Influence of Water Treatment and Wastewater Treatment on the Changes in Residues of Important Elements in Drinking Water
Abstract
:1. Introduction
2. Results
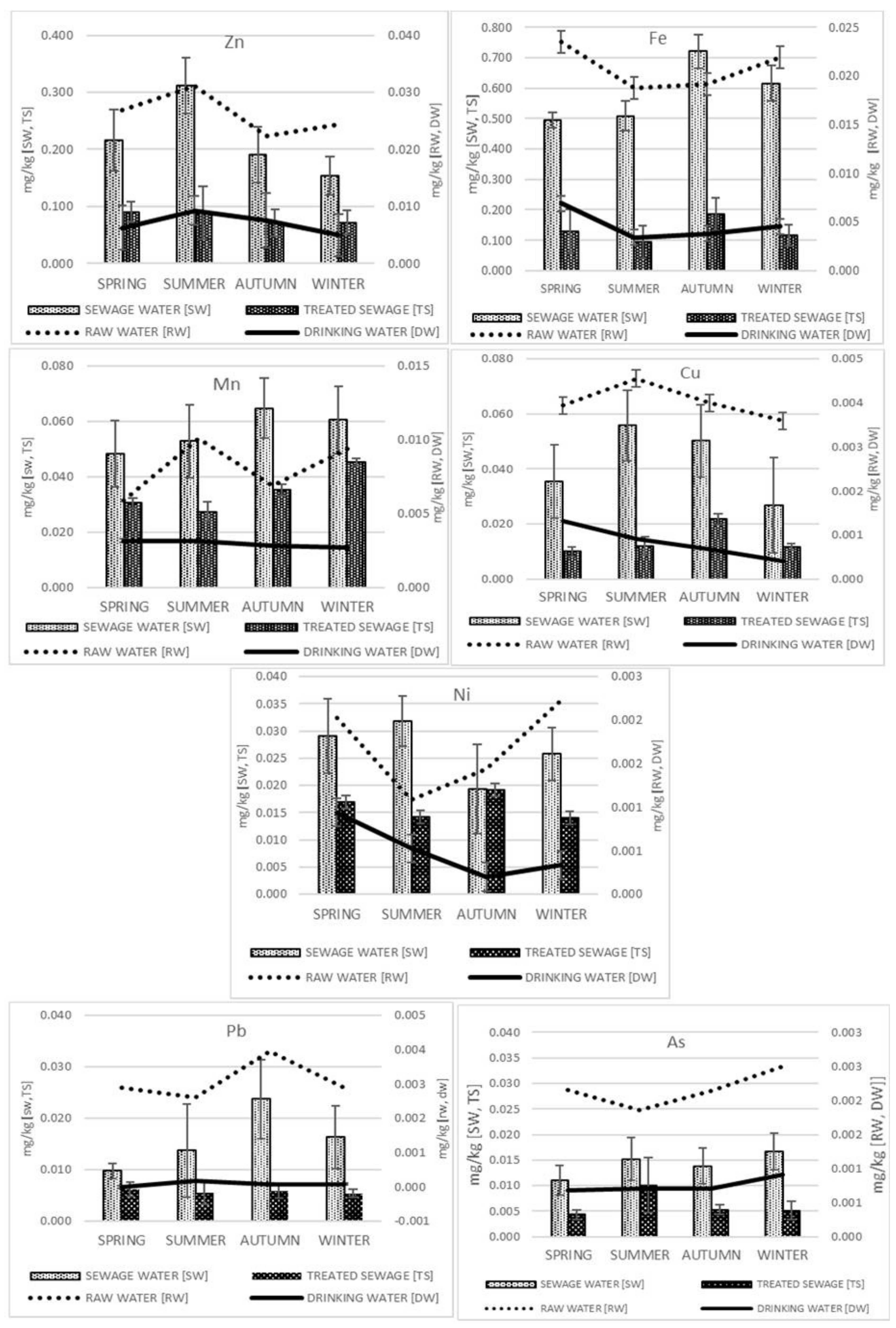
2.1. Analyzed Elements in Drinking Water and Wastewater
2.2. Analyzed Elements in Drinking Water and Wastewater
2.3. Estimating Health Risks Associated with Drinking Water Consumption
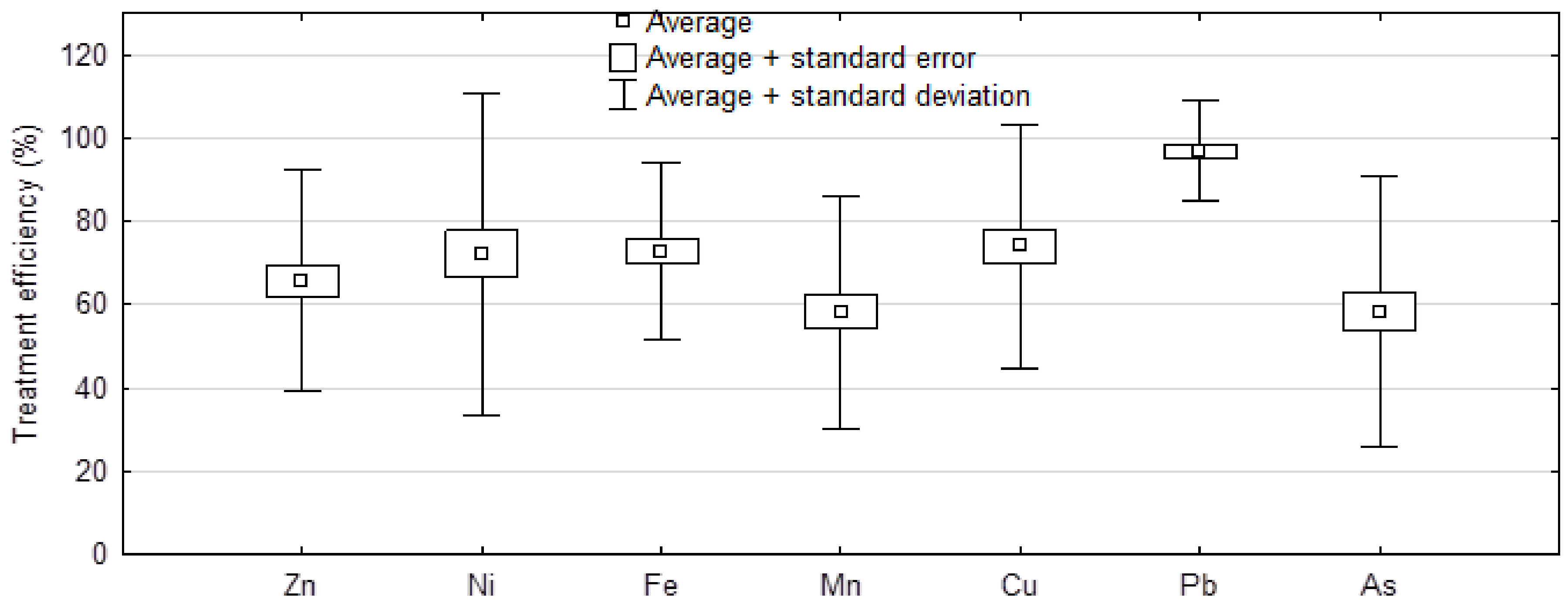
2.4. Effectiveness of Treating Wastewaters in Different Seasons of the Year
3. Discussion
4. Materials and Methods
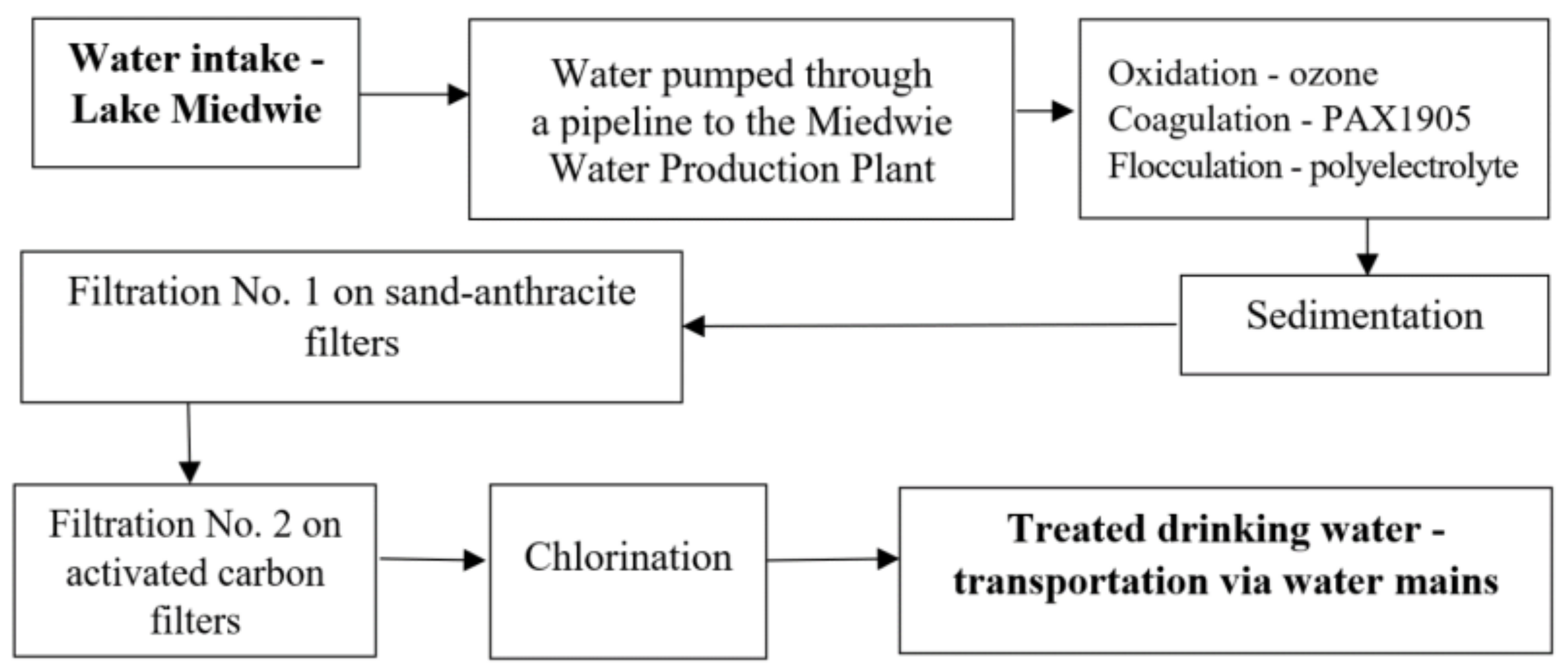
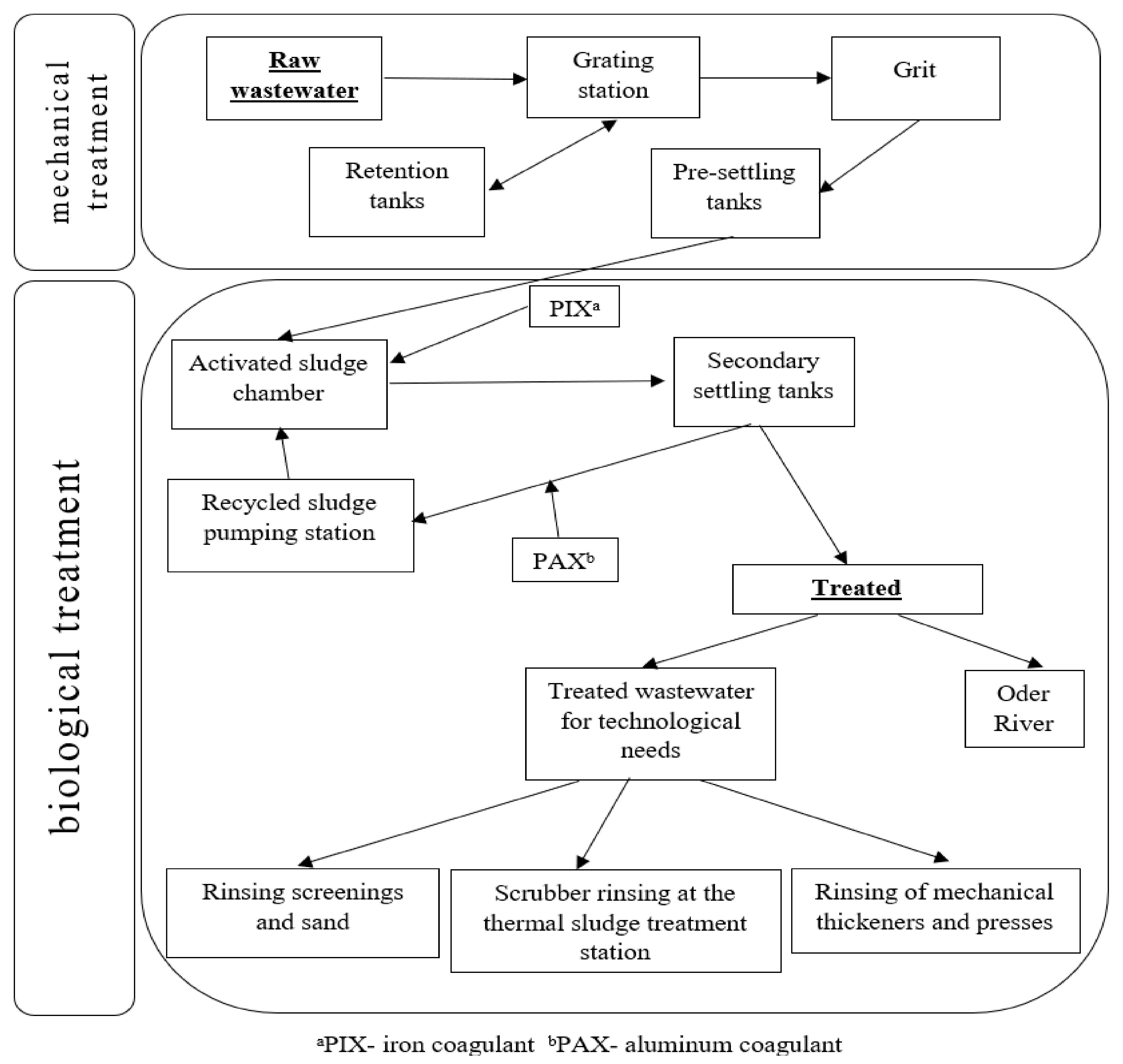
4.1. Study Material
4.1.1. Drinking Water Treatment
4.1.2. Treating Wastewater
4.2. Methods
4.2.1. Digestion and ICP-AES Analysis
4.2.2. Estimating Consumer Exposure Risk
4.2.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef] [Green Version]
- Vriens, B.; Voegelin, A.; Hug, D.J.; Kaegi, R.; Winkel, L.H.E.; Buser, M.A.; Berg, M. Quantification of element fluxes in wastewaters: A nationwide survey in Switzerland. Environ. Sci. Technol. 2017, 51, 10943–10953. [Google Scholar] [CrossRef] [PubMed]
- Stoliarenko, V.; Chernova, M.; Yakovchuk, O. Monitoring of trace element content in tap water from Karachuny Reservoir, Kryvyi Rih city. E3S Web Conf. 2020, 166, 01005. [Google Scholar] [CrossRef] [Green Version]
- Directive (EU) 2020/2184 of the European Parliament and of the Council of 16 December 2020 on the Quality of Water Intended for Human Consumption. 2020. Available online: http://data.europa.eu/eli/dir/2020/2184/oj (accessed on 25 September 2021).
- Journal of Laws, Item. 2294. Regulation of the Minister of Health of 7 December 2017 on the Quality of Water Intended for Human Consumption. 2017. Available online: http://prawo.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 19 September 2021). (In Polish)
- European Food Safety Authority (EFSA). Scientific opinion on dietary reference values for water. EFSA J. 2017, 18–38. [Google Scholar]
- Cybulski, J.; Witczak, A.; Pokorska-Niewiada, K. The effect of water and sewage treatment on reducing residues of selected organochlorine pesticides in Szczecin (Poland). Water Air Soil Pollut. 2021, 232, 310. [Google Scholar] [CrossRef]
