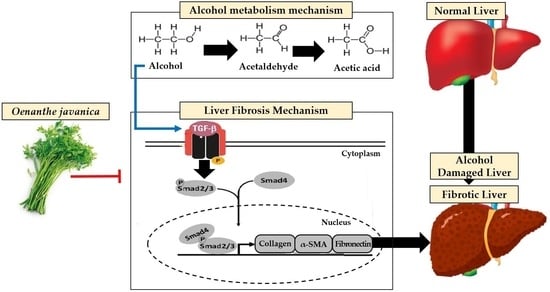
Production of Bioactive Substances to Alleviates Hangover and Ethanol-Induced Liver Damage through Fermentation of Oenanthe javanica Using Lactiplantibacillus plantarum
Abstract
1. Introduction
2. Results and Discussion
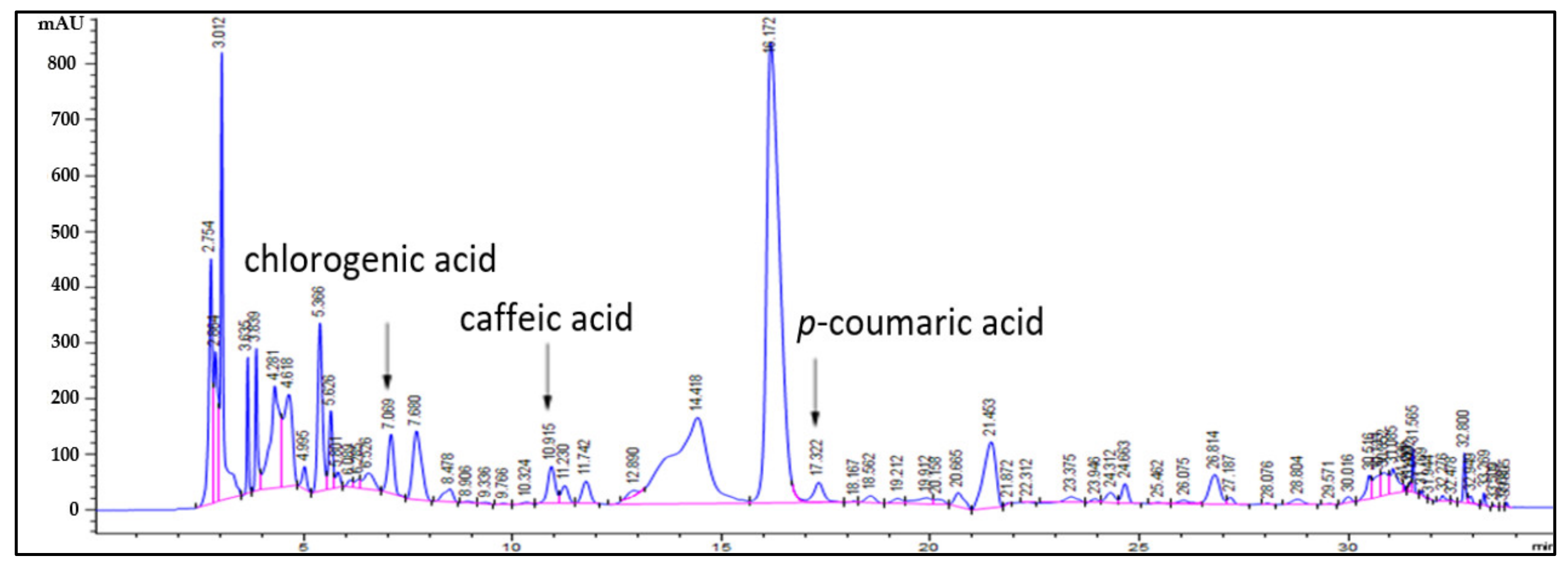
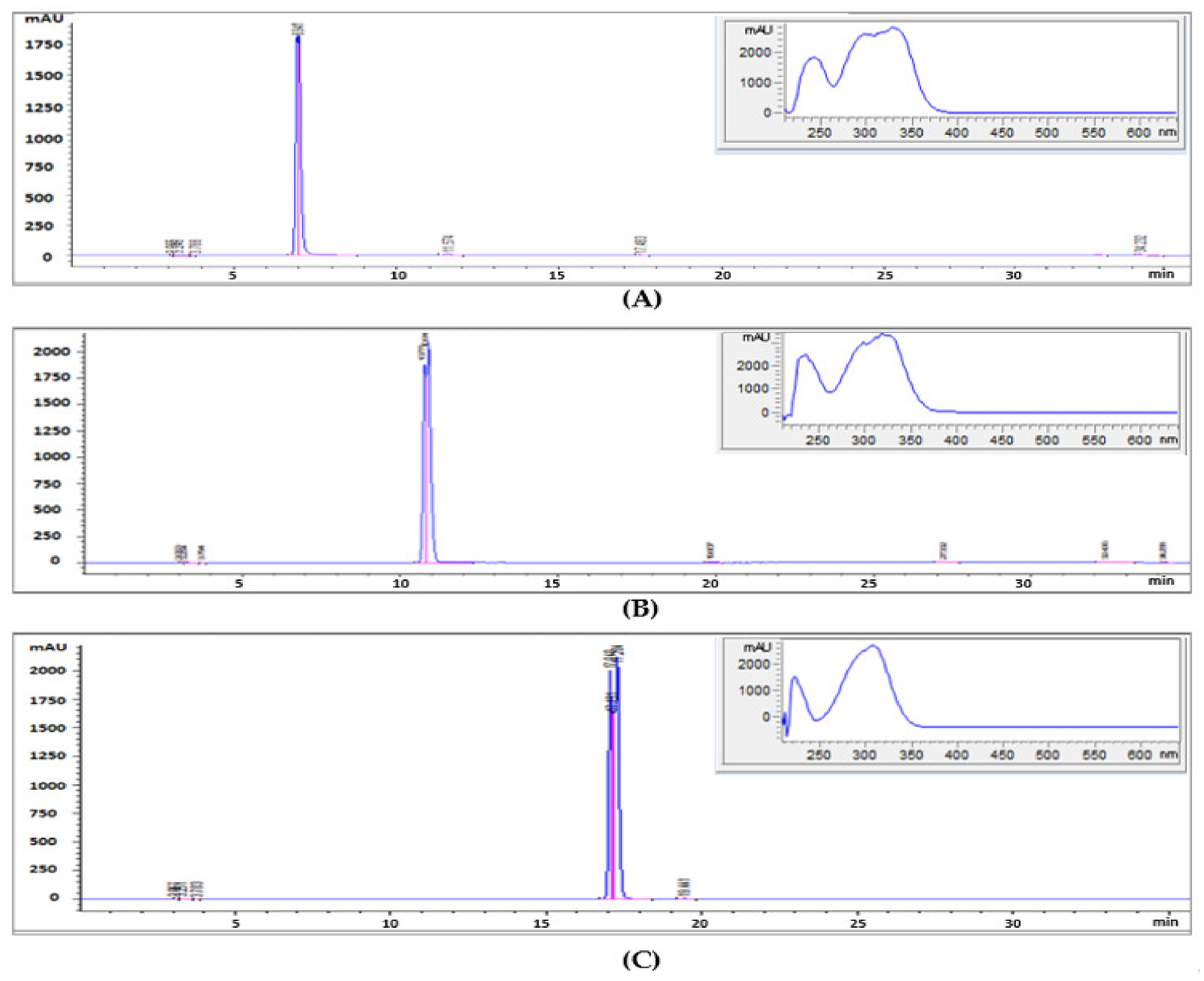
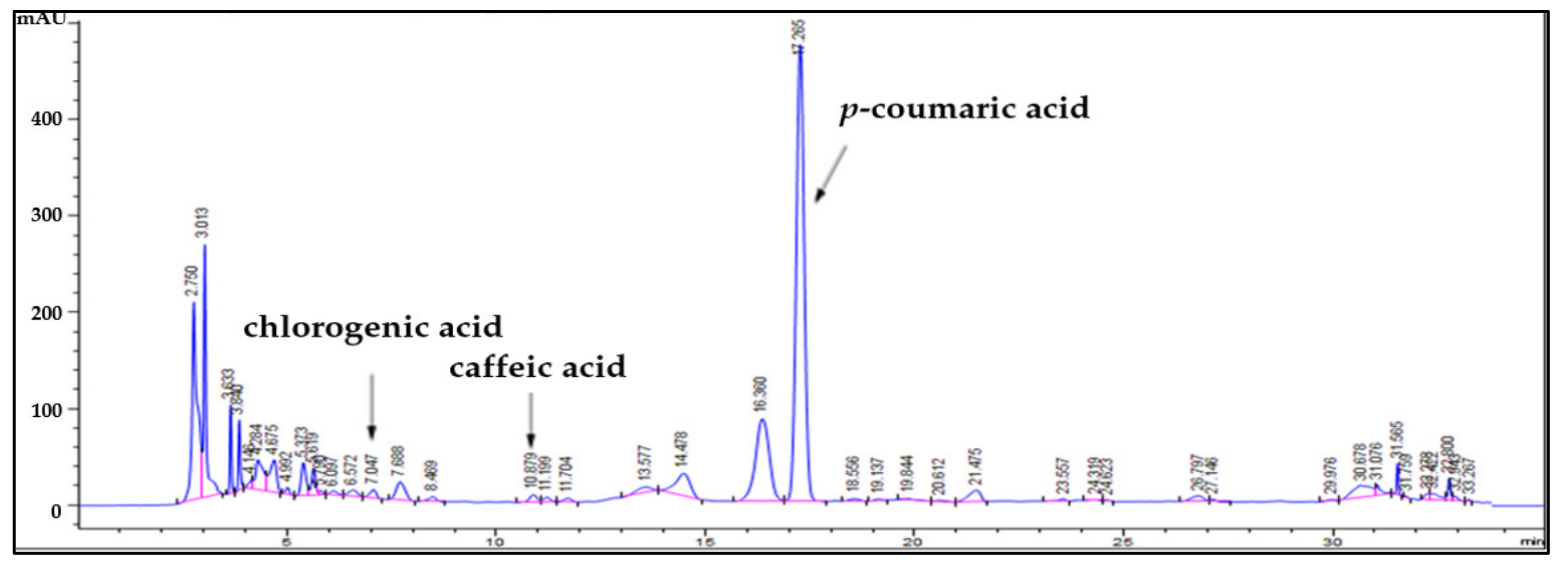
2.1. Identification of Bioactive Substances
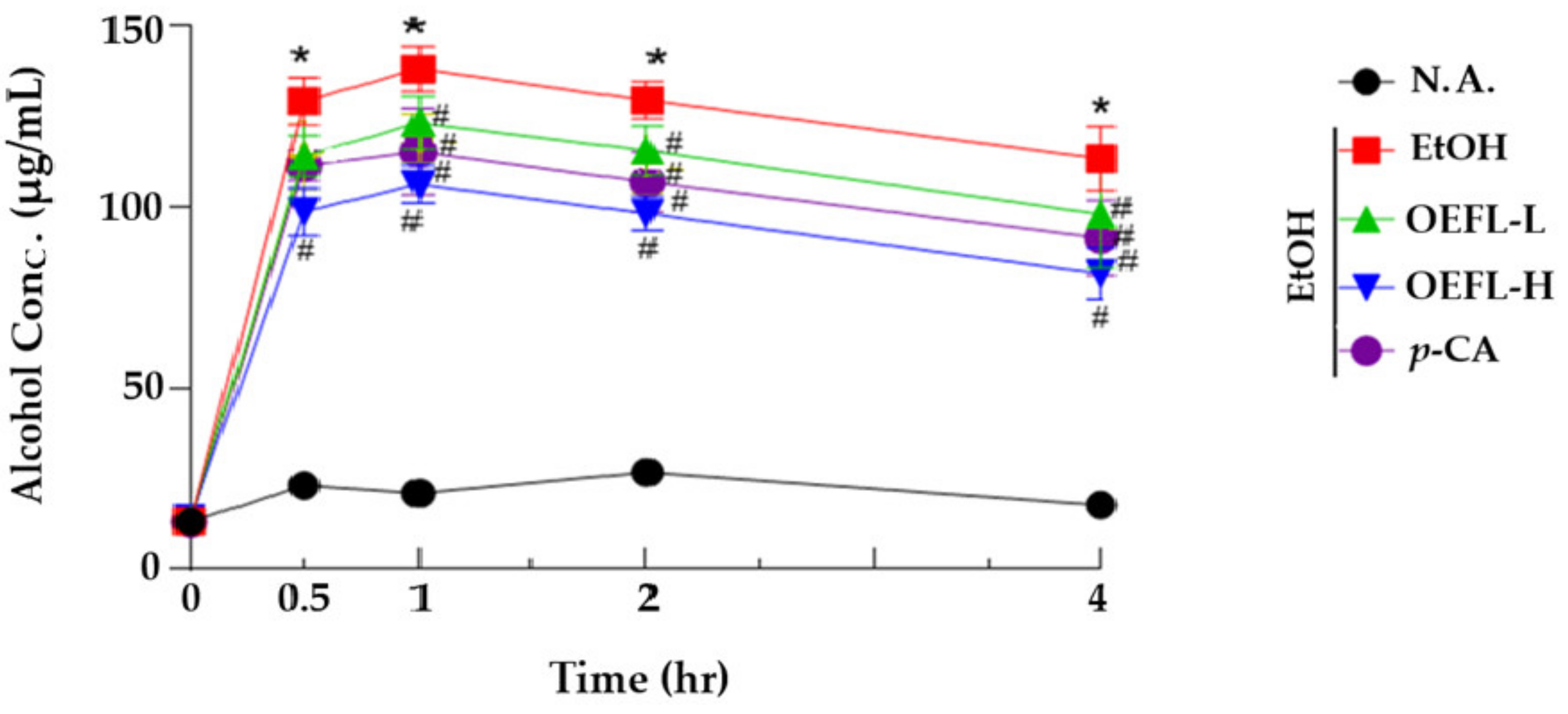
2.2. Blood Ethanol Concentration Analysis
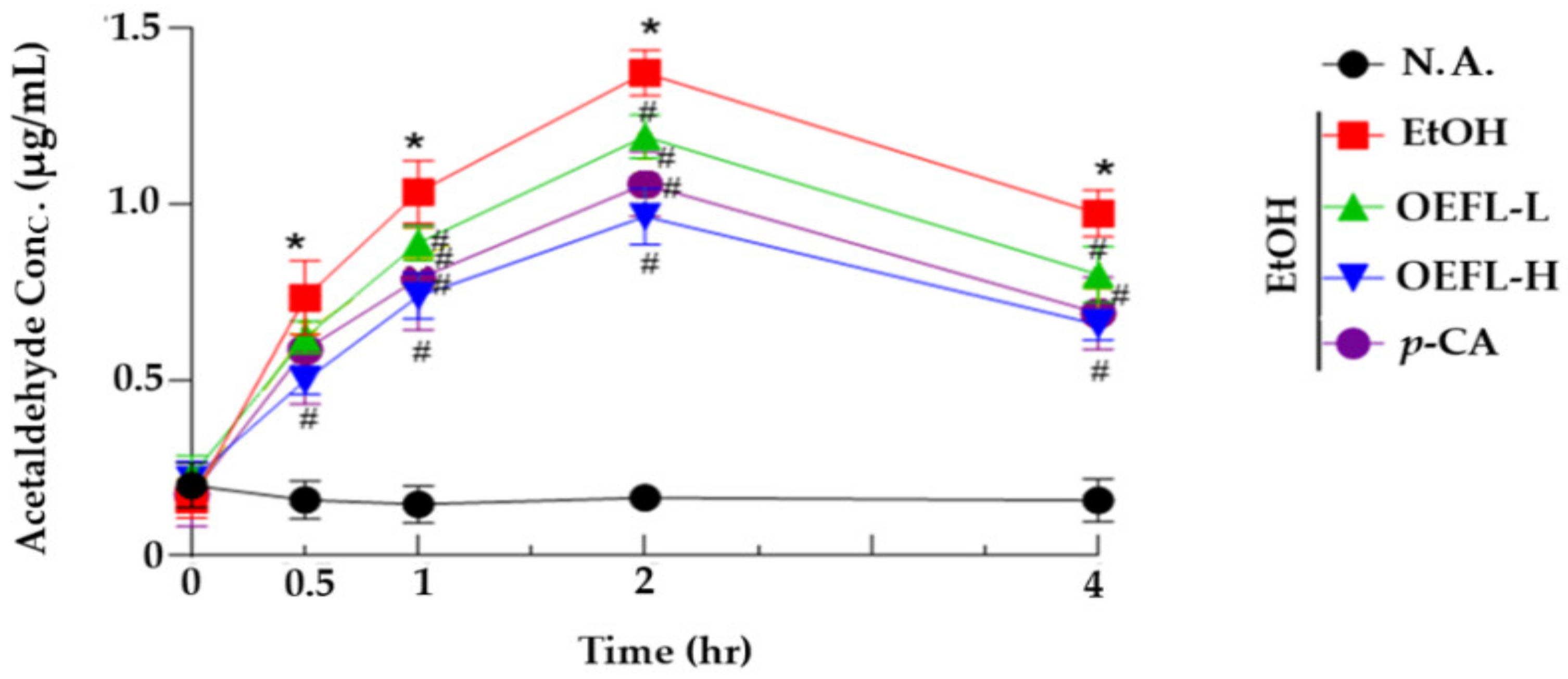
2.3. Blood Acetaldehyde Concentration
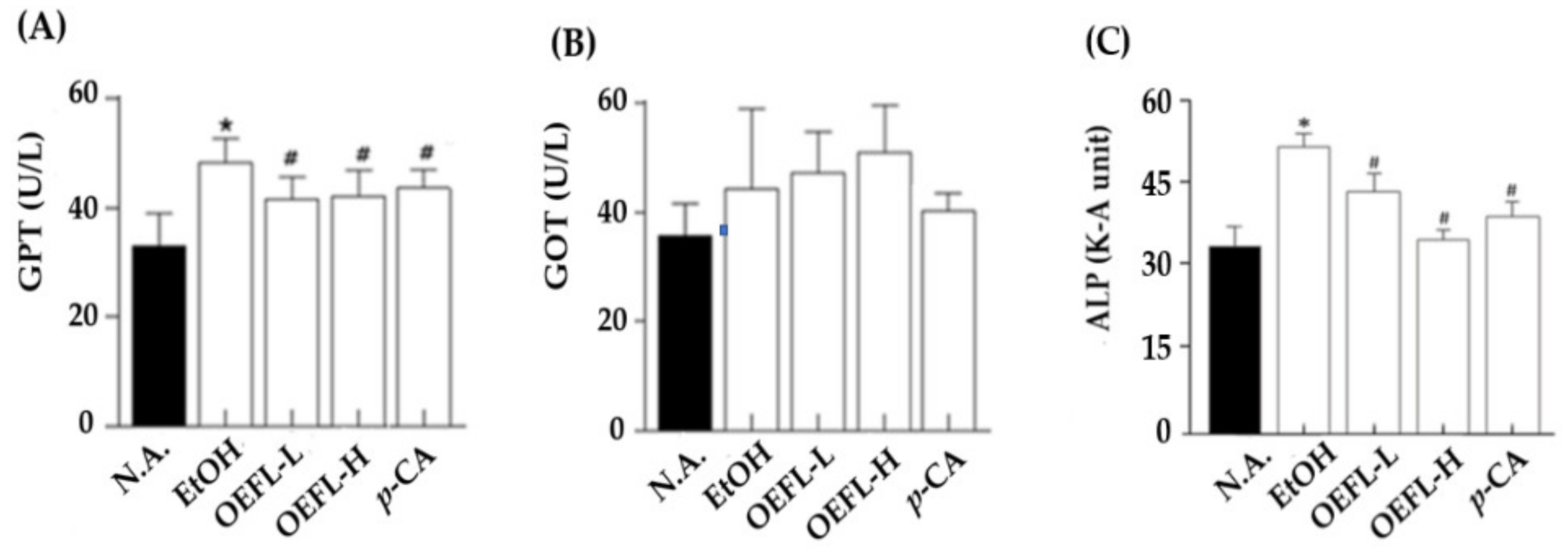
2.4. Blood GPT, GOT, and ALP Concentrations
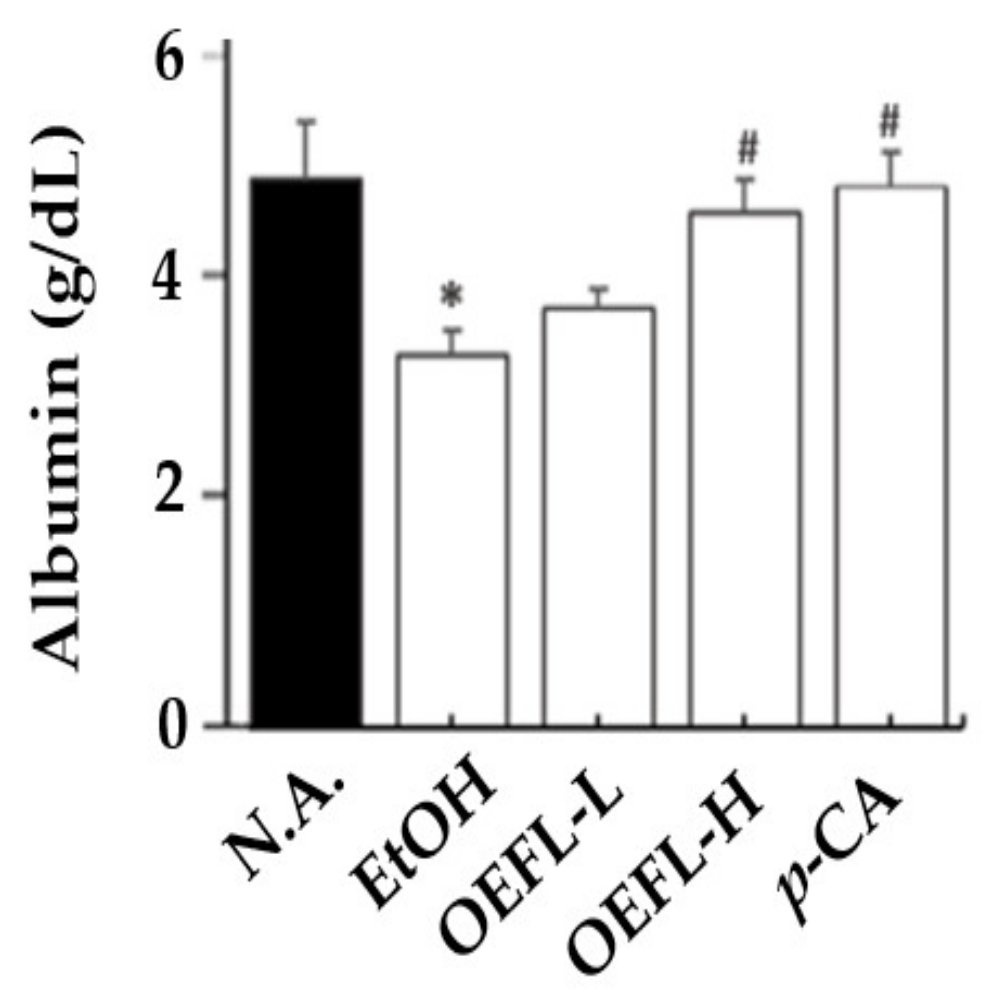
2.5. Blood Albumin Concentration
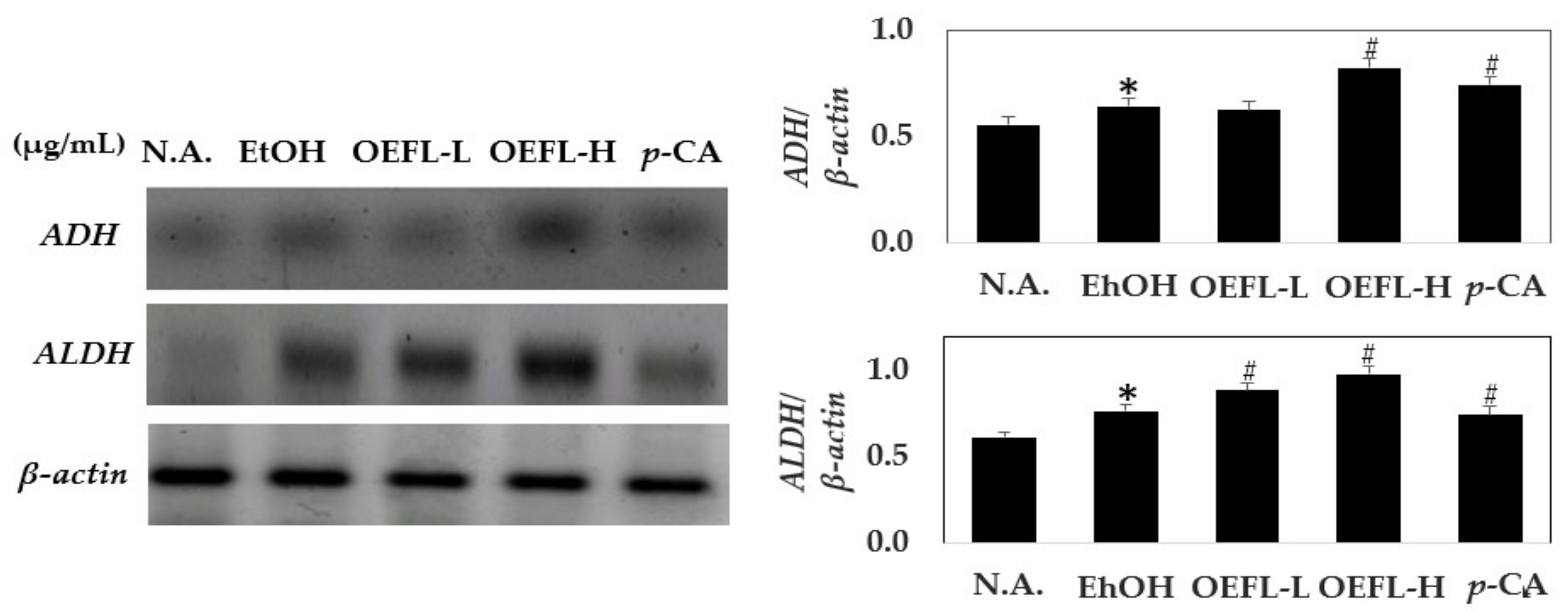
2.6. Comparison of Gene Expression Related to Ethanol Oxidation in Liver Tissue
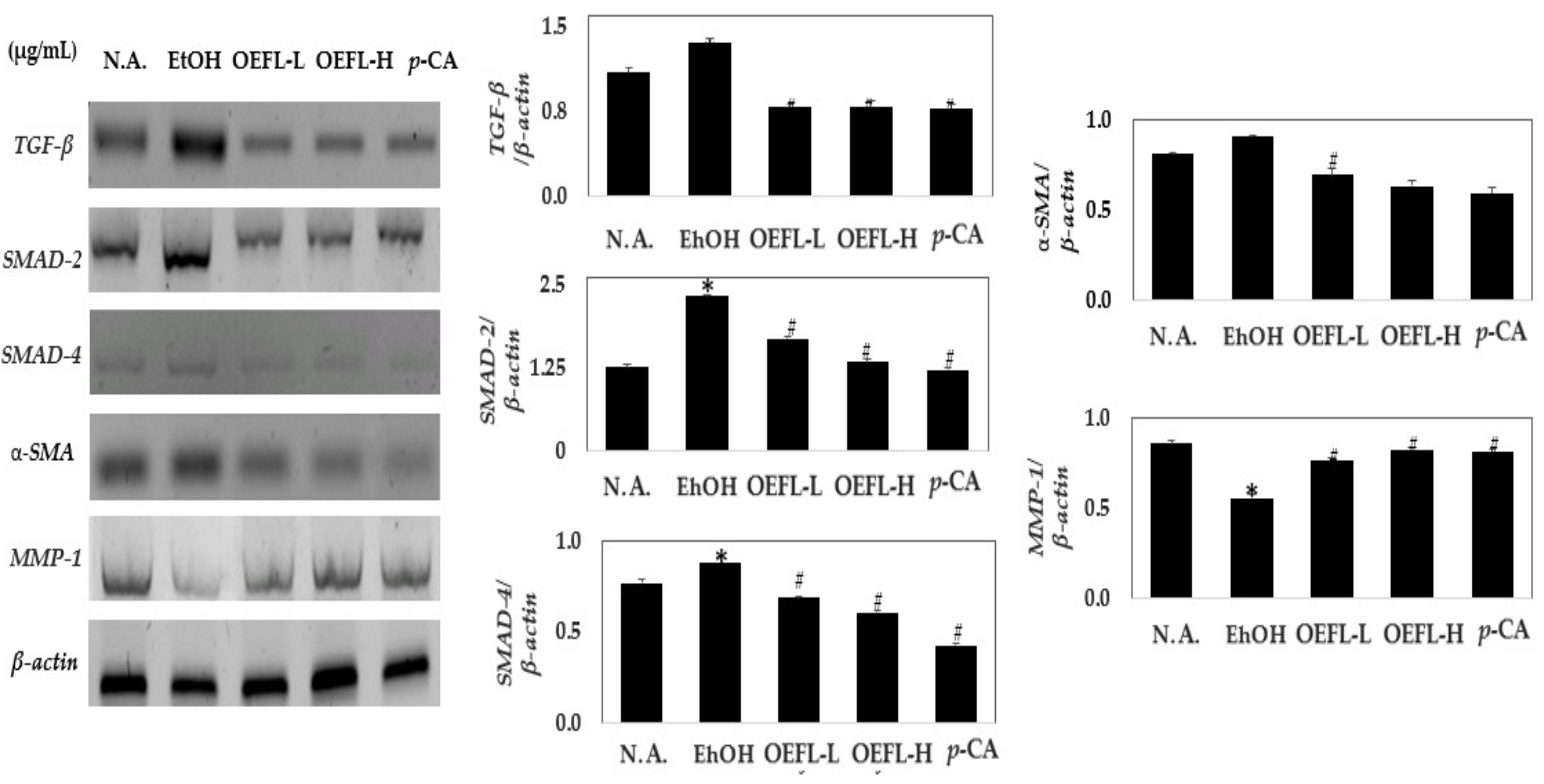
2.7. Comparison of Expression of Genes Related to Fibrosis
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Extract
3.3. Microorganism Isolation and Identification
3.4. Bioconversion Using L. plantarum
3.5. HPLC Analysis
3.6. Experimental Animal Models
3.7. Blood and Liver Tissue Sampling
3.8. Blood Ethanol Concentration Analysis
3.9. Blood Aldehyde Concentration Analysis
3.10. Blood GPT and GOT Concentrations Analysis
3.11. Blood Albumin Concentration Analysis
3.12. Blood ALP Analysis
