Determination of Thymol in Commercial Formulation, Essential Oils, Traditional, and Ultrasound-Based Extracts of Thymus vulgaris and Origanum vulgare Using a Greener HPTLC Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials
2.3. Chromatography and Analysis
2.4. Derivatization and Densitometry Scanning
2.5. Preparation of Thymol Standard Solutions for Calibration and Quality Control (QC)
2.6. Sample Processing of Thymol from Commercial Formulation
2.7. Isolation of the Essential Oil from T. vulgaris and O. vulgare of Different Geographical Regions
2.8. Traditional Extraction of Thymol from T. vulgaris and O. vulgare of Different Geographical Regions
2.9. Ultrasound-Based Extraction of Thymol from T. vulgaris and O. vulgare of Different Geographical Regions
2.10. Validation Studies
2.11. Analysis of Thymol in Commercial Formulation, Essential Oils, TE, and UBE of Different Geographical Regions
2.12. Greenness Assessment
3. Results and Discussion
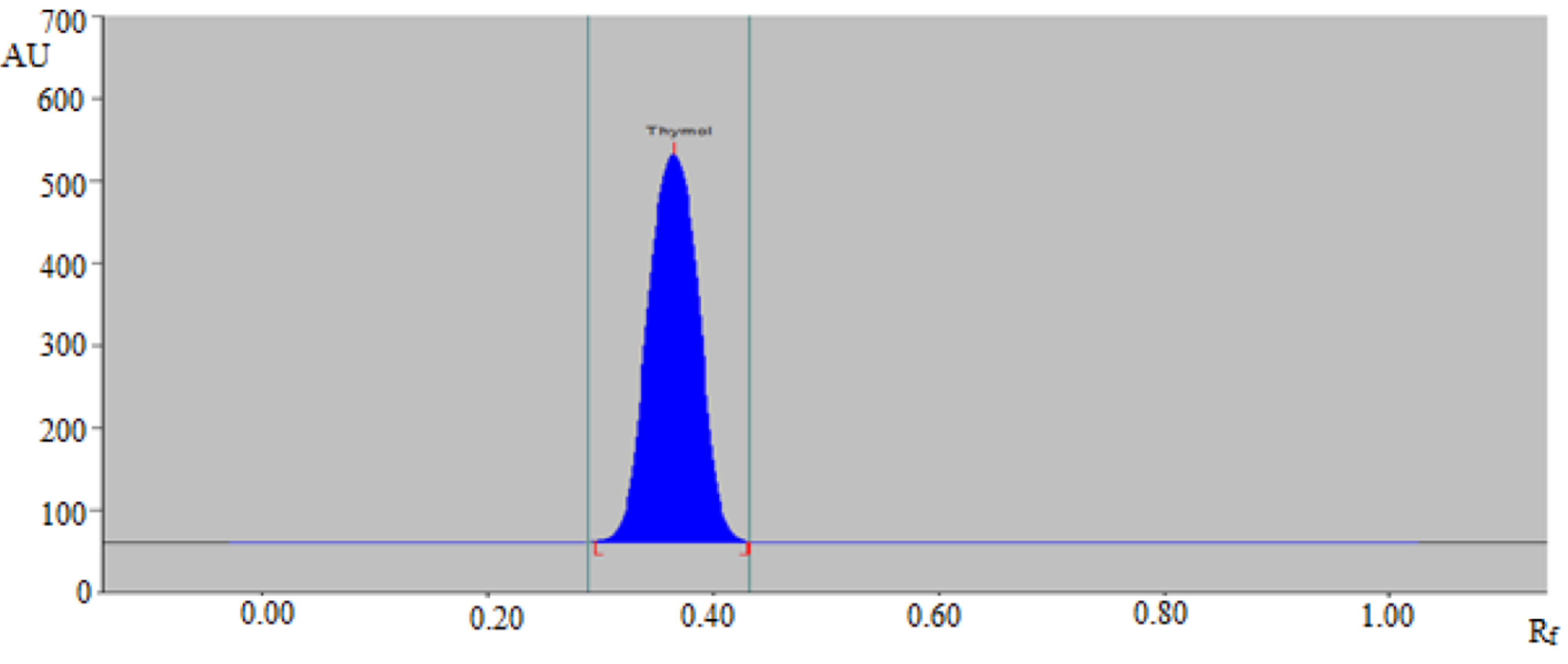
3.1. Method Development
3.2. Validation Studies
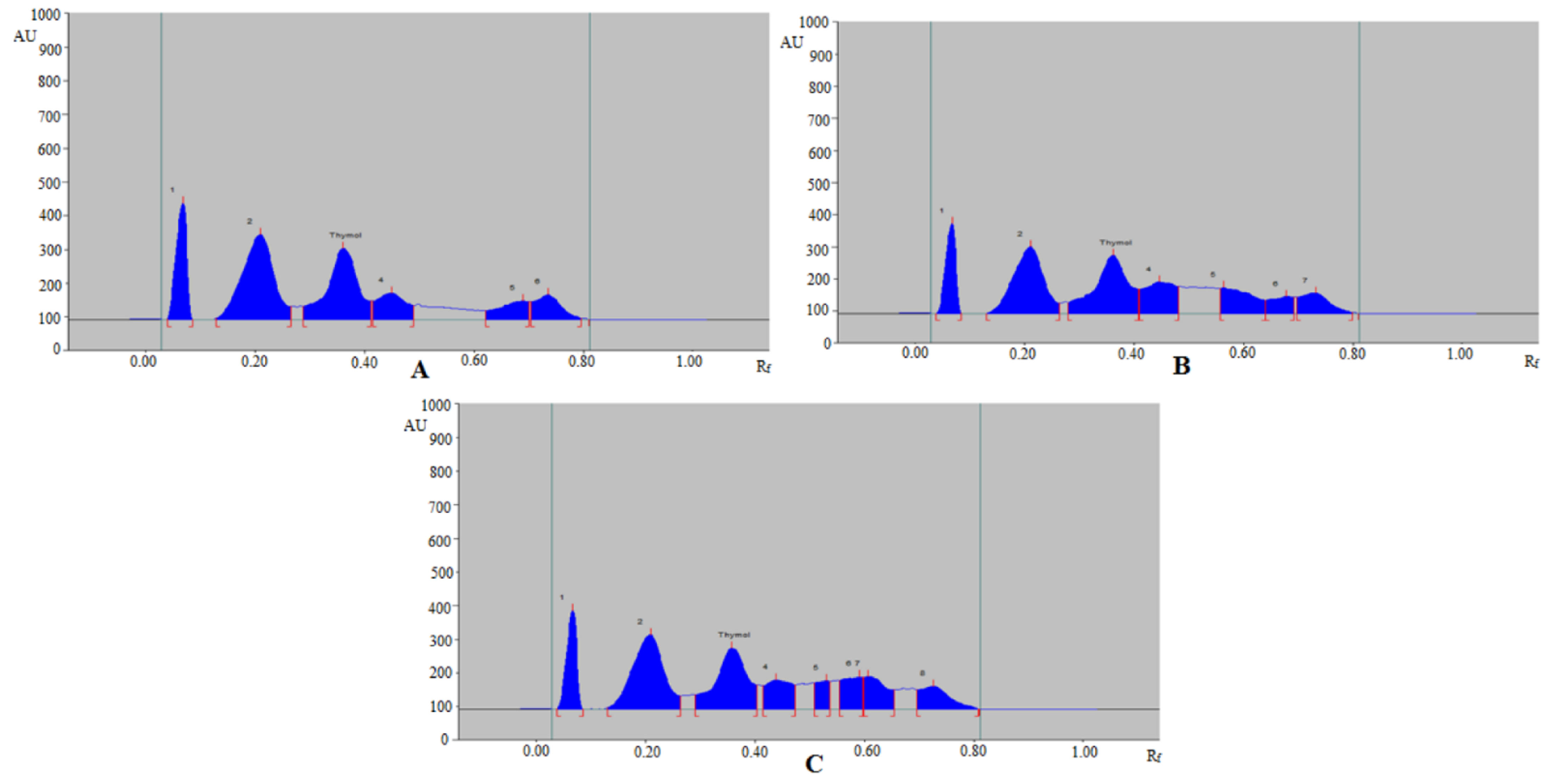
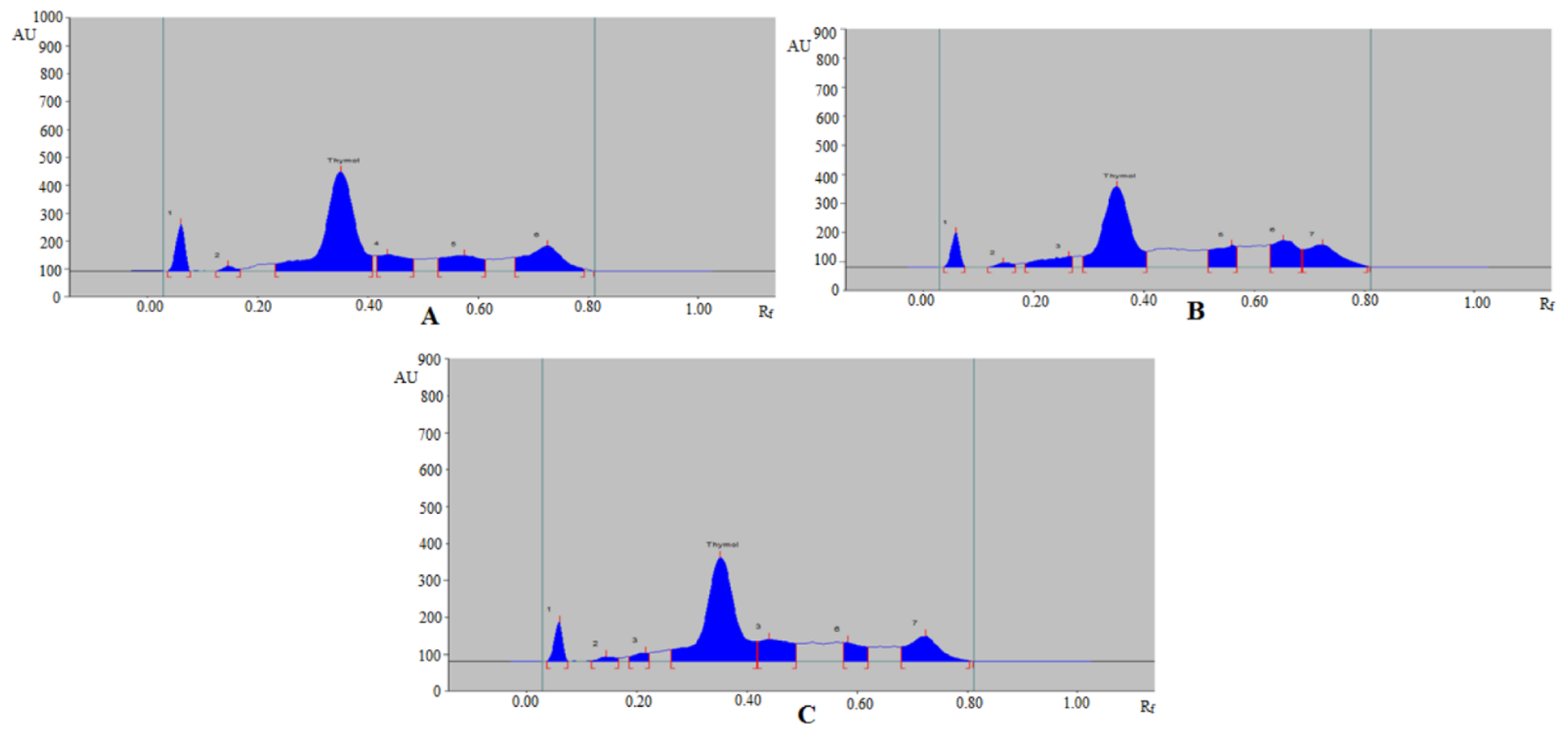
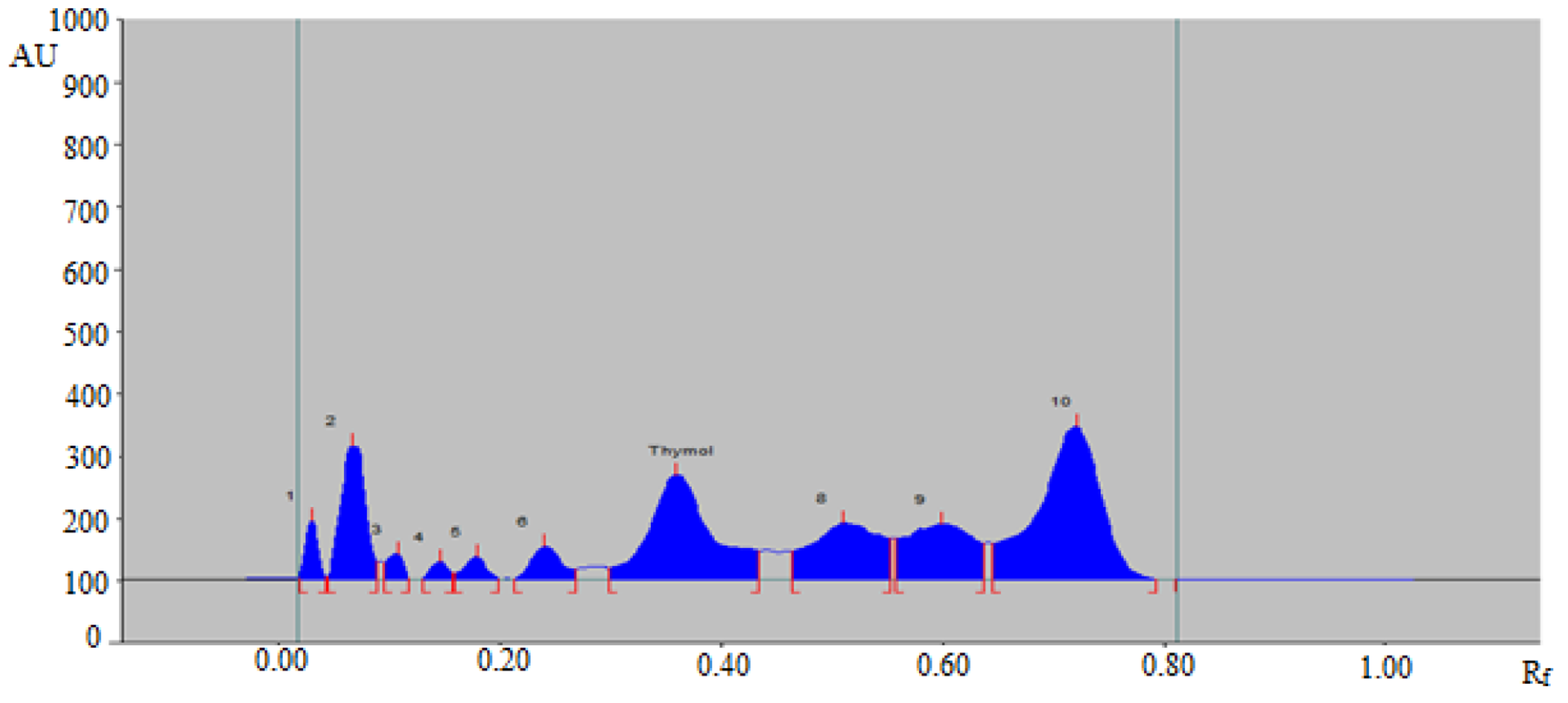
3.3. Analysis of Thymol in Commercial Formulation, Essential oils, TE, and UBE of Different Geographical Regions
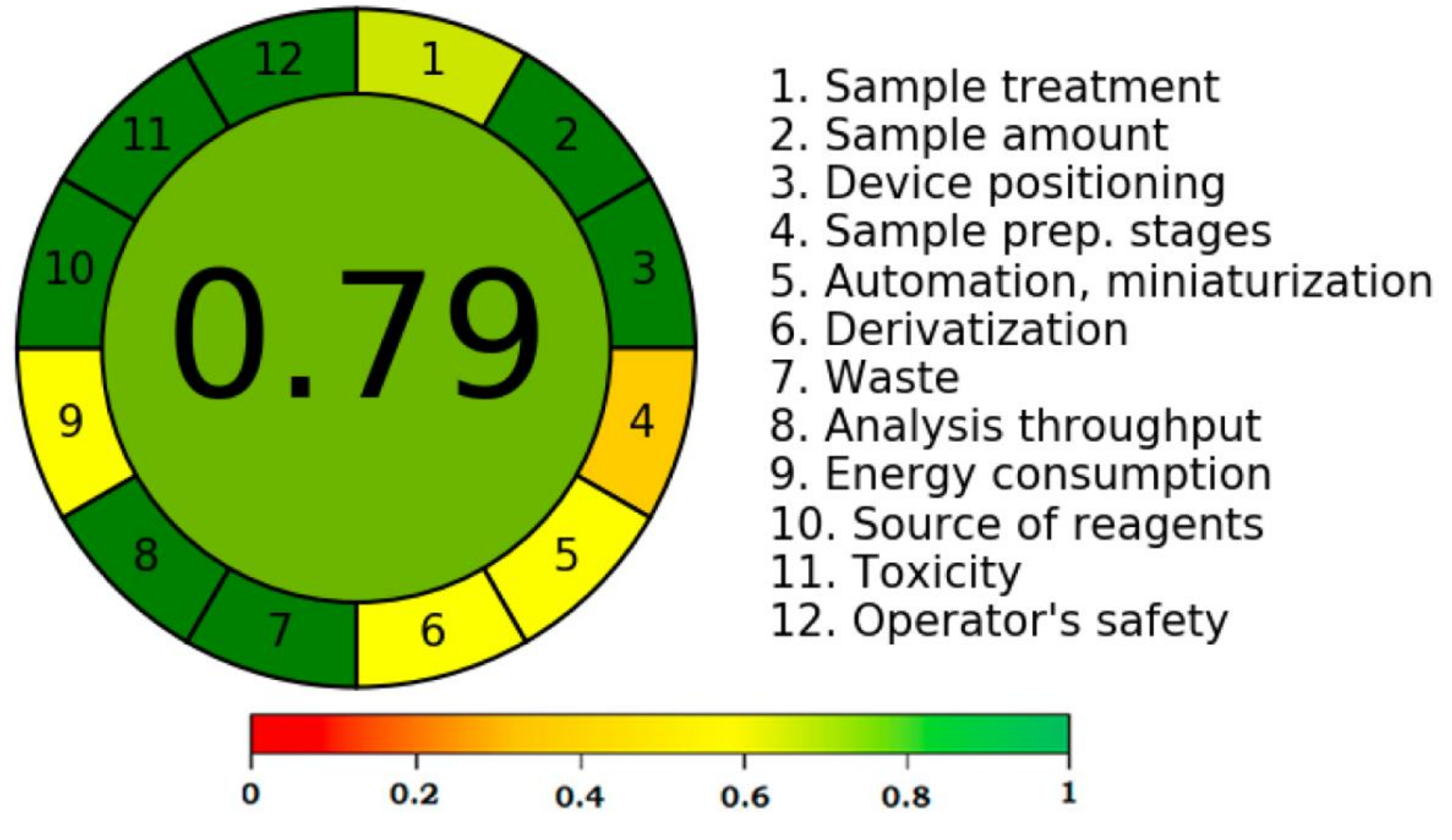
3.4. Greenness Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Verma, R.S.; Verma, R.K.; Chauhan, A.; Yadav, A.K. Seasonal variation in essential oil content and composition of Thyme, Thymus serpyllum L. cultivated in uttarakhand hills. Indian J. Pharm. Sci. 2011, 73, 233–235. [Google Scholar] [CrossRef]