- Yang, F.; Zhao, L.; Yan, X.; Wang, Y. Bioaccumulation of trace elements in Ruditapes philippinarum from China: Public health risk assessment implications. Int. J. Environ. Res. Public Health 2013, 10, 1392–1405. [Google Scholar] [CrossRef] [Green Version]
- Journal of Laws, Item. 1311. Regulation of the Minister of Maritime Economy and Inland Navigation of 12 July 2019 on Substances Particularly Harmful to the Aquatic Environment and the Conditions to be Met When Discharging Sewage into Waters or Ground, as Well as When Discharging Rainwater or Meltwater into Waters or into Aquatic Devices. 2019. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001311 (accessed on 28 January 2021). (In Polish)
- McComb, J.; Alexander, T.C.; Han, F.X.; Tchounwou, P.B. Understanding biogeochemical cycling of trace elements and heavy metals in estuarine ecosystems. Int. J. Environ. Bioremediat. Biodegrad. 2014, 5, 1000e148. [Google Scholar] [CrossRef]
- Janyasuthiwong, S.; Phiri, S.M.; Kijjanapanich, P.; Rene, E.R.; Esposito, G.; Lens, P.N.L. Copper, lead and zinc removal from metal contaminated wastewater by adsorption onto agricultural wastes. Environ. Technol. 2015, 36, 3071–3083. [Google Scholar] [CrossRef]
- Szczuko, M.; Splinter, J.; Zapałowska-Chwyć, M.; Ziętek, M.; Maciejewska, D. Fluorine may intensify the mechanisms of polycystic ovary syndrome (PCOS) development via increased insulin resistance and disturbed thyroid-stimulating hormone (TSH) synthesis even at reference levels. Med. Hypotheses 2019, 128, 58–63. [Google Scholar] [CrossRef]
- Al-Fartusie, F.S.; Mohssan, S.N. Essential trace elements and their vital roles in human body. Indian J. Adv. Chem. Sci. 2017, 5, 127–136. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (USEPA). Edition of the Drinking Water Standards and Health Advisories. 2006. Available online: https://agri.idaho.gov/main/wp-content/uploads/2018/03/epa_drinking_water_standard-2006.pdf (accessed on 1 December 2021).