3.13. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, Y.H.; Lee, H.M.; Chang, S.C.; Lee, M.J.; Woo, J.J. Effect of mixture including hot water extract of Houttuynia cordata thunb on ethanol-induced hangover in rats. J. Korean Soc. Food. Sci. Nutr. 2016, 45, 1508–1512. [Google Scholar] [CrossRef]
- Marsland, P.; Parrella, A.; Vore, A.S.; Barney, T.M.; Varlinskaya, E.I.; Deak, T. Male, but not female, Sprague Dawley rats display enhanced fear learning following acute ethanol withdrawal (hangover). Pharmacol. Biochem. Behav. 2021, 208, 173229. [Google Scholar] [CrossRef] [PubMed]
- Verster, J.C.; Loo, A.J.; Benson, S.; Scholey, A.; Stock, A.K. The assessment of overall hangover severity. J. Clin. Med. 2020, 9, 786. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.Y.; Bae, J.H.; Kim, D.E.; Kim, D.S.; Cho, H.K.; Kim, S.Y. Evaluation of clinical usefulness of herbal mixture ho-series for improving hangover. Korean J. Pharmacogn. 2020, 51, 278–290. [Google Scholar]
- Edenberg, H.J. The genetics of ethanol metabolism: Role of ethanol dehydrogenase and aldehyde dehydrogenase variants. Ethanol Res. Health 2007, 30, 5. [Google Scholar]
- Teschke, R. Ethanolic steatohepatitis (ASH) and ethanolic hepatitis (AH): Cascade of events, clinical aspects, and pharmacotherapy options. Expert Opin. Pharmacother. 2018, 19, 779–793. [Google Scholar] [CrossRef]
