou, B.; Djouonzo, P.; Mfopa, A.; Legac, J.; Tsabang, N.; Gut, J.; et al. Extracts from Annona muricata L. and Annona reticulata L. (Annonaceae) Potently and Selectively Inhibit Plasmodium Falciparum. Medicines 2015, 2, 55–66. [Google Scholar] [CrossRef]
- Adefegha, S.A.; Oyeleye, S.I.; Oboh, G. Distribution of Phenolic Contents, Antidiabetic Potentials, Antihypertensive Properties, and Antioxidative Effects of Soursop (Annona muricata L.) Fruit Parts in Vitro. Biochem. Res. Int. 2015, 2015, 347673. [Google Scholar] [CrossRef] [PubMed]
- De Pinto, N.C.C.; Campos, L.M.; Evangelista, A.C.S.; Lemos, A.S.O.; Silva, T.P.; Melo, R.C.N.; de Lourenço, C.C.; Salvador, M.J.; Apolônio, A.C.M.; Scio, E.; et al. Antimicrobial Annona muricata L. (Soursop) Extract Targets the Cell Membranes of Gram-Positive and Gram-Negative Bacteria. Ind. Crops Prod. 2017, 107, 332–340. [Google Scholar] [CrossRef]
- Nwokocha, C.R.; Owu, D.U.; Gordon, A.; Thaxter, K.; Mccalla, G.; Ozolua, R.I.; Young, L. Possible Mechanisms of Action of the Hypotensive Effect of Annona muricata (Soursop) in Normotensive SpragueDawley Rats. Pharm. Biol. 2012, 50, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Vijayameena, C.; Subhashini, G.; Loganayagi, M.; Ramesh, B. Phytochemical Screening and Assessment of Antibacterial Activity for the Bioactive Compounds in Annona muricata. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 1–3. [Google Scholar]
- Gyamfi, K.; Sarfo, D.K.; Nyarko, B.J.B.; Akaho, E.K.H. Assessment of Elemental Content in the Fruit of Graviola Plant, Annona muricata, from Some Selected Communities in Ghana by Instrumental Neutron Activation Analysis. Elixir Food Sci. 2011, 41, 5676–5680. [Google Scholar]
- Landolt, J.L.; Ahammadsahib, K.I.; Hollingworth, R.M.; Barr, R.; Crane, F.L.; Buerckv, N.L.; McCabe, G.P.; McLaughlin, J.L. Determination of Structure-Activity Relationships of Annonaceous Acetogenins by Inhibition of Oxygen Uptake in Rat Liver Mitochondria. Chem. Biol. Interact. 1995, 98, 1–13. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Zhou, N.; Zhu, W.; Dou, Q.P.; Zhou, K. Isolation of Three New Annonaceous Acetogenins from Graviola Fruit (Annona muricata) and Their Anti-Proliferation on Human Prostate Cancer Cell PC-3. Bioorganic Med. Chem. Lett. 2016, 26, 4382–4385. [Google Scholar] [CrossRef]
- Mohanty, S.; Hollinsheadb, J.; Jonesb, L.; Jonesb, P.W.; Thomasb, D.; Watsonb, A.A.; Watsona, D.G.; Graya, A.I.; Molyneuxc, R.J.; Nashb, R.J. Annona muricata (Graviola): Toxic or Therapeutic. Nat. Prod. Commun. 2008, 3, 31–33. [Google Scholar] [CrossRef]
- Nawwar, M.; Ayoub, N.; Hussein, S.; Hashim, A.; El-Sharawy, R.; Wende, K.; Harms, M.; Lindequist, U. A Flavonol Triglycoside and Investigation of the Antioxidant and Cell Stimulating Activities of Annona muricata Linn. Arch. Pharm. Res. 2012, 35, 761–765. [Google Scholar] [CrossRef]
- Oliveira Souza, D.; dos Santos Sales, V.; Kelly de Souza Rodrigues, C.; Rolim de Oliveira, L.; Cristina Santiago Lemos, I.; de Araújo Delmondes, G.; Brito Monteiro, Á.; Petícia do Nascimento, E.; Rodolpho Sobreira Dantas Nóbrega de Figuêiredo, F.; Galberto Martins da Costa, J.; et al. Phytochemical Analysis and Central Effects of Annona muricata Linnaeus: Possible Involvement of the Gabaergic and Monoaminergic Systems. Iran. J. Pharm. Res. 2018, 17, 1306–1317. [Google Scholar]