- Khan, S.T.; Khan, M.; Ahmad, J.; Wahab, R.; Abd-Elkader, O.H.; Musarrat, J.; Alkhathlan, H.Z.; Al-Kedhairy, A.A. Thymol and carvacrol induce autolysis, stress, growth inhibition and reduce the biofilm formation by Streptococcus mutans. AMB Express. 2017, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.M.; Sequra-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, S.T.; Khan, M.; Mousa, A.A.; Mahmood, A.; Alkhathlan, H.Z. Chemical diversity in leaf and stem essential oils of Origanum vulgare L. and their effects on microbicidal activities. AMB Express. 2019, 9, 176. [Google Scholar] [CrossRef] [PubMed]
- Grulova, D.; Caputo, L.; Elshafie, H.S.; Baranova, B.; De Martino, L.; Sedlak, V.; Gogalova, Z.; Poracova, J.; Camele, I.; De Feo, V. Thymol chemotype Origanum vulgare L. essential oil as a potential selective bio-based herbicide on monocot plant species. Molecules 2020, 25, 595. [Google Scholar] [CrossRef] [PubMed]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Foe, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef] [PubMed]
- Meeran, M.F.N.; Javed, H.; Al Taee, H.; Azimullah, S.; Ojha, S.K. Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Sancheti, J.; Shaikh, M.F.; Chaudhari, R.; Somani, G.; Patil, S.; Jain, P.; Sathaye, S. Characterization of anticonvulsant and antiepileptogenic potential of thymol in various experimental models. Arch. Pharmacol. 2014, 387, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Bashir, W.A.; Mansour, S.S. Spectrophotometric assay of thymol in various samples by coupling with diazotized p-nitroaniline. Raf. J. Sci. 2007, 18, 28–37. [Google Scholar]
- Dhahir, S.A. Determination of thymol in pure and pharmaceutical preparation by diazotization-coupling method with 2,4-dichloroaniline as the coupling agent. J. Kufa Chem. Sci. 2011, 2, 99–108. [Google Scholar]