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Standards for the Polish Population and Their Application; National Institute of Public Health—National Institute of Hygiene: Warsaw, Poland, 2020; ISBN 978-83-65870-28-5. (In Polish) [Google Scholar]
- Journal of Laws, Item. 1747. Regulation of the Minister of Maritime Economy and Inland Navigation of 29 August 2019 on the Requirements for Surface Waters Used to Supply the Population with Water Intended for Human Consumption. 2019. Available online: https://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20190001747 (accessed on 17 October 2021). (In Polish)
- Herasymowicz-Bąk, M.M.; Brzeski, K. Contamination of drinking water with metals in the city of Szczecin. Probl. Hig. Epidemiol. 2009, 90, 146–150. [Google Scholar]
- Rahman, M.A.; Kumar, S.; Lamb, D.; Rahman, M.M. Health risk assessment of arsenic, manganese, and iron from drinking water for high school children. Water Air Soil Pollut. 2021, 232, 269. [Google Scholar] [CrossRef]
- Birke, M.; Rauch, U.; Harazim, B.; Lorenz, H.; Glatte, W. Major and trace elements in German bottled water, their regional distribution, and accordance with national and international standards. J. Geochem. Explor. 2010, 107, 245–271. [Google Scholar] [CrossRef]
- Richir, J.; Gobert, S. Trace elements in marine environments: Occurrence, threats and monitoring with special focus on the Coastal Mediterranean. J. Environ. Anal. Toxicol. 2016, 6, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Poonkothai, M.; Vijayavathi, S. Nickel as an essential element and a toxicant. Int. J. Eng. Sci. Technol. 2012, 1, 285–288. [Google Scholar]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef] [Green Version]
- Ibezim-Ezeani, M.U.; Ihunwo, O.C. Assessment of Pb, Cd, Cr and Ni in water and water hyacinth (Eichhornia crassipes) plant from Woji Creek, Rivers State, Nigeria. J. Appl. Sci. Environ. Manag. 2020, 24, 719–727. [Google Scholar] [CrossRef]
- Turkish Water Standard (TDWS). Water Intended for Human Consumption. 2005. Available online: http://rega.basbakanlik.gov.tr/eskiler/2005/02/20050217-3.htm (accessed on 12 November 2021).
