The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide
Abstract
:1. Introduction
2. Results
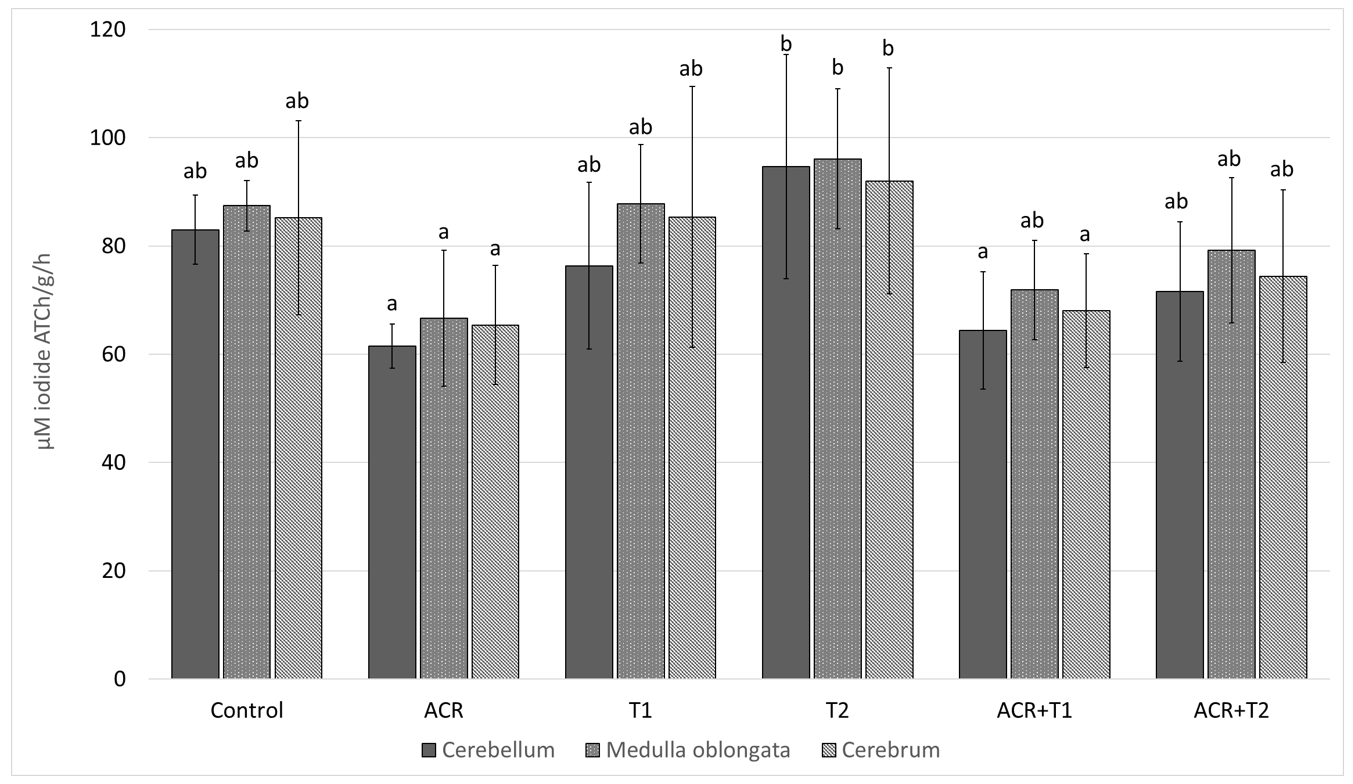
2.1. The Influence of Tocopherol on Acetylcholinesterase Activity
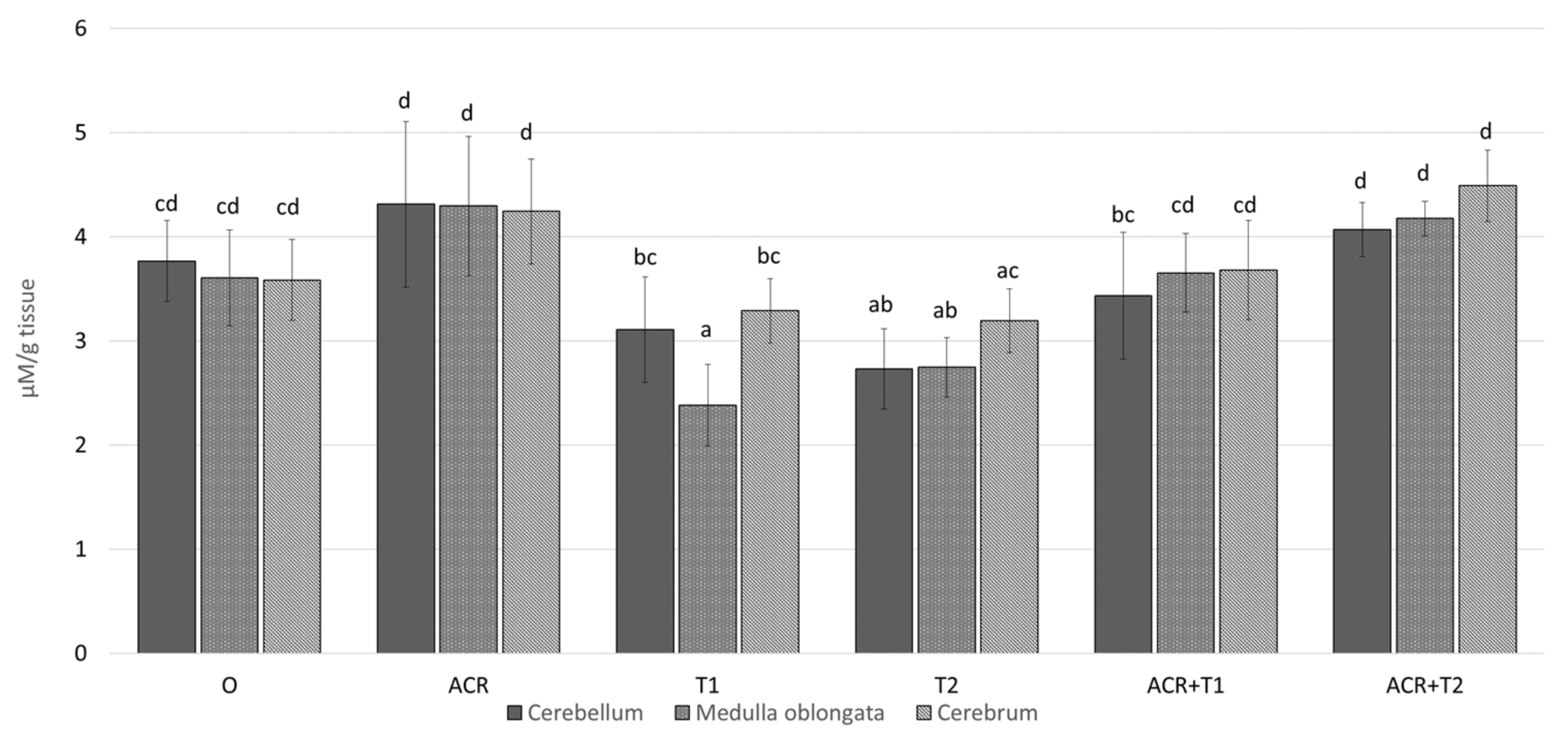
2.2. The Influence of Tocopherol on Malondialdehyde