- Fadhil, G. Spectrophotometric determination of thymol in pharmaceutical preparation via oxidative coupling reaction with 2,4-dinitrophenylhydrazine in the presence of potassium periodate. Iraqi J. Sci. 2014, 55, 27–34. [Google Scholar]
- Barrak, M.H.; Al-Rufaie, M.M.; Motaweq, Z.Y. Thymol quantitative analysis in medicinal formulation types through employing of nano-technology and antimicrobial activity in some pathogenic bacterial isolates. Nusant. Biosci. 2021, 13, 129–137. [Google Scholar] [CrossRef]
- Fibranz, L.; Blake, M.I.; Miller, C.E. Colorimetric determination of thymol in thyme oil. J. Am. Pharm. Assoc. 1958, 47, 133–135. [Google Scholar] [CrossRef] [PubMed]
- Al-Abachi, M.Q.; Al-Najjar, N.A. Flow injection spectrophotometric determination of thymol using 4-aminoantipyrene and copper (II) nitrate. Baghdad Sci. J. 2015, 12, 332–339. [Google Scholar]
- Al-Abachi, M.Q.; Hadi, H.; Fadhil, G. Flow injection determination of thymol in pharmaceutical samples via oxidative coupling reaction with 2,4-dinitrophenylhydrazine. J. Al-Nahrain Univ. 2015, 18, 18–24. [Google Scholar] [CrossRef]
- Ziyatdinova, G.; Ziganshina, E.; Cong, P.; Budnikov, H. Voltametric determination of thymol in Oregano using CeO2 modified electrode in Brij® 35 micellar medium. Food Anal. Methods 2017, 10, 129–136. [Google Scholar] [CrossRef]
- Naskar, H.; Biswas, S.; Tudu, B.; Bandopadhyay, R.; Pamanik, P. Voltammetric detection of thymol (THY) using polyacrylamide embedded graphite molecular imprinted polymer (PAM@G-MIP) electrode. IEEE Sens. J. 2019, 19, 8583–8589. [Google Scholar] [CrossRef]
- Zima, J.; Cienciala, M.; Barek, J.; Moreira, J.C. Determination of thymol using HPLC-ED with glassy carbon paste electrode. Chem. Anal. 2007, 52, 1049–1057. [Google Scholar]