- Yuce, G.; Alptekin, C. In situ and laboratory treatment tests for lowering of excess manganese and iron in drinking water sourced from river–groundwater interaction. Environ. Earth Sci. 2013, 70, 2827–2837. [Google Scholar] [CrossRef]
- Rio, S.; Martin, P. Removal of metal ions from aqueous solution by adsorption onto low-cost biosorbent. Environ. Technol. 2012, 33, 2211–2215. [Google Scholar] [CrossRef]
- Etxabe, I.Z.; Cotin, K.C.; Olalde, C.O.; Alonso, J.V. Release of lead and other metals from piping into drinking water in the Basque Country (Spain). Gac. Sanit. 2010, 24, 460–465. [Google Scholar] [CrossRef] [Green Version]
- Haider, T.; Haider, M.; Wruss, W.; Sommer, R.; Kundi, M. Lead in drinking water of Vienna in comparison to other European countries and accordance with recent guidelines. Int. J. Hyg. Environ. Health 2002, 205, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.; Pittman, C.U., Jr. Arsenic removal from water/wastewater using adsorbents—A critical review. J. Hazard. Mater. 2007, 142, 1–53. [Google Scholar] [CrossRef] [PubMed]
- Kelepertsis, A.; Alexakis, D.; Skordas, K. Arsenic, antimony and other toxic elements in the drinking water of Eastern Thessaly in Greece and its possible effects on human health. Environ. Geol. 2006, 50, 76–84. [Google Scholar] [CrossRef]
- Jovanovic, D.; Jakovijevic, B.; Ragic-Milutinovic, Z.; Paunovic, K.; Pekovic, G.; Knezevic, T. Arsenic occurrence in drinking water supply systems in ten municipalities in Vojvodina Region, Serbia. Environ. Res. 2011, 111, 315–318. [Google Scholar] [CrossRef]
- Cavar, S.; Klapec, T.; Grubesic, R.J.; Valek, M. High exposure to arsenic from drinking water at several localities in eastern Croatia. Sci. Total Environ. 2005, 339, 277–282. [Google Scholar] [CrossRef]
- Tamasi, G.; Cini, R. Heavy metals in drinking water from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the province of Siena. Sci. Total Environ. 2004, 327, 41–51. [Google Scholar] [CrossRef]
- Szczecin Water and Sewerage Department. Parameters of the water tested. Unpublished work. ZWiK: Szczecin, Poland, 2019. [Google Scholar]
- Górski, J.; Siepak, M. Assessment of metal concentrations in tap-water—From source to the tap: A case study from Szczecin, Poland. Geologos 2014, 20, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Polish Norm (PN-EN ISO 15587-2:2005). Water Quality—Digestion for the Determination of Selected Elements in Water—Part 2: Nitric acid Digestion. 2005. (Polska Norma (PN-EN ISO 15587-2:2005), Jakość Wody—Mineralizacja w Celu Oznaczania Wybranych Pierwiastków w Wodzie—Część 2: Mineralizacja Kwasem Azotowym ISO 15587-2:2005). (Polish Article). Available online: https://sklep.pkn.pl/pn-en-iso-15587-2-2005p.html (accessed on 12 November 2021).
- Szczecin Water and Sewerage Department. Parameters of the water tested. Unpublished work. ZWiK: Szczecin, Poland, 2018. [Google Scholar]


| Trace Elements | Raw Drinking Water n = 288 | Drinking Water n = 288 | Raw Wastewater n = 288 | Treated Wastewater n = 288 | |
|---|---|---|---|---|---|
| Zn | ± SD Me (xmin–xmax) > LOD% | 0.026 ± 0.014 0.023 (0.008–0.069) 100% | 0.007 ± 0.004 0.008 (< LOD–0.021) 98% | 0.218 ± 0.096 0.207 (0.088–0.536) 100% | 0.080 ± 0.030 0.074 (0.035–0.192) 100% |
| Ni | ± SD Me (xmin–xmax) > LOD% | 0.002 ± 0.001 0.002 (< LOD–0.004) 98% | 0.001 ± 0.001 0.0004 (< LOD–0.003) 52% | 0.026 ± 0.012 0.023 (0.010–0.068) 100% | 0.016 ± 0.006 0.015 (0.006–0.041) 100% |
| Fe | ± SD Me (xmin–xmax) > LOD% | 0.021 ± 0.012 0.019 (0.002–0.068) 100% | 0.005 ± 0.003 0.004 (0.001–0.010) 100% | 0.585 ± 0.480 0.344 (0.058–1.863) 100% | 0.131 ± 0.077 0.116 (0.028–0.377) 100% |
| Mn | ± SD Me (xmin–xmax) > LOD% | 0.008 ± 0.004 0.007 (0.003–0.020) 100% | 0.003 ± 0.002 0.003 (< LOD–0.007) 91% | 0.057 ± 0.022 0.053 (0.021–0.135) 100% | 0.035 ± 0.033 0.029 (0.012–0.240) 100% |
| Cu | ± SD Me (xmin–xmax) > LOD% | 0.004 ± 0.002 0.004 (< LOD–0.008) 98% | 0.001 ± 0.001 0.001 (< LOD–0.005) 69% | 0.042 ± 0.023 0.039 (0.011–0.090) 100% | 0.014 ± 0.007 0.011 (0.008–0.040) 100% |