- Zhong, Z.; Lemasters, J.J. A unifying hypothesis linking hepatic adaptations for ethanol metabolism to the proinflammatory and profibrotic events of ethanolic liver disease. Alcohol. Clin. Exp. Res. 2018, 42, 2072–2089. [Google Scholar] [CrossRef]
- Jung, Y.J.; Han, D.O.; Choi, B.H.; Park, C.; Lee, H.J.; Kim, S.H.; Ham, D.H. Effect of fermented herbal extract, HP-1 on enzyme activities and gene expressions related to ethanol Metabolism in ethanol-loaded rats. Korean J. Med. Physiol. Pathol. 2007, 21, 387–391. [Google Scholar]
- Lee, H.I.; Lee, M.K. Effects of scopoletin supplementation on insulin resistance and antioxidant defense system in chronic ethanol-fed rats. J. Korean Soc. Food Sci. Nutr. 2015, 44, 173–181. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Dongiovanni, P. Ethanol or gut microbiota: Who is the guilty? Int. J. Mol. Sci. 2019, 20, 4568. [Google Scholar] [CrossRef]
- Bajaj, J.S. Ethanol, liver disease and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 235–246. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, Y.J.; Kim, H.J.; Yang, J.W.; Kim, J.S.; Jee, Y.H. Sasa quelpaertensis Nakai ethyl acetate fraction protects the liver against chronic ethanol-induced liver injury and fat accumulation in mice. Korean J. Vet. Res. 2020, 60, 215–223. [Google Scholar] [CrossRef]
- Hyun, C.S.; Park, G.Y.; Oh, Y.M.; Lee, Y.J.; Han, C.H. Effect of medicinal plant extract for hangover relief. Korean J. Vet. Res. 2014, 54, 233–238. [Google Scholar] [CrossRef][Green Version]