- Hajimehdipoor, H.; Shekarchi, M.; Khanavi, M.; Adib, N.; Amri, M. A validated high performance liquid chromatography method for the analysis of thymol and carvacrol in Thymus vulgaris L. volatile oil. Phcog. Mag. 2010, 6, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Angelo, T.; Pires, F.Q.; Gelfuso, G.M.; da Sila, J.K.R.; Gratieri, T.; Cunha-Filho, M.S.S. Development and validation of a selective HPLC-UV method thymol determination in skin permeation experiments. J. Chromatogr. B 2016, 1022, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, A.; Azadbakht, M.; Hosseinimehr, S.J. Quantification of thymol content in different extracts of Zataria multiflora by HPLC method. Pharm. Biol. Res. 2016, 2, 8–13. [Google Scholar] [CrossRef][Green Version]
- Louchard, B.O.; Costa, L.C.; Silva, A.R.A.; Leal, L.K.A.M. Validation of a high performance liquid chromatography method to quantify thymol in nanocapsules of bioactive essential from Lippia sidoides. Int. J. Complement. Alt. Med. 2017, 10, 00330. [Google Scholar]
- Dedic, M.; Becic, E.; Imamovic, B.; Ziga, N.; Medanhodzic-Vuk, S.; Sober, M. HPLC method for determination the content of thymol and carvacrol in thyme tincture. Bull. Chem. Technol. Bos. Herz. 2018, 50, 1–6. [Google Scholar]
- Shahariz, M.; Adzib, M.; Ilham, Z. Simultaneous analytical determination of methyl salicylate and thymol in selected Malaysian traditional medicines. AIMS Med. Sci. 2020, 7, 43–56. [Google Scholar] [CrossRef]
- Ghobadloo, P.A.; Hamidi, S.; Nemati, M.; Jahed, F.S. Ultrasound assisted dispersive solid phase microextraction of thymol and carvacrol in pharmaceutical products using graphene oxide as an adsorbent prior to analysis by high performance liquid chromatography. Curr. Pharm. Anal. 2020, 16, 578–584. [Google Scholar] [CrossRef]