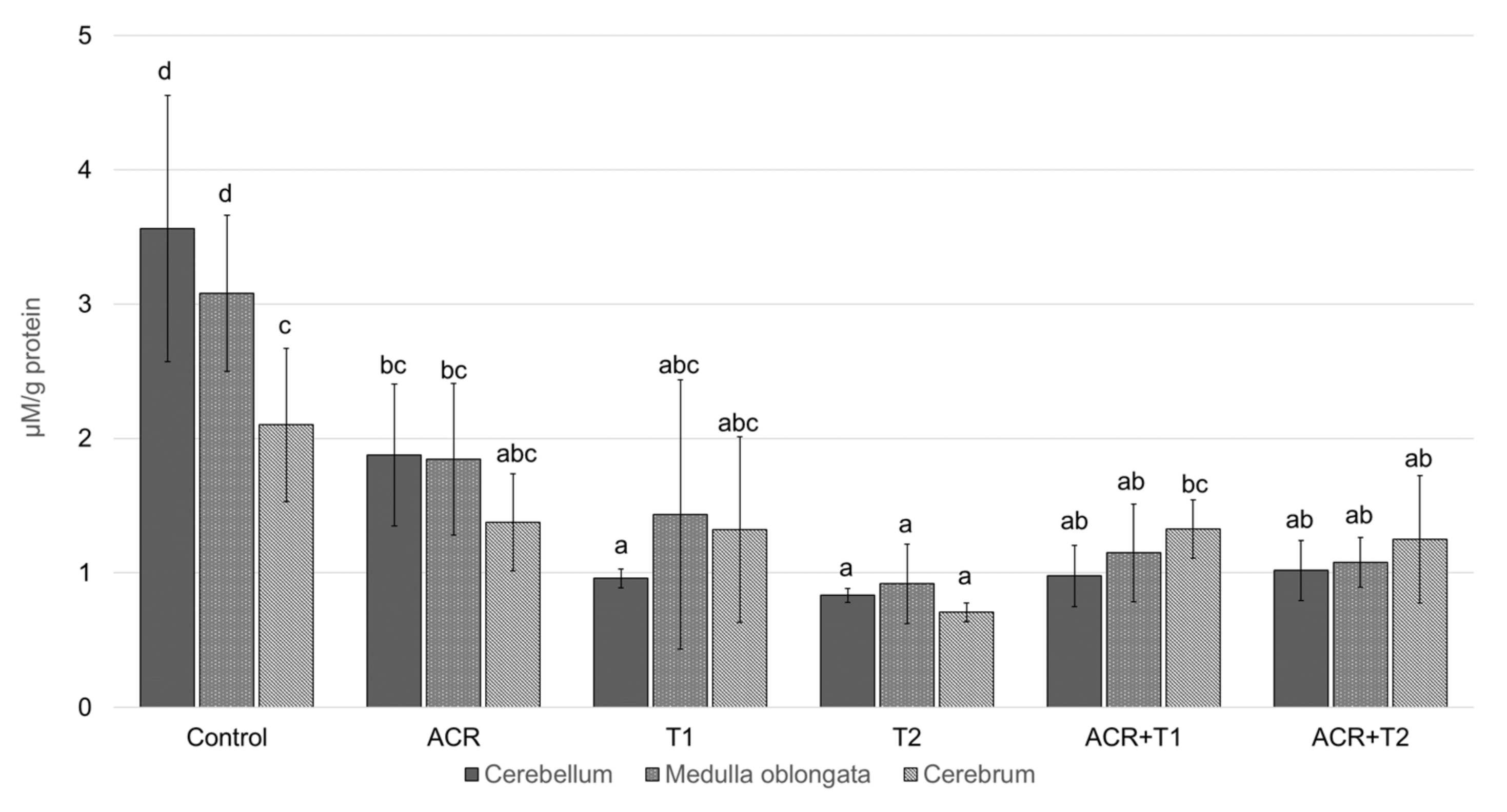
2.3. The Influence of Tocopherol on the Analyzed Glutathione
3. Discussion
4. Materials and Methods
Statistical Analyses
5. Conclusions
- Relationships between exposure to acrylamide an a decrease in AChE and GSH activity were demonstrated.
- α-Tocopherol reduces the harmful effects of acrylamide on AChE.
- Relationships between exposure to acrylamide and an increase in MDA, one of the markers of oxidative stress, were demonstrated.
- α-Tocopherol can reduce MDA concentration.
- α-Tocopherol can reduce the toxic effects of acrylamide, thus increasing the level of GSH.