- Kang, K.Y.; Lee, J.H. A case of ethanolic liver cirrhosis treated with injinoryeong-san. Korean J. Int. Med. 2016, 37, 135–142. [Google Scholar]
- Park, G.H.; Park, J.Y.; Jang, Y.H. Changes in flavonoid aglycone contents and antioxidant activities of citrus peel depending on enzyme treatment times. Korean J. Food Sci. Technol. 2019, 48, 542–550. [Google Scholar]
- Yang, X.; Ma, F.; Yu, H.; Zhang, X.; Chen, S. Effects of biopretreatment of corn stover with white-rot fungus on low-temperature pyrolysis products. Bioresour. Technol. 2011, 102, 3498–3503. [Google Scholar] [CrossRef]
- Lee, B.J. Development of functional food using fermented marine organism. Food Sci. Nutr. 2013, 18, 8–12. [Google Scholar]
- Won, B.Y.; Shin, K.Y.; Ha, H.; Kim, Y.S.; Gun, H. Changes in nutritional composition of dropwort (Oenanthe javanica) ethanol extracts. J. Korean Soc. Food Sci. Nutr. 2015, 44, 882–887. [Google Scholar] [CrossRef]
- Tsikrika, K.; O’Brien, N.; Rai, D.K. The effect of high pressure processing on polyphenol oxidase activity, phytochemicals and proximate composition of irish potato cultivars. Foods 2019, 8, 517. [Google Scholar] [CrossRef]
- Liu, Y.; Hui, X.; Ibrahim, S.A.; Huang, W. Increasing antiradical activity of polyphenols from lotus seed epicarp by probiotic bacteria bioconversion. Molecules 2018, 23, 2667. [Google Scholar] [CrossRef]