- Cijo George, V.; Naveen Kumar, D.R.; Rajkumar, V.; Suresh, P.K.; Ashok Kumar, R. Quantitative Assessment of the Relative Antineoplastic Potential of the N-Butanolic Leaf Extract of Annona muricata Linn. in Normal and Immortalized Human Cell Lines. Asian Pacific J. Cancer Prev. 2012, 13, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, M.; Legueut, C.; Cavé, A.; Desconclois, J.F.; Forgacs, P.; Jacquemin, H. Alkaloids of Annonaceae. XXIX. Alkaloids of Annona muricata. Planta Med. 1981, 42, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Fofana, S.; Keita, A.; Balde, S.; Ziyaev, R.; Aripova, S.F. Alkaloids from Leaves of Annona muricata. Chem. Nat. Compd. 2012, 48, 714. [Google Scholar] [CrossRef]
- Hasrat, J.A.; De Bruyne, T.; De Backer, J.P.; Vauquelin, G.; Vlietinck, A.J. Isoquinoline Derivatives Isolated from the Fruit of Annona muricata as 5-HTergic 5-HT(1A) Receptor Agonists in Rats: Unexploited Antidepressive (Lad) Products. J. Pharm. Pharmacol. 1997, 49, 1145–1149. [Google Scholar] [CrossRef]
- Matsushige, A.; Kotake, Y.; Matsunami, K.; Otsuka, H.; Ohta, S.; Takeda, Y. Annonamine, a New Aporphine Alkaloid from the Leaves of Annona muricata. Chem. Pharm. Bull. 2012, 60, 257–259. [Google Scholar] [CrossRef]
- Fofana, S.; Ziyaev, R.; Abdusamatov, A.; Zakirov, S.K. Alkaloids from Annona muricata Leaves. Chem. Nat. Compd. 2011, 47, 321. [Google Scholar] [CrossRef]
- Agu, K.C.; Okolie, N.P.; Falodun, A.; Erharuyi, O.; Igbe, I.; Elekofehinti, O.O.; Edosa, R.O.; Oghagbon, S.E. Isolation and Elucidation of 15-Acetylguanacone from Soursop (Annona muricata Linn) Fruit and Molecular Docking Experiments. J. Appl. Sci. Environ. Manag. 2017, 21, 236–243. [Google Scholar] [CrossRef]
- Liaw, C.C.; Chang, F.R.; Lin, C.Y.; Chou, C.J.; Chiu, H.F.; Wu, M.J.; Wu, Y.C. New Cytotoxic Monotetrahydrofuran Annonaceous Acetogenins from Annona muricata. J. Nat. Prod. 2002, 65, 470–475. [Google Scholar] [CrossRef]
- Chang, F.R.; Liaw, C.C.; Lin, C.Y.; Chou, C.J.; Chiu, H.F.; Wu, Y.C. New Adjacent Bis-Tetrahydrofuran Annonaceous Acetogenins from Annona muricata. Planta Med. 2003, 69, 24–246. [Google Scholar] [CrossRef]
- Kim, G.S.; Zeng, L.; Alali, F.; Rogers, L.L.; Wu, F.E.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Mono-Tetrahydrofuran Ring Acetogenins, Annomuricin E and Muricapentocin, from the Leaves of Annona muricata. J. Nat. Prod. 1998, 61, 432–436. [Google Scholar] [CrossRef]
- Wu, F.E.; Gu, Z.M.; Zeng, L.; Zhao, G.X.; Zhang, Y.; McLaughlin, J.L.; Sastrodihardjo, S. Two New Cytotoxic Monotetrahydrofuran Annonaceous Acetogenins, Annomuricins A and B, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.E.; Zhao, G.X.; Zeng, L.; Zhang, Y.; Schwedler, J.T.; McLaughlin, J.L.; Sastrodihardjo, S. Additional Bioactive Acetogenins, Annomutacin and (2,4-Trans and Cis-10r-Annonacin-a-Ones, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 1430–1437. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Soriano, G.; Torres, O.B.; Don, M.J.; Shen, C.C. Acetogenins from Annona muricata. Pharmacogn. J. 2012, 4, 32–37. [Google Scholar] [CrossRef]