- Soran, M.-L.; Lung, I. HPTLC analysis of thymol in extracts of Satureja hortensis L. obtained by different techniques. J. Planar Chromatogr. 2010, 23, 320–322. [Google Scholar] [CrossRef]
- Haque, M.R.; Ansari, S.H.; Najmi, A.K.; Naquvi, M.J. Validated HPTLC analysis method for quantification of thymol content in Trachyspermum ammi and polyherbal Unani formulation arq zerra. Int. J. Pharm. Pharm. Sci. 2012, 4, 478–482. [Google Scholar]
- Patil, A.G.; Ansari, A.Y.; Koli, S.P.; Patil, D.A. HPTLC method development and validation of secondary metabolite-thymol from Sufof-E-Mohazzil: A Unani formulation. Asian J. Chem. 2013, 25, 3827–3830. [Google Scholar] [CrossRef]
- Alqarni, M.H.; Foudah, A.I.; Alam, A.; Salkini, M.A.; Alam, P.; Yusufoglu, H.S. Novel HPTLC-densitometric method for concurrent quantification of linalool and thymol in essential oils. Arabian J. Chem. 2021, 14, 102916. [Google Scholar] [CrossRef]
- Porcaro, P.J.; Johnston, V.D. Determination of thymol isomers by gas liquid chromatography using lanolin. Anal. Chem. 1962, 34, 1071–1073. [Google Scholar] [CrossRef]
- Noall, M.W.; Knight, V.; Hargrove, M.W.; Elledge, B.W. Gas chromatographic determination of thymol. Anal. Biochem. 1975, 69, 10–15. [Google Scholar] [CrossRef]
- Raghad, H.; Fadi, A.; Saleh, T.; Yaser, B. Determination of thymol in thyme extract and its pharmaceutical forms using gas chromatography method. Res. J. Pharm. Technol. 2020, 13, 4055–4060. [Google Scholar]
- Nan, G.; Zhang, L.; Liu, Z.; Liu, Y.; Du, Y.; Zhao, H.; Zheng, H.; Lin, R.; Yang, G.; Zheng, S. Quantitative determination of p-cymene, thymol, neryl acetate, and β-caryophyllene in different growth periods and and parts of Eupatorium fortunei Turcz. by GC-MS/MS. J. Anal. Methods Chem. 2021, 2021, 2174667. [Google Scholar] [CrossRef]