- Choi, S.I.; Hwang, S.J.; Lee, O.H.; Kim, J.D. Antioxidant activity and component analysis of Populus Tomentiglandulosa extract. Korean J. Food Sci. Technol. 2020, 52, 119–124. [Google Scholar]
- Park, J.C.; Yu, Y.B.; Lee, J.H.; Kim, N.J. Anti-inflammatory and analgesic effects of the components from some edible plants. J. Korean Soc. Food Sci. Nutr. 1994, 23, 671–674. [Google Scholar]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic acid: Recent advances on its dual role as a food additive and a nutraceutical against metabolic syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Yoon, T.J.; Jo, S.Y. Effect of Acanthopanax senticosus extracts on ethanol degradation and anti-inflammatory activity in mice. Korean J. Food Nutr. 2010, 23, 542–548. [Google Scholar]
- Yang, S.T. Effects of aged black garlic extract on ethanol induced hangover in rats. J. Life Sci. 2010, 20, 225–230. [Google Scholar] [CrossRef]
- Han, C.K.; Seong, K.S.; Lee, K.W.; Park, S.S.; Jeong, J.Y.; Kim, S.S. Effects of omija (Schizandra chinensis) concentrate on blood ethanol clearance and hepatoprotective function in rats induced by acute ethanol intoxication and chronic ethanol treatment. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1139–1147. [Google Scholar] [CrossRef]
- Choi, J.H.; Jung, S.M.; Kang, I.S.; Choi, S. Effects of intramuscular injection of Taurine-F TM on anti-hepatotoxicity and innate immunity in olive flounder, Paralichthys olivaceus. J. Fish Pathol. 2020, 33, 83–89. [Google Scholar]