- Gleye, C.; Laurens, A.; Hocquemiller, R.; Laprévote, O.; Serani, L.; Cavé, A. Cohibins A and B, Acetogenins from Roots of Annona muricata. Phytochemistry 1997, 44, 1541–1545. [Google Scholar] [CrossRef]
- Gleye, C.; Raynaud, S.; Fourneau, C.; Laurens, A.; Laprévote, O.; Serani, L.; Fournet, A.; Hocquemiller, R. Cohibins C and D, Two Important Metabolites in the Biogenesis of Acetogenins from Annona muricata and Annona Nutans. J. Nat. Prod. 2000, 63, 1192–1196. [Google Scholar] [CrossRef]
- Gromek, D.; Hocquemiller, R.; Cavé, A. Qualitative and Quantitative Evaluation of Annonaceous Acetogenins by High Performance Liquid Chromatography. Phytochem. Anal. 1994, 5, 133–140. [Google Scholar] [CrossRef]
- Chang, F.R.; Wu, Y.C. Novel Cytotoxic Annonaceous Acetogenins from Annona muricata. J. Nat. Prod. 2001, 64, 925–931. [Google Scholar] [CrossRef]
- Champy, P.; Guérineau, V.; Laprévote, O. MALDI-TOF MS Profiling of Annonaceous Acetogenins in Annona muricata Products for Human Consumption. Molecules 2009, 14, 5235–5246. [Google Scholar] [CrossRef] [PubMed]
- Melot, A.; Fall, D.; Gleye, C.; Champy, P. Apolar Annonaceous Acetogenins from the Fruit Pulp of Annona muricata. Molecules 2009, 14, 4387–4395. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Yu, J.G.; Zhu, J.X.; Yu, D.L.; Luo, X.Z.; Sun, L.; Yang, S.L. Annonaceous Acetogenins of the Seeds from Annona muricata. J. Asian Nat. Prod. Res. 2001, 3, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.E.; Zeng, L.; Gu, Z.M.; Zhao, G.X.; Zhang, Y.; Schwedler, J.T.; McLaughlin, J.L.; Sastrodihardjo, S. New Bioactive Monotetrahydrofuran Annonaceous Acetogenins, Annomuricin C and Muricatocin C, from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 909–915. [Google Scholar] [CrossRef]
- Rieser, M.J.; Gu, Z.M.; Fang, X.P.; Zeng, L.; Wood, K.V.; McLaughlin, J.L. Five Novel Mono-Tetrahydrofuran Ring Acetogenins from the Seeds of Annona muricata. J. Nat. Prod. 1996, 59, 100–108. [Google Scholar] [CrossRef]
- Li, D.Y.; Yu, J.G.; Luo, X.Z.; Sun, L.; Yang, S.L. Muricatenol, a Linear Acetogenin from Annona muricata (Annonaceae). Chinese Chem. Lett. 2000, 11, 239–242. [Google Scholar]
- Wu, F.E.; Zeng, L.; Gu, Z.M.; Zhao, G.X.; Zhang, Y.; Schwedler, J.T.; McLaughlin, J.L.; Sastrodihardjo, S. Muricatocins A and B, Two New Bioactive Monotetrahydrofuran Annonaceous Acetogenins from the Leaves of Annona muricata. J. Nat. Prod. 1995, 58, 902–908. [Google Scholar] [CrossRef]
- Sun, S.; Liu, J.; Kadouh, H.; Sun, X.; Zhou, K. Three New Anti-Proliferative Annonaceous Acetogenins with Mono-Tetrahydrofuran Ring from Graviola Fruit (Annona muricata). Bioorganic Med. Chem. Lett. 2014, 58, 902–908. [Google Scholar] [CrossRef]
- George, V.C.; Kumar, D.R.N.; Suresh, P.K.; Kumar, R.A. Antioxidant, DNA Protective Efficacy and HPLC Analysis of Annona muricata (Soursop) Extracts. J. Food Sci. Technol. 2015, 52, 2328–2335. [Google Scholar] [CrossRef]
- Jiménez, V.M.; Gruschwitz, M.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Identification of Phenolic Compounds in Soursop (Annona muricata) Pulp by High-Performance Liquid Chromatography with Diode Array and Electrospray Ionization Mass Spectrometric Detection. Food Res. Int. 2014, 65, 42–46. [Google Scholar] [CrossRef]