| Pb | ± SD Me (xmin–xmax) > LOD% | 0.003 ± 0.001 0.003 (0.0005–0.006) 100% | 0.0001 ± 0.0003 0 (< LOD–0.001) 8% | 0.016 ± 0.013 0.01 (< LOD–0.056) 98% | 0.006 ± 0.001 0.006 (0.003–0.011) 100% |
| As | ± SD Me (xmin–xmax) > LOD% | 0.002 ± 0.001 0.002 (0.0001–0.004) 100% | 0.001 ± 0.0005 0.001 (< LOD–0.002) 77% | 0.014 ± 0.004 0.014 (0.007–0.022) 100% | 0.006 ± 0.004 0.005 (0.003–0.018) 100% |
| Parameter | Zn | Ni | Fe | Mn | Cu | Pb | As | Zn | Ni | Fe | Mn | Cu | Pb | As |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Summer | |||||||||||||
| Drinking Water | ||||||||||||||
| NH4+ (mg L−1) | −0.377 | 0.165 | 0.100 | 0.087 | 0.381 | n.d. | −0.193 | 0.000 | −0.210 | −0.110 | −0.078 | −0.187 | 0.194 | 0.272 |
| NO3− (mg L−1) | 0.242 | −0.246 | −0.135 | 0.121 | −0.371 | n.d. | 0.137 | 0.360 | 0.126 | −0.284 | −0.329 | 0.169 | −0.316 | 0.093 |
| NO2− (mg L−1) | −0.377 | 0.165 | 0.100 | 0.087 | 0.381 | n.d. | −0.193 | 0.089 | −0.037 | −0.331 | −0.425 | −0.307 | 0.226 | 0.425 |
| COD (mg O2 L−1) | 0.119 | −0.015 | 0.130 | 0.235 | −0.210 | n.d. | 0.136 | 0.294 | −0.401 | 0.179 | 0.057 | 0.323 | −0.387 | 0.189 |
| ClO2 (mg L−1) | −0.382 | 0.156 | 0.082 | 0.098 | 0.374 | n.d. | −0.191 | 0.072 | 0.108 | 0.749 | 0.542 | 0.180 | −0.158 | −0.083 |
| Raw Drinking Water | ||||||||||||||
| NH4+ (mg L−1) | −0.436 | −0.134 | −0.129 | 0.377 | −0.833 | −0.418 | −0.375 | 0.836 | 0.254 | 0.029 | −0.465 | 0.193 | −0.630 | −0.307 |
| NO3− (mg L−1) | 0.029 | 0.488 | 0.485 | −0.319 | 0.533 | 0.666 | 0.739 | −0.734 | −0.274 | −0.622 | −0.252 | −0.186 | 0.452 | 0.308 |
| NO2− (mg L−1) | −0.162 | −0.558 | −0.459 | 0.300 | −0.531 | −0.781 | −0.640 | 0.854 | 0.223 | −0.063 | −0.489 | 0.088 | −0.634 | −0.057 |
| COD (mg O2 L−1) | −0.394 | −0.252 | −0.286 | 0.407 | −0.401 | −0.622 | −0.498 | 0.056 | 0.084 | 0.547 | 0.528 | 0.040 | −0.279 | −0.578 |
| Treated Wastewater | ||||||||||||||
| COD (mg O2 L−1) | 0.073 | −0.476 | −0.086 | −0.012 | −0.378 | 0.283 | 0.363 | 0.918 | −0.452 | −0.392 | 0.168 | −0.427 | −0.208 | −0.507 |
| BOD5 (mg L−1) | 0.720 | 0.261 | −0.155 | 0.059 | 0.291 | −0.237 | −0.033 | 0.510 | 0.011 | 0.331 | 0.541 | −0.309 | −0.292 | −0.528 |
| P,total (mg L−1) | −0.046 | 0.492 | 0.081 | 0.014 | 0.394 | −0.296 | −0.368 | 0.463 | −0.241 | −0.109 | −0.101 | −0.237 | −0.199 | −0.547 |
| N,total (mg L−1) | −0.738 | −0.417 | 0.138 | −0.066 | −0.419 | 0.334 | 0.143 | 0.133 | −0.078 | 0.120 | −0.155 | −0.081 | −0.186 | −0.425 |
| Raw Wastewater | ||||||||||||||
| COD (mg O2 L−1) | −0.363 | −0.027 | −0.192 | 0.072 | −0.435 | −0.236 | −0.139 | 0.113 | −0.080 | −0.385 | −0.452 | −0.001 | 0.173 | −0.406 |
| BOD5 (mg L−1) | −0.319 | −0.300 | 0.015 | 0.239 | −0.246 | −0.244 | −0.070 | 0.050 | 0.068 | −0.310 | −0.392 | 0.016 | 0.254 | −0.302 |
| P,total (mg L−1) | −0.331 | 0.174 | −0.307 | −0.060 | −0.494 | −0.188 | −0.165 | −0.009 | 0.077 | −0.661 | −0.650 | −0.249 | 0.304 | −0.240 |
| N,total (mg L−1) | −0.178 | 0.440 | −0.395 | −0.252 | −0.436 | −0.054 | −0.155 | −0.100 | 0.239 | −0.670 | −0.637 | −0.313 | 0.394 | −0.078 |
| Autumn | Winter | |||||||||||||
| Drinking Water | ||||||||||||||
| NH4 + (mg L−1) | −0.108 | −0.015 | −0.546 | 0.073 | 0.714 | −0.213 | −0.158 | −0.544 | 0.158 | −0.211 | 0.234 | 0.076 | −0.135 | 0.058 |
| NO3− (mg L−1) | −0.161 | 0.238 | −0.528 | 0.197 | 0.611 | −0.254 | −0.332 | −0.494 | 0.060 | 0.012 | 0.017 | 0.033 | −0.484 | −0.245 |
| NO2− (mg L−1) | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 0.038 | 0.125 | 0.593 | 0.626 | −0.070 | −0.426 | −0.368 |
| COD (mg O2 L−1) | −0.096 | −0.457 | −0.069 | −0.274 | −0.127 | −0.161 | −0.030 | −0.089 | −0.263 | 0.005 | 0.024 | −0.195 | 0.056 | −0.024 |
| ClO2 (mg L−1) | 0.352 | 0.157 | 0.328 | 0.205 | 0.115 | 0.302 | 0.310 | −0.038 | −0.125 | −0.593 | −0.626 | 0.070 | 0.426 | 0.368 |
| Raw Drinking Water | ||||||||||||||
| NH4 + (mg L−1) | 0.576 | −0.234 | 0.109 | −0.686 | 0.000 | 0.117 | 0.405 | 0.008 | 0.064 | 0.143 | −0.057 | 0.599 | −0.176 | 0.000 |