- Acrylamide is a substance that negatively affects the cholinergic system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- FAO; WHO. Evaluation of Certain Food Contaminants-Sixty-Fourth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Rice, J.M. The carcinogenicity of acrylamide. Mutat. Res.-Genet. Toxicol. Environ. Mutagen. 2005, 580, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Sengul, E.; Gelen, V.; Yildirim, S.; Tekin, S.; Dag, Y. The Effects of Selenium in Acrylamide-Induced Nephrotoxicity in Rats: Roles of Oxidative Stress, Inflammation, Apoptosis, and DNA Damage. Biol. Trace Element Res. 2020, 199, 173–184. [Google Scholar] [CrossRef]
- Zeki, A.; Ince, S.; Arslan-Acaroz, D.; Gurler, Z.; Kucukkurt, I.; Demirel, H.H.; Arslan, H.O.; Varol, N.; Zhu, K. The ameliorative effects of boron against acrylamide-induced oxidative stress, inflammatory response, and metabolic changes in rats. Food Chem. Toxicol. 2018, 118, 745–752. [Google Scholar] [CrossRef]
- Bo, N.; Yilin, H.; Chaoyue, Y.; Lu, L.; Yuan, Y. Acrylamide induces NLRP3 inflammasome activation via oxidative stress- and endoplasmic reticulum stress-mediated MAPK pathway in HepG2 cells. Food Che. Toxicol. 2020, 145, 111679. [Google Scholar] [CrossRef] [PubMed]
- Amirshahrokhi, K. Acrylamide exposure aggravates the development of ulcerative colitis in mice through activation of NF-κB, inflammatory cytokines, iNOS, and oxidative stress. Iran. J. Basic Med. Sci. 2021, 24, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Mashimo, M.; Moriwaki, Y.; Misawa, H.; Ono, S.; Horiguchi, K.; Kawashima, K. Expression and Function of the Cholinergic System in Immune Cells. Front. Immunol. 2017, 8, 1085. [Google Scholar] [CrossRef] [Green Version]
- Cox, M.A.; Bassi, C.; Saunders, M.E.; Nechanitzky, R.; Morgado-Palacin, I.; Zheng, C.; Mak, T.W. Beyond neurotransmission: Acetylcholine in immunity and inflammation. J. Intern. Med. 2019, 287, 120–133. [Google Scholar] [CrossRef]
- Borovikova, L.V.; Ivanovaa, S.; Nardia, D.; Zhanga, M.; Yanga, H.; Ombrellinob, M.; Tracey, K.J. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton. Neurosci. 2000, 85, 141–147. [Google Scholar] [CrossRef]
- Han, B.; Li, X.; Hao, J. The cholinergic anti-inflammatory pathway: An innovative treatment strategy for neurological diseases. Neurosci. Biobehav. Rev. 2017, 77, 358–368. [Google Scholar] [CrossRef]
- Wallert, M.; Ziegler, M.; Wang, X.; Maluenda, A.; Xu, X.; Yap, M.L.; Witt, R.; Giles, C.; Kluge, S.; Hortmann, M.; et al. α-Tocopherol preserves cardiac function by reducing oxidative stress and inflammation in ischemia/reperfusion injury. Redox Biol. 2019, 26, 101292. [Google Scholar] [CrossRef]
- Nakamura, Y.K.; Omaye, S.T. α-Tocopherol modulates human umbilical vein endothelial cell expression of Cu/Zn superoxide dismutase and catalase and lipid peroxidation. Nutr. Res. 2008, 28, 671–680. [Google Scholar] [CrossRef]
- Uemura, M.; Manabe, H.; Yoshida, N.; Fujita, N.; Ochiai, J.; Matsumoto, N.; Takagi, T.; Naito, Y.; Yoshikawa, T. Alpha-tocopherol prevents apoptosis of vascular endothelial cells via a mechanism exceeding that of mere antioxidation. Eur. J. Pharmacol. 2002, 456, 29–37. [Google Scholar] [CrossRef]
- Wallert, M.; Schmölz, L.; Galli, F.; Birringer, M.; Lorkowski, S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014, 2, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [Green Version]
- Traber, M.G. Vitamin E Regulatory Mechanisms. Annu. Rev. Nutr. 2007, 27, 347–362. [Google Scholar] [CrossRef]