- Seo, H.B.; Seo, H.J.; Shim, S.H.; Lee, C.; Hak, C.W.; Kim, S.Y.; Choi, J.Y.; Park, S.H.; Yun, Y.J.; Hong, J.W.; et al. Effect of herbal medicine on liver function in korean medical hospital inpatients: A retrospective chart review. Korean J. Intern. Med. 2019, 40, 1145–1151. [Google Scholar] [CrossRef]
- Kim, S.E. Optimal evaluation of the results of liver function test. Korean J. Intern. Med. 2019, 94, 89–95. [Google Scholar] [CrossRef][Green Version]
- Kang, M.J.; Shin, J.H.; Lee, S.J.; Chung, M.J.; Sung, N.J. Effect of garlic and medicinal plants composites on the liver function and lipid metabolism of rats administered with ethanol during the short-term. J. Life Sci. 2009, 19, 934–942. [Google Scholar]
- Bae, H.J.; Kim, K.A.; Kim, A.J.; Park, M.J.; Hahn, H.J.; Son, I.J.; Lee, H.S. Development of the SNUH nutritional assessment tool using objective parameters. J. Korean Soc. Health Syst. Pharm. 2010, 27, 21–28. [Google Scholar]
- Akirov, A.; Masri-Iraqi, H.; Atamna, A.; Shimon, I. Low albumin levels are associated with mortality risk in hospitalized patients. Am. J. Med. Sci. 2017, 130, 1465.e11–1465.e19. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.Y.; Choung, S.Y. Effects of plant vinegar extract on the reduction of blood concentration of ethanol and acetaldehyde in ethanol administrated rats. Biomol. Ther. 2005, 13, 107–112. [Google Scholar]