- Matsushige, A.; Matsunami, K.; Kotake, Y.; Otsuka, H.; Ohta, S. Three New Megastigmanes from the Leaves of Annona muricata. J. Nat. Med. 2012, 66, 284–291. [Google Scholar] [CrossRef]
- Li, C.M.; Tan, N.H.; Lu, Y.P.; Liang, H.L.; Mu, Q.; Zheng, H.L.; Hao, X.J.; Zhou, J. Annomuricatin A, a New Cyclopeptide from the Seeds of Annona muricata. Acta Bot. Yunnanica 1995, 17, 459–462. [Google Scholar]
- Li, C.M.; Tan, N.H.; Zheng, H.L.; Mu, Q.; Hao, X.J.; He, Y.N.; Zou, J. Cyclopeptide from the Seeds of Annona muricata. Phytochemistry 1998, 48, 555–556. [Google Scholar] [CrossRef]
- Son, Y.R.; Choi, E.H.; Kim, G.T.; Park, T.S.; Shim, S.M. Bioefficacy of Graviola Leaf Extracts in Scavenging Free Radicals and Upregulating Antioxidant Genes. Food Funct. 2016, 7, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Hansra, D.M.; Silva, O.; Mehta, A.; Ahn, E. Patient with Metastatic Breast Cancer Achieves Stable Disease for 5 Years on Graviola and Xeloda after Progressing on Multiple Lines of Therapy. Adv. Breast Cancer Res. 2014, 3, 47526. [Google Scholar] [CrossRef]
- Parmar, N.S.; Parmar, S. Anti-Ulcer Potential of Flavonoids. Ind. J. Physiol. Pharmacol. 1998, 42, 343–351. [Google Scholar]
- Quilez, A.M.; Montserrat-de la Paz, S.; De la Puerta, R.; Fernández-Arche, M.A.; García-Giménez, M.D. Validation of Ethnopharmacological Use as Anti-Inflammatory of a Decoction from Annona muricata Leaves. African J. Tradit. Complement. Altern. Med. 2015, 12, 14–20. [Google Scholar] [CrossRef][Green Version]
- Ngnameko, C.R.; Njayou, F.N.; Fowora, M.; Nemg, F.B.S.; Moundipa Fewou, P.; Smith, S.I. Inhibitory Effect of Medicinal Plants from Cameroon on the Growth and Adhesion of Helicobacter Pylori. Eur. J. Integr. Med. 2019, 30, 100957. [Google Scholar] [CrossRef]
- Laksmitawati, D.R.; Prasanti, A.P.; Larasinta, N.; Syauta, G.A.; Hilda, R.; Ramadaniati, H.U.; Widyastuti, A.; Karami, N.; Afni, M.; Rihibiha, D.D.; et al. Anti-Inflammatory Potential of Gandarusa (Gendarussa Vulgaris Nees) and Soursoup (Annona muricata L.) Extracts in LPS Stimulated-Macrophage Cell (RAW264.7). J. Nat. Remedies 2016, 16, 73–81. [Google Scholar] [CrossRef]
- Ezekwesili, C.; Obiora, K.; Ugwu, O. Evaluation of Anti-Diarrhoeal Property of Crude Aqueous Extract of Ocimum gratissimum L. (Labiatae) In Rats. Biokemistri 2005, 16, 122–131. [Google Scholar] [CrossRef]
- Mabeku Laure B., K.; Kouam, J.; Beng, V.P.; Ngadjui, B.T.; Fomum, Z.T.; Etoa, F.X. Evaluation of Antimicrobial Activity of the Stem Bark of Cylicodiscus Gabunensis (Mimosaceae). Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 87–93. [Google Scholar] [CrossRef]
- Ouachinou, J.M.A.S.; Dassou, G.H.; Idohou, R.; Adomou, A.C.; Yédomonhan, H. National Inventory and Usage of Plant-Based Medicine to Treat Gastrointestinal Disorders with Cattle in Benin (West Africa). S. Afr. J. Bot. 2019, 122, 432–446. [Google Scholar] [CrossRef]
- Ngoua-Meye-Misso, R.L.; Sima-Obiang, C.; Ndong, J.D.L.C.; Ndong-Atome, G.R.; Ondo, J.P.; Ovono Abessolo, F.; Obame-Engonga, L.C. Medicinal Plants Used in Management of Cancer and Other Related Diseases in Woleu-Ntem Province, Gabon. Eur. J. Integr. Med. 2019, 29, 100924. [Google Scholar] [CrossRef]