| NO3− (mg L−1) | −0.375 | 0.189 | −0.375 | −0.095 | −0.110 | −0.226 | 0.015 | 0.103 | 0.454 | 0.218 | −0.609 | −0.419 | −0.287 | −0.346 |
| NO2− (mg L−1) | 0.314 | 0.097 | 0.234 | −0.025 | 0.366 | 0.500 | 0.108 | 0.393 | 0.483 | 0.190 | 0.820 | −0.068 | 0.325 | 0.170 |
| COD (mg O2 L−1) | 0.576 | −0.234 | 0.109 | −0.686 | 0.000 | 0.117 | 0.405 | 0.075 | −0.403 | 0.145 | −0.067 | 0.387 | 0.300 | 0.204 |
| Treated Wastewater | ||||||||||||||
| COD (mg O2 L−1) | −0.738 | 0.639 | −0.515 | −0.226 | 0.067 | 0.345 | 0.288 | −0.241 | 0.756 | 0.610 | 0.025 | −0.486 | 0.566 | 0.445 |
| BOD5 (mg L−1) | 0.047 | −0.245 | 0.107 | −0.621 | 0.293 | 0.290 | 0.053 | 0.072 | −0.401 | −0.706 | 0.463 | −0.055 | −0.686 | −0.147 |
| P,total (mg L−1) | −0.721 | 0.278 | −0.485 | −0.675 | 0.131 | 0.301 | 0.137 | 0.504 | 0.068 | 0.538 | −0.103 | 0.547 | 0.118 | 0.110 |
| N,total (mg L−1) | −0.046 | 0.744 | 0.080 | 0.078 | 0.469 | 0.708 | 0.479 | −0.149 | −0.693 | −0.409 | −0.421 | 0.277 | −0.119 | −0.603 |
| Raw Wastewater | ||||||||||||||
| COD (mg O2 L−1) | −0.328 | −0.316 | 0.063 | 0.398 | 0.303 | 0.131 | −0.348 | 0.272 | −0.406 | −0.056 | −0.163 | 0.378 | 0.088 | −0.637 |
| BOD5 (mg L−1) | −0.367 | −0.136 | −0.094 | 0.417 | 0.203 | −0.043 | −0.197 | 0.006 | −0.405 | −0.579 | −0.579 | 0.363 | −0.421 | −0.766 |
| P,total (mg L−1) | 0.084 | −0.201 | 0.367 | 0.508 | 0.359 | 0.349 | −0.183 | 0.355 | −0.159 | −0.027 | −0.057 | 0.276 | 0.010 | −0.182 |
| N,total (mg L−1) | −0.103 | −0.452 | 0.344 | 0.380 | 0.469 | 0.380 | −0.449 | 0.265 | 0.031 | −0.035 | −0.004 | 0.165 | −0.102 | 0.140 |
| Trace Element | p-Value | Trace Element | p-Value | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spring | Spring | Spring | Summer | Summer | Autumn | Spring | Spring | Spring | Summer | Summer | Autumn | ||
| vs. | vs. | vs. | vs. | vs. | vs. | vs. | vs. | vs. | vs. | vs. | vs. | ||
| Summer | Autumn | Winter | Autumn | Winter | Winter | Summer | Autumn | Winter | Autumn | Winter | Winter | ||
| Drinking Water | Treated Wastewater | ||||||||||||
| Zn | 0.236 | 0.87 | 0.833 | 0.659 | 0.040 | 0.384 | Zn | 0.998 | 0.413 | 0.397 | 0.52 | 0.503 | 1 |
| Ni | 0.357 | 0.024 | 0.085 | 0.56 | 0.865 | 0.95 | Ni | 0.719 | 0.828 | 0.687 | 0.239 | 1 | 0.218 |
| Fe | 0.779 | 0.915 | 0.948 | 0.921 | 0.935 | 0.927 | Fe | 0.658 | 0.223 | 0.972 | 0.017 | 0.888 | 0.096 |
| Mn | 1 | 0.969 | 0.918 | 0.978 | 0.935 | 0.998 | Mn | 0.995 | 0.985 | 0.695 | 0.937 | 0.549 | 0.878 |
| Cu | 0.736 | 0.371 | 0.109 | 0.929 | 0.571 | 0.902 | Cu | 0.832 | 0 | 0.902 | 0.001 | 0.999 | 0.001 |
| Pb | 0.477 | 0.887 | 0.887 | 0.887 | 0.887 | 0.854 | Pb | 0.76 | 0.928 | 0.577 | 0.982 | 0.99 | 0.906 |
| As | 1 | 1 | 0.676 | 1 | 0.744 | 0.725 | As | 0 | 0.837 | 0.898 | 0.001 | 0.001 | 0.999 |
| Raw Drinking Water | Raw Wastewater | ||||||||||||
| Zn | 0.871 | 0.851 | 0.972 | 0.407 | 0.632 | 0.982 | Zn | 0.021 | 0.857 | 0.207 | 0.002 | 0 | 0.631 |
| Ni | 0.05 | 0.353 | 0.944 | 0.75 | 0.012 | 0.133 | Ni | 0.93 | 0.153 | 0.884 | 0.041 | 0.546 | 0.493 |
| Fe | 0.775 | 0.812 | 0.988 | 1 | 0.922 | 0.943 | Fe | 1 | 0.666 | 0.927 | 0.711 | 0.949 | 0.952 |
| Mn | 0.027 | 0.895 | 0.078 | 0.133 | 0.968 | 0.301 | Mn | 0.959 | 0.276 | 0.534 | 0.553 | 0.828 | 0.965 |
| Cu | 0.854 | 1 | 0.969 | 0.887 | 0.598 | 0.952 | Cu | 0.078 | 0.288 | 0.726 | 0.905 | 0.006 | 0.033 |
| Pb | 0.938 | 0.131 | 1 | 0.036 | 0.942 | 0.127 | Pb | 0.848 | 0.034 | 0.545 | 0.194 | 0.953 | 0.446 |
| As | 0.867 | 1 | 0.807 | 0.885 | 0.352 | 0.784 | As | 0.032 | 0.225 | 0.002 | 0.797 | 0.733 | 0.225 |
| Spring | Summer | Autumn | Winter | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zn | Ni | Fe | Mn | Cu | Pb | Zn | Ni | Fe | Mn | Cu | Pb | Zn | Ni | Fe | Mn | Cu | Pb | Zn | Ni | Fe | Mn | Cu | Pb | |
| Raw Wastewater | ||||||||||||||||||||||||
| Ni | −0.248 | −0.104 | 0.132 | −0.111 | ||||||||||||||||||||
| Fe | −0.147 | −0.471 | 0.004 | −0.028 | 0.571 | −0.363 | 0.111 | 0.157 | ||||||||||||||||