- Tsigouri, A.; Passaloglou-Katrali, M.; Sabatakou, O. Determination of eucalyptol, camphor, menthol and thymol in thyme honey by GC-FID. Acta Aliment. 2008, 37, 181–189. [Google Scholar] [CrossRef]
- Kohlert, C.; Abel, G.; Schmid, E.; Veit, M. Determination of thymol in human plasma by automated headspace solid-phase microextraction-gas chromatographic analysis. J. Chromatogr. B 2002, 767, 11–18. [Google Scholar] [CrossRef]
- Fiori, G.M.L.; Bonato, P.S.; Pereira, M.P.M.; Contini, S.H.T.; Pereira, A.M.S. Determination of thymol and carvacrol in plasma and milk of dairy cows using solid-phase microextraction. J. Braz. Chem. Soc. 2013, 24, 837–846. [Google Scholar] [CrossRef]
- Zhao, X.; Du, Y.; Ye, W.; Lu, D.; Xia, X.; Wang, C. Sensitive determination of thymol based on CeO2 nanoparticle-decorated graphene hybrid film. New J. Chem. 2013, 37, 4045–4051. [Google Scholar] [CrossRef]
- Vinas, P.; Soler-Romera, M.J.; Hernandez-Cordoba, M. Liquid chromatographic determination of phenol, thymol and carvacrol in honey using fluorimetric detection. Talanta 2006, 69, 1063–1067. [Google Scholar] [CrossRef]
- Chang, X.; Sun, P.; Ma, Y.; Han, D.; Zhao, Y.; Bai, Y.; Zhang, D.; Yang, L. A new method for determination of thymol and carvacrol in Thymi herba by ultraperformance convergence chromatography (UPC2). Molecules 2020, 25, 502. [Google Scholar] [CrossRef] [PubMed]
- Alqarni, M.H.; Alam, P.; Foudah, A.I.; Muharram, M.M.; Shakeel, F. Combining normal/reversed-phase HPTLC with univariate calibration for piperine quantification with traditional and ultrasound-assisted extracts of various food spices of Piper nigrum L. under green analytical chemistry viewpoint. Molecules 2021, 26, 732. [Google Scholar] [CrossRef]