- Yan, Y.; Zeng, J.; Xing, L.; Li, C. Extra- and intra-cellular mechanisms of hepatic stellate cell activation. Biomedicines 2021, 9, 1014. [Google Scholar] [CrossRef]
- Elnagdy, M.; Barve, S.; Mcclain, C.; Gobejishvili, L. cAMP signaling in pathobiology of ethanol associated liver disease. Biomolecules 2020, 10, 1433. [Google Scholar] [CrossRef]
- Bi, J.; Ge, S. Potential roles of BMP9 in liver fibrosis. Int. J. Mol. Sci. 2014, 15, 20656–20667. [Google Scholar] [CrossRef]
- Jang, Y.A.; Lee, J.T. Anti-wrinkle effect of berberine by inhibition of MMP-2 and MMP-9 activity in fibroblasts. J. Appl. Biol. Chem. 2018, 61, 9–15. [Google Scholar] [CrossRef]
- Gam, D.H.; Park, J.H.; Hong, J.W.; Jeon, S.J.; Kim, J.H.; Kim, J.W. Effects of Sargassum thunbergii extract on skin whitening and anti-wrinkling through inhibition of TRP-1 and MMPs. Molecules 2021, 26, 7381. [Google Scholar] [CrossRef]
- Cecchini, S.; Fazio, F. Assessment of total antioxidant capacity in serum of heathy and stressed hens. SD Rats. Animals 2020, 10, 2019. [Google Scholar] [CrossRef]
| Primer | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| 1ADH | CGAGGGAGCTGGCATTGTT | CGGGTGCTTGCAGATCCT |
| 2ALDH | GCTGTGAAGGCCGCAAGA | GCCAGCAGCAGACGATCTC |
| 3TGF-β | CCCTGCCCCTACATTTGGA | GCACGCAGCACGGTGAT |
| SMAD-2 | TGTGCAGAGCCCCAACTGT | CTGAGCCAGAAGAGCAGCAA |
| SMAD-4 | CGACGCTGTGGGAAATGC | CTCCTCGCTGCGGTTCTG |
| 4α-SMA | TCCAGGGCTCCAACGAGAT | CCCCAAGTTCCGGTGTGA |