| Mn | 0.163 | −0.567 | 0.484 | 0.156 | 0.157 | 0.811 | 0.093 | 0.218 | 0.510 | 0.456 | 0.528 | 0.769 | ||||||||||||
| Cu | 0.072 | −0.409 | 0.328 | 0.265 | 0.555 | 0.045 | 0.485 | 0.438 | 0.050 | −0.666 | 0.390 | 0.064 | 0.340 | −0.406 | 0.231 | 0.050 | ||||||||
| Pb | −0.435 | 0.313 | 0.355 | 0.252 | 0.116 | −0.229 | 0.017 | −0.307 | −0.256 | 0.007 | 0.535 | −0.399 | 0.568 | −0.045 | 0.054 | −0.196 | 0.047 | 0.666 | 0.389 | −0.031 | ||||
| As | 0.056 | −0.315 | 0.048 | 0.197 | 0.781 | −0.113 | −0.526 | 0.292 | 0.036 | 0.145 | −0.186 | 0.487 | 0.385 | 0.815 | −0.199 | 0.258 | −0.606 | −0.106 | 0.043 | 0.330 | 0.494 | 0.489 | 0.083 | 0.414 |
| Treated Wastewater | ||||||||||||||||||||||||
| Ni | 0.343 | −0.647 | −0.284 | −0.064 | ||||||||||||||||||||
| Fe | −0.449 | 0.183 | −0.512 | 0.453 | 0.566 | −0.032 | 0.003 | 0.347 | ||||||||||||||||
| Mn | −0.118 | −0.324 | −0.283 | 0.156 | 0.263 | 0.449 | 0.264 | 0.108 | 0.509 | −0.279 | 0.227 | −0.233 | ||||||||||||
| Cu | 0.218 | 0.034 | −0.424 | 0.645 | −0.335 | 0.205 | 0.116 | −0.051 | 0.132 | 0.411 | 0.744 | 0.231 | 0.316 | −0.521 | 0.228 | −0.332 | ||||||||
| Pb | −0.254 | −0.034 | −0.094 | 0.311 | −0.210 | −0.246 | 0.521 | −0.278 | −0.305 | 0.383 | −0.048 | 0.464 | 0.031 | −0.370 | 0.258 | 0.018 | 0.344 | 0.665 | −0.154 | −0.130 | ||||
| As | −0.156 | −0.108 | 0.598 | −0.211 | −0.387 | 0.243 | −0.292 | −0.421 | 0.169 | −0.162 | 0.049 | −0.415 | −0.170 | 0.073 | −0.209 | 0.004 | −0.114 | 0.558 | −0.104 | 0.400 | 0.385 | 0.545 | −0.184 | 0.404 |
| Raw Water | ||||||||||||||||||||||||
| Ni | −0.052 | 0.160 | −0.137 | 0.305 | ||||||||||||||||||||
| Fe | −0.167 | 0.648 | 0.315 | 0.101 | 0.274 | 0.032 | 0.593 | 0.112 | ||||||||||||||||
| Mn | −0.782 | −0.121 | 0.248 | −0.206 | −0.016 | 0.508 | −0.097 | −0.069 | 0.374 | 0.313 | 0.210 | 0.070 | ||||||||||||
| Cu | 0.331 | 0.382 | 0.203 | −0.256 | 0.247 | 0.633 | 0.343 | 0.184 | 0.111 | −0.192 | 0.581 | 0.273 | 0.063 | 0.131 | −0.004 | 0.231 | ||||||||
| Pb | 0.005 | 0.533 | 0.336 | −0.111 | −0.562 | 0.205 | −0.169 | 0.124 | 0.141 | −0.094 | 0.641 | 0.093 | −0.349 | −0.064 | −0.025 | −0.310 | −0.192 | 0.230 | −0.313 | |||||
| As | −0.145 | 0.695 | 0.673 | −0.002 | 0.566 | 0.774 | 0.015 | −0.244 | −0.162 | 0.002 | −0.177 | 0.088 | 0.356 | 0.244 | −0.154 | −0.268 | −0.301 | 0.070 | 0.042 | −0.263 | 0.091 | 0.207 | 0.490 | 0.143 |
| Drinking Water | ||||||||||||||||||||||||
| Ni | −0.018 | −0.458 | 0.067 | −0.106 | ||||||||||||||||||||
| Fe | −0.117 | 0.433 | 0.159 | 0.124 | −0.016 | 0.377 | 0.424 | −0.097 | ||||||||||||||||
| Mn | −0.492 | 0.060 | 0.207 | −0.095 | 0.074 | 0.160 | 0.064 | 0.022 | −0.418 | −0.198 | 0.371 | 0.458 | ||||||||||||
| Cu | −0.106 | 0.235 | 0.437 | 0.467 | 0.208 | 0.034 | 0.086 | 0.246 | −0.148 | 0.025 | −0.278 | 0.175 | 0.234 | −0.191 | 0.013 | −0.453 | ||||||||
| Pb | 0.016 | 0.141 | 0.288 | 0.241 | 0.153 | −0.866 | 0.548 | −0.301 | 0.090 | −0.033 | 0.036 | 0.516 | 0.272 | 0.031 | 0.170 | 0.090 | −0.213 | −0.386 | −0.326 | 0.465 | ||||
| As | 0.125 | 0.038 | 0.015 | 0.185 | 0.213 | 0.108 | 0.554 | −0.561 | 0.066 | −0.266 | −0.457 | −0.550 | 0.111 | −0.091 | 0.387 | −0.259 | −0.331 | 0.214 | 0.145 | −0.460 | −0.256 | −0.307 | 0.221 | 0.039 |
| Trace Element | ADI (mg kg−1) | PTWI (µg per kg per Week) | PTWI1 | PTWI2 | PTWI3 | PTWI4 | Mean Concentration (µg L−1 of Drinking Water) | PTWI1 (%) | PTWI2 (%) | PTWI3 (%) | PTWI4 (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 0.0021 | 15 | 900 | 1050 | 180 | 570 | 1 | 1.56 | 1.67 | 5.06 | 2.46 |
| Pb | 0.0036 | 25 | 1500 | 1750 | 300 | 950 | 0.1 | 0.09 | 0.10 | 0.30 | 0.15 |
| Ni | 0.005 | 35 | 2100 | 2450 | 420 | 1330 | 1 | 0.67 | 0.71 | 2.17 | 1.05 |
| Zn | 0.1 | 700 | 42,000 | 49,000 | 8400 | 26,600 | 7 | 0.23 | 0.25 | 0.76 | 0.37 |
| Mn | 0.14 | 980 | 58,800 | 68,600 | 11,760 | 37,240 | 3 | 0.07 | 0.08 | 0.23 | 0.11 |
| Cu | 0.5 | 3500 | 210,000 | 245,000 | 42,000 | 133,000 | 1 | 0.01 | 0.01 | 0.02 | 0.01 |
| Fe | 0.8 | 5600 | 336,000 | 392,000 | 67,200 | 212,800 | 5 | 0.02 | 0.02 | 0.07 | 0.03 |