- Alam, P.; Iqbal, M.; Ezzeldin, E.; Khalil, N.Y.; Foudah, A.I.; Alqarni, M.H.; Shakeel, F. Simple and accurate HPTLC-densitometry method for quantification of delafloxacin (a novel fluoroquinolone antibiotic) in plasma samples: Application to pharmacokinetic study in rats. Antibiotics 2020, 9, 134. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.A.; Elmansi, H.; Fathy, M.E. Green RP-HPLC method for simultaneous determination of moxifloxacin combinations: Investigation of the greenness for the proposed method. Microchem. J. 2019, 148, 151–161. [Google Scholar] [CrossRef]
- Abou-Taleb, N.H.; Al-Enany, N.M.; El-Sherbiny, D.T.; El-Subbagh, H.I. Digitally enhanced thin layer chromatography for simultaneous determination of norfloxacin tinidazole with the aid of Taguchi orthogonal array and desirability function approach: Greenness assessment by analytical eco-scale. J. Sep. Sci. 2020, 43, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously administered madness drops (tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, 105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A green HPLC method for determination of nine sulfonamides in milk and beef, and its greenness assessment with analytical eco-scale and greenness profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.M.; Koscielniak, P. What color is your method? Adaptation of the RGB additive color model to analytical method evaluation. Anal. Chem. 2019, 91, 10343–10352. [Google Scholar] [CrossRef]
- Byrne, F.P.; Jin, S.; Paggiola, G.; Petchey, T.H.M.; Clark, J.H.; Farmer, T.J.; Hunt, A.J.; McElory, C.R.; Sherwood, J. Tools and techniques for solvent selection: Green solvent selection guide. Sustain. Chem. Proc. 2016, 4, 7. [Google Scholar] [CrossRef]
- Validation of Analytical Procedures–Text and Methodology; International Conference on Harmonization (ICH): Geneva, Switzerland, 2005.