- Ball, G.F.M. Vitamin E. In Bioavailability and Analysis of Vitamins in Foods; Springer: Boston, MA, USA, 1998. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G.; Pratt, C.W. Fundamentals of Biochemistry: Life at the Molecular Level, 5th ed.; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Rosilene, R.R.; Corrêa, M.C.; Spanevello, R.M.; Morsch, V.M.; Mazzanti, C.M.; Gonçalves, J.F.; Schetinger, M.R. Acetylcholinesterase activation and enhanced lipid peroxidation after long-term exposure to low levels of aluminum on different mouse brain regions. J. Inorg. Biochem. 2005, 99, 1865–1870. [Google Scholar] [CrossRef]
- Wallert, M.; Börmel, L.; Lorkowski, S. Inflammatory Diseases and Vitamin E—What Do We Know and Where Do We Go? Mol. Nutr. Food Res. 2021, 65, 2000097. [Google Scholar] [CrossRef]
- Bano, S.; Parihar, M.S. Reduction of lipid peroxidation in different brain regions by a combination of α-tocopherol and ascorbic acid. J. Neural Transm. 1997, 104, 1277–1286. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, Y.; Sun, G.; Wang, P.; Hu, X.; Chen, F. Role of glutathione on acrylamide inhibition: Transformation products and mechanism. Food Chem. 2020, 326, 126982. [Google Scholar] [CrossRef]
- Tirmenstein, M.; Reed, D.J. Effects of glutathione on the alpha-tocopherol-dependent inhibition of nuclear lipid peroxidation. J. Lipid Res. 1989, 30, 959–965. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E Isoforms as Modulators of Lung Inflammation. Nutrients 2013, 5, 4347–4363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devaraj, S.; Tang, R.; Adams-Huet, B.; Harris, A.; Seenivasan, T.; De Lemos, J.A.; Jialal, I. Effect of high-dose α-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery diseasew. Am. J. Clin. Nutr. 2007, 86, 1392–1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazrun, A.S.; Norazlina, M.; Norliza, M.; Nirwana, S.I. The Anti-Inflammatory Role of Vitamin E in Prevention of Osteoporosis. Adv. Pharmacol. Sci. 2011, 2012, 142702. [Google Scholar] [CrossRef]
- Reiter, E.; Jiang, Q.; Christen, S. Anti-inflammatory properties of α-and γ-tocopherol. Mol. Asp. Med. 2007, 28, 668–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Batoryna, M.; Lis, M.; Formicki, G. Acrylamide-induced disturbance of the redox balance in the chick embryonic brain. J. Environ. Sci. Health Part B 2017, 52, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Lis, M.W.; Głodek, K.; Sechman, A.; Wator, D.; Pawlak, K.; Niedziółka, J.W. Effect of in ovo injection of aroclor 1254 on embryonic development, time of hatching, and blood thyroid hormone levels in one-day-old chicken. Bull. Vet. Inst. Pulawy 2011, 55, 293–298. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
| Group | α-Tocopherol | Dissolvent to α-Tocopherol (Archid Oil) | Acrylamide | Dissolvent to Acrylamide (0.7% NaCl) |
|---|---|---|---|---|
| C (control) | 0.0 mg | 0.1 mL | 0.0 mg | 0.1 mL |
| ACR | 0.0 mg | 0.1 mL | 2.4 mg | 0.1 mL |
| T1 | 0.5 mg | 0.1 mL | 0.0 mg | 0.1 mL |
| T2 | 5.0 mg | 0.1 mL | 0.0 mg | 0.1 mL |
| ACR + T1 | 0.5 mg | 0.1 mL | 2.4 mg | 0.1 mL |
| ACR + T2 | 5.0 mg | 0.1 mL | 2.4 mg | 0.1 mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopańska, M.; Batoryna, M.; Banaś-Ząbczyk, A.; Błajda, J.; Lis, M.W. The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide. Molecules 2022, 27, 965. https://doi.org/10.3390/molecules27030965
Kopańska M, Batoryna M, Banaś-Ząbczyk A, Błajda J, Lis MW. The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide. Molecules. 2022; 27(3):965. https://doi.org/10.3390/molecules27030965
Chicago/Turabian StyleKopańska, Marta, Marta Batoryna, Agnieszka Banaś-Ząbczyk, Joanna Błajda, and Marcin W. Lis. 