| Trace Element | RW (mg) | DW (mg) | NRV DW (mg) | NRV RW (%) | NRV DW (%) | Difference in NRV (%) | Difference in NRV (%) |
|---|---|---|---|---|---|---|---|
| Zn | 0.026 | 0.007 | 8 (W) 11 (M) | 0.32 0.23 | 0.09 0.06 | 0.23 0.17 | 71.88 73.91 |
| Ni | 0.002 | 0.001 | - | - | - | - | |
| Fe | 0.021 | 0.005 | 10 (M) 18 (W) | 0.21 0.11 | 0.05 0.03 | 0.16 0.08 | 76.19 72.72 |
| Mn | 0.008 | 0.003 | 1.8 (W) 2.3 (M) | 0.44 0.34 | 0.16 0.13 | 0.28 0.21 | 63.63 61.76 |
| Cu | 0.004 | 0.001 | 0.9 (W,M) | 0.44 | 0.11 | 0.33 | 75.0 |
| Pb | 0.003 | 0.0001 | - | - | - | - | |
| As | 0.002 | 0.001 | - | - | - | - |
| Trace Element | MAC—Raw Drinking Water a | MAC—Drinking Water b | MAC—Treated Wastewater c |
|---|---|---|---|
| Content, mg L−1 | |||
| As | 0.05 | 0.01 | 0.1 |
| Cu | 0.05 | 2 | 0.5 |
| Ni | 0.05 | 0.02 | 0.5 |
| Pb | 0.05 | 0.005 | 0.5 |
| Mn | 0.05 | 0.05 | - |
| Fe | 0.3 | 0.2 | 10 |
| Zn | 3 | - | 2 |
| Parameter | Spring | Summer | Autumn | Winter | ||||
|---|---|---|---|---|---|---|---|---|
| SD | SD | SD | SD | |||||
| Drinking Water | ||||||||
| pH | 6.80 | 0.20 | 7.50 | 0.06 | 7.55 | 0.10 | 7.77 | 0.05 |
| Alkalinity (mmol L−1) | 3.052 | 0.442 | 3.383 | 0.072 | 3.100 | 0.135 | 3.017 | 0.039 |
| NH4+ (mg L−1) | 0.388 | 0.814 | 0.027 | 0.010 | 0.027 | 0.010 | 0.023 | 0.008 |
| NO3− (mg L−1) | 2.963 | 0.405 | 3.487 | 0.172 | 2.360 | 0.744 | 1.898 | 0.051 |
| NO2− (mg L−1) | 0.359 | 0.837 | n.d. | n.d. | n.d. | n.d. | 0.001 | 0.000 |
| COD (mg O2 L−1) | 2.73 | 0.29 | 2.63 | 0.10 | 2.67 | 0.13 | 2.48 | 0.09 |
| Raw Drinking Water | ||||||||
| pH | 8.22 | 0.04 | 7.75 | 0.13 | 7.78 | 0.19 | 8.13 | 0.08 |
| NH4+ (mg L−1) | 0.097 | 0.014 | 0.093 | 0.023 | 0.093 | 0.010 | 0.090 | 0.010 |
| NO3− (mg L−1) | 2.570 | 0.216 | 3.027 | 0.277 | 2.145 | 0.930 | 1.692 | 0.070 |
| NO2− (mg L−1) | 0.010 | 0.004 | 0.018 | 0.021 | 0.009 | 0.002 | 0.005 | 0.002 |
| COD (mg O2 L−1) | 6.98 | 0.13 | 6.82 | 0.14 | 6.67 | 0.05 | 6.57 | 0.10 |
| Treated Wastewater | ||||||||
| pH | 7.70 | 0.06 | 7.76 | 0.10 | 7.69 | 0.14 | 7.66 | 0.10 |
| Alkalinity (mmol L−1) | 4.967 | 2.403 | 2.467 | 1.822 | 4.683 | 2.805 | 4.905 | 1.716 |
| NO3− (mg L−1) | 0.762 | 0.137 | 0.587 | 0.200 | 0.659 | 0.066 | 0.599 | 0.087 |
| NO2− (mg L−1) | 7.53 | 0.36 | 6.74 | 1.32 | 8.20 | 1.08 | 8.67 | 0.99 |
| COD (mg O2 L−1) | 34.50 | 1.17 | 27.56 | 3.15 | 27.14 | 1.58 | 26.28 | 1.88 |
| BOD5 (mg O2 L−1) | 3.58 | 0.60 | 2.95 | 0.75 | 4.95 | 1.56 | 8.01 | 2.51 |
| Raw Wastewater | ||||||||
| pH | 7.92 | 0.04 | 7.82 | 0.21 | 7.81 | 0.12 | 8.00 | 0.11 |
| Alkalinity (mmol L−1) | 310.89 | 17.34 | 294.78 | 107.07 | 397.22 | 34.40 | 409.67 | 64.98 |
| NO3− (mg L−1) | 10.11 | 0.97 | 8.46 | 2.55 | 9.49 | 1.02 | 8.89 | 0.85 |
| NO2− (mg L−1) | 82.09 | 0.61 | 73.53 | 21.04 | 85.13 | 9.79 | 76.23 | 9.46 |
| COD (mg O2 L−1) | 885.6 | 60.48 | 778.1 | 185.02 | 909.4 | 106.71 | 924.1 | 94.77 |
| BOD5 (mg O2 L−1) | 437.50 | 64.40 | 385.14 | 132.04 | 425.14 | 96.22 | 378.61 | 95.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cybulski, J.; Witczak, A.; Pokorska-Niewiada, K. Influence of Water Treatment and Wastewater Treatment on the Changes in Residues of Important Elements in Drinking Water. Molecules 2022, 27, 972. https://doi.org/10.3390/molecules27030972
Cybulski J, Witczak A, Pokorska-Niewiada K. Influence of Water Treatment and Wastewater Treatment on the Changes in Residues of Important Elements in Drinking Water. Molecules. 2022; 27(3):972. https://doi.org/10.3390/molecules27030972
Chicago/Turabian StyleCybulski, Jacek, Agata Witczak, and Kamila Pokorska-Niewiada. 2022. "Influence of Water Treatment and Wastewater Treatment on the Changes in Residues of Important Elements in Drinking Water" Molecules 27, no. 3: 972. https://doi.org/10.3390/molecules27030972
APA StyleCybulski, J., Witczak, A., & Pokorska-Niewiada, K. (2022). Influence of Water Treatment and Wastewater Treatment on the Changes in Residues of Important Elements in Drinking Water. Molecules, 27(3), 972. https://doi.org/10.3390/molecules27030972