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem. J. 2021, 164, 105960. [Google Scholar] [CrossRef]



| Parameters | Values a |
|---|---|
| Linearity range (ng/band) | 10–2000 |
| Regression equation | y = 14.214x + 663.79 |
| R2 | 0.9991 |
| R | 0.9995 |
| Slope ± SD | 14.214 ± 0.84000 |
| Intercept ± SD | 663.79 ± 8.3900 |
| Standard error of slope | 0.34299 |
| Standard error of intercept | 3.4258 |
| 95% confidence interval of slope | 12.738–15.689 |
| 95% confidence interval of intercept | 649.04–678.53 |
| LOD ± SD (ng/band) | 10.31 ± 0.24 |
| LOQ ± SD (ng/band) | 30.93 ± 0.72 |
| Conc. (ng/band) | Conc. Found (ng/band) ± SD | Recovery (%) | CV (%) |
|---|---|---|---|
| 50 | 49.21 ± 0.54 | 98.42 | 1.09 |
| 500 | 494.65 ± 3.14 | 98.93 | 0.63 |
| 2000 | 2022.32 ± 7.21 | 101.11 | 0.35 |
| Conc. (ng/band) | Intraday Precision | Interday Precision | ||||
|---|---|---|---|---|---|---|
| Conc. (ng/band) ± SD | Standard Error | CV (%) | Conc. (ng/band) ± SD | Standard Error | CV (%) | |
| 50 | 51.23 ± 0.48 | 0.19 | 0.93 | 50.68 ± 0.50 | 0.20 | 0.98 |
| 500 | 504.64 ± 3.25 | 1.32 | 0.64 | 493.65 ± 3.08 | 1.25 | 0.62 |
| 2000 | 1987.24 ± 6.84 | 2.79 | 0.34 | 2024.21 ± 7.38 | 3.01 | 0.36 |
| Conc. (ng/band) | Mobile Phase Composition (CY-EtOAc) | Results | ||||
|---|---|---|---|---|---|---|
| Original | Used | Level | Conc. (ng/band) ± SD | % CV | Rf | |
| 87:13 | +2.0 | 485.31 ± 3.24 | 0.66 | 0.36 | ||
| 500 | 85:15 | 85:15 | 0.0 | 491.31 ± 3.54 | 0.72 | 0.37 |
| 83:17 | −2.0 | 511.25 ± 3.91 | 0.76 | 0.38 | ||
| Samples | Traditional Extraction | Ultrasound-Based Extraction |
|---|---|---|
| Amount of Thymol (%w/w) | ||
| Commercial formulation | 7.61 ± 0.62 | NA * |
| Essential oil of T. vulgaris (SA) | 40.72 ± 1.84 | NA * |
| Essential oil of T. vulgaris (IND) | 31.67 ± 1.64 | NA * |
| Essential oil of T. vulgaris (UK) | 33.81 ± 2.12 | NA * |
| Essential oil of O. vulgare (SA) | 54.32 ± 2.64 | NA * |
| Essential oil of O. vulgare (IND) | 41.10 ± 1.48 | NA * |
| Essential oil of O. vulgare (UK) | 45.60 ± 1.34 | NA * |
| Extract of T. vulgaris (SA) | 8.60 ± 0.76 | 10.55 ± 0.79 |
| Extract of T. vulgaris (IND) | 5.44 ± 0.32 | 6.85 ± 0.37 |
| Extract of T. vulgaris (UK) | 6.31 ± 0.41 | 8.49 ± 0.41 |
| Extract of O. vulgare (SA) | 18.43 ± 0.91 | 22.08 ± 1.09 |
| Extract of O. vulgare (IND) | 12.96 ± 0.84 | 15.38 ± 0.68 |
| Extract of O. vulgare (UK) | 13.58 ± 0.87 | 15.93 ± 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Ali, A.; Alshehri, S.; Ghoneim, M.M.; Alam, P. Determination of Thymol in Commercial Formulation, Essential Oils, Traditional, and Ultrasound-Based Extracts of Thymus vulgaris and Origanum vulgare Using a Greener HPTLC Approach. Molecules 2022, 27, 1164. https://doi.org/10.3390/molecules27041164
Foudah AI, Shakeel F, Alqarni MH, Ali A, Alshehri S, Ghoneim MM, Alam P. Determination of Thymol in Commercial Formulation, Essential Oils, Traditional, and Ultrasound-Based Extracts of Thymus vulgaris and Origanum vulgare Using a Greener HPTLC Approach. Molecules. 2022; 27(4):1164. https://doi.org/10.3390/molecules27041164
Chicago/Turabian StyleFoudah, Ahmed I., Faiyaz Shakeel, Mohammed H. Alqarni, Abuzer Ali, Sultan Alshehri, Mohammed M. Ghoneim, and Prawez Alam. 2022. "Determination of Thymol in Commercial Formulation, Essential Oils, Traditional, and Ultrasound-Based Extracts of Thymus vulgaris and Origanum vulgare Using a Greener HPTLC Approach" Molecules 27, no. 4: 1164. https://doi.org/10.3390/molecules27041164
APA StyleFoudah, A. I., Shakeel, F., Alqarni, M. H., Ali, A., Alshehri, S., Ghoneim, M. M., & Alam, P. (2022). Determination of Thymol in Commercial Formulation, Essential Oils, Traditional, and Ultrasound-Based Extracts of Thymus vulgaris and Origanum vulgare Using a Greener HPTLC Approach. Molecules, 27(4), 1164. https://doi.org/10.3390/molecules27041164