| 5MMP-1 | CTGAAAAGCTGAGGCAAATGC | TGGTCCAACGAGGATTGTTGT |
| β-actin | CCCTGGCTCCTAGCACCAT | GAGCCACCAATCCACACAGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gam, D.H.; Park, J.H.; Kim, S.H.; Kang, M.H.; Kim, S.B.; Kim, J.W. Production of Bioactive Substances to Alleviates Hangover and Ethanol-Induced Liver Damage through Fermentation of Oenanthe javanica Using Lactiplantibacillus plantarum. Molecules 2022, 27, 1175. https://doi.org/10.3390/molecules27041175
Gam DH, Park JH, Kim SH, Kang MH, Kim SB, Kim JW. Production of Bioactive Substances to Alleviates Hangover and Ethanol-Induced Liver Damage through Fermentation of Oenanthe javanica Using Lactiplantibacillus plantarum. Molecules. 2022; 27(4):1175. https://doi.org/10.3390/molecules27041175
Chicago/Turabian StyleGam, Da Hye, Jae Hyun Park, So Hee Kim, Min Ho Kang, Se Bin Kim, and Jin Woo Kim. 2022. "Production of Bioactive Substances to Alleviates Hangover and Ethanol-Induced Liver Damage through Fermentation of Oenanthe javanica Using Lactiplantibacillus plantarum" Molecules 27, no. 4: 1175. https://doi.org/10.3390/molecules27041175
APA StyleGam, D. H., Park, J. H., Kim, S. H., Kang, M. H., Kim, S. B., & Kim, J. W. (2022). Production of Bioactive Substances to Alleviates Hangover and Ethanol-Induced Liver Damage through Fermentation of Oenanthe javanica Using Lactiplantibacillus plantarum. Molecules, 27(4), 1175. https://doi.org/10.3390/molecules27041175