- Hardoko, Y.H.; Halim, Y.; Wijoyo, S.V. In Vitro Antidiabetic Activity of “Green Tea” Soursop Leaves Brew through α-Glucosidase Inhibition. Int. J. PharmTech Res. 2015, 8, 30–37. [Google Scholar]
- Agu, K.C.; Eluehike, N.; Ofeimun, R.O.; Abile, D.; Ideho, G.; Ogedengbe, M.O.; Onose, P.O.; Elekofehinti, O.O. Possible Anti-Diabetic Potentials of Annona muricata (Soursop): Inhibition of α-Amylase and α-Glucosidase Activities. Clin. Phytosci. 2019, 5, 1–13. [Google Scholar] [CrossRef]
- Pinto, L.C.; Cerqueira-Lima, A.T.; Suzarth, S.D.S.; de Souza, R.; Tosta, B.R.; da Silva, H.B.; Pires, A.D.O.; Queiroz, G.D.A.; Teixeira, T.O.; Dourado, K.M.C.; et al. Anonna muricata L. (Soursop) Seed Oil Improves Type 1 Diabetes Parameters in Vivo and in Vitro. PharmaNutrition 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Adeyemi, D.O.; Komolafe, O.A.; Adewole, O.S.; Martins, E.M.; Kehinde, A.T. Anti Hyperglycemic Activities of Annona muricata (Linn). Afr. J. Tradit. Complement. Altern. Med. 2009, 6, 62. [Google Scholar] [CrossRef]
- Vila-Nova, N.S.; de Morais, S.M.; Falcão, M.J.C.; Machado, L.K.A.; Beviláqua, C.M.L.; Costa, I.R.S.; Brasil, N.V.G.P.D.S.; de Andrade, H.F. Atividade Leishmanicida e Citotoxicidade de Constituintes Quimicos de Duas Especies de Annonaceae Cultivadas No Nordeste Do Brasil. Rev. Soc. Bras. Med. Trop. 2011, 44, 567–571. [Google Scholar] [CrossRef]
- Vieira, G.H.F.; Mourão, J.A.; Ângelo, Â.M.; Costa, R.A.; Vieira, R.H.S.D.F. Antibacterial Effect (in Vitro) of Moringa Oleifera and Annona muricata against Gram Positive and Gram Negative Bacteria. Rev. Inst. Med. Trop. Sao Paulo 2010, 52, 129–132. [Google Scholar] [CrossRef]
- Bento, E.B.; Matias, E.F.F.; Brito, F.E.; Oliveira, D.R.; Coutinho, H.D.M.; Costa, J.G.M.; Kerntopf, M.R.; Menezes, I.R.A. Association between Food and Drugs: Antimicrobial and Synergistic Activity of Annona muricata L. Int. J. Food Prop. 2013, 16, 738–744. [Google Scholar] [CrossRef]
- Solomon-Wisdom, G.O.; Ugoh, S.C.; Mohammed, B. Phytochemical Screening and Antimicrobial Activities of Annona muricata (L) Leaf Extract. Am. J. Biol. Chem. Pharm. Sci. 2014, 2, 1–7. [Google Scholar]
- Padma, P.; Pramod, N.P.; Thyagarajan, S.P.; Khosa, R.L. Effect of the Extract of Annona muricata and Petunia Nyctaginiflora on Herpes Simplex Virus. J. Ethnopharmacol. 1998, 61, 81–83. [Google Scholar] [CrossRef]
- Helfer, M.; Koppensteiner, H.; Schneider, M.; Rebensburg, S.; Forcisi, S.; Müller, C.; Schmitt-Kopplin, P.; Schindler, M.; Brack-Werner, R. The Root Extract of the Medicinal Plant Pelargonium Sidoides Is a Potent HIV-1 Attachment Inhibitor. PLoS ONE 2014, 9, e87487. [Google Scholar] [CrossRef]
- Balderrama-Carmona, A.P.; Silva-Beltrán, N.P.; Gálvez-Ruiz, J.C.; Ruíz-Cruz, S.; Chaidez-Quiroz, C.; Morán-Palacio, E.F. Antiviral, Antioxidant, and Antihemolytic Effect of Annona muricata L. Leaves Extracts. Plants 2020, 9, 1650. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.K.; Pradeep, S.; Shimavallu, C.; Kollur, S.P.; Syed, A.; Marraiki, N.; Egbuna, C.; Gaman, M.A.; Kosakowska, O.; Cho, W.C.; et al. Evaluation of Annona muricata Acetogenins as Potential Anti-SARS-CoV-2 Agents Through Computational Approaches. Front. Chem. 2021, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Sokpe, A.; Mensah, M.L.K.; Koffuor, G.A.; Thomford, K.P.; Arthur, R.; Jibira, Y.; Baah, M.K.; Adedi, B.; Agbemenyah, H.Y. Hypotensive and Antihypertensive Properties and Safety for Use of Annona muricata and Persea Americana and Their Combination Products. Evid.-Based Complement. Altern. Med. 2020, 2020, 8833828. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Rouhollahi, E.; Hajrezaie, M.; Karimian, H.; Abdulla, M.A.; Kadir, H.A. Annona muricata Leaves Accelerate Wound Healing in Rats via Involvement of Hsp70 and Antioxidant Defence. Int. J. Surg. 2015, 18, 110–117. [Google Scholar] [CrossRef]
- Paarakh, P.M.; Chansouria, J.P.N.; Khosa, R.L. Wound Healing Activity of Annona muricata Extract. J. Pharm. Res. 2009, 2, 404–406. [Google Scholar]
- Arthur, F.; Woode, E.; Terlabi, E.L. C. Evaluation of Acute and Subchronic Toxicity of Annona muricata (Linn.) Aqueous Extract in Animals. Eur. J. Exp. Biol. 2011, 1, 115–124. [Google Scholar]
- Escobar-Khondiker, M.; Höllerhage, M.; Muriel, M.P.; Champy, P.; Bach, A.; Depienne, C.; Respondek, G.; Yamada, E.S.; Lannuzel, A.; Yagi, T.; et al. Annonacin, a Natural Mitochondrial Complex I Inhibitor, Causes Tau Pathology in Cultured Neurons. J. Neurosci. 2007, 27, 7827–7837. [Google Scholar] [CrossRef]
- Höllerhage, M.; Rösler, T.W.; Berjas, M.; Luo, R.; Tran, K.; Richards, K.M.; Sabaa-Srur, A.U.; Maia, J.G.S.; De Moraes, M.R.; Godoy, H.T.; et al. Neurotoxicity of Dietary Supplements from Annonaceae Species. Int. J. Toxicol. 2015, 34, 543–550. [Google Scholar] [CrossRef]
- Lannuzel, A.; Höglinger, G.U.; Champy, P.; Michel, P.P.; Hirsch, E.C.; Ruberg, M. Is Atypical Parkinsonism in the Caribbean Caused by the Consumption of Annonacae? J. Neural Transm. Suppl. 2006, 153–157. [Google Scholar] [CrossRef]
- Rady, I.; Bloch, M.B.; Chamcheu, R.C.N.; Banang Mbeumi, S.; Anwar, M.R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxid. Med. Cell. Longev. 2018, 2018, 1826170. [Google Scholar] [CrossRef]
- Wahab, S.M.A.; Jantan, I.; Haque, M.A.; Arshad, L. Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-Inflammatory and Anticancer Agents. Front. Pharm. 2018, 9, 661. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mutakin, M.; Fauziati, R.; Fadhilah, F.N.; Zuhrotun, A.; Amalia, R.; Hadisaputri, Y.E. Pharmacological Activities of Soursop (Annona muricata Lin.). Molecules 2022, 27, 1201. https://doi.org/10.3390/molecules27041201
Mutakin M, Fauziati R, Fadhilah FN, Zuhrotun A, Amalia R, Hadisaputri YE. Pharmacological Activities of Soursop (Annona muricata Lin.). Molecules. 2022; 27(4):1201. https://doi.org/10.3390/molecules27041201
Chicago/Turabian StyleMutakin, Mutakin, Rizky Fauziati, Fahrina Nur Fadhilah, Ade Zuhrotun, Riezki Amalia, and Yuni Elsa Hadisaputri. 2022. "Pharmacological Activities of Soursop (Annona muricata Lin.)" Molecules 27, no. 4: 1201. https://doi.org/10.3390/molecules27041201
APA StyleMutakin, M., Fauziati, R., Fadhilah, F. N., Zuhrotun, A., Amalia, R., & Hadisaputri, Y. E. (2022). Pharmacological Activities of Soursop (Annona muricata Lin.). Molecules, 27(4), 1201. https://doi.org/10.3390/molecules27041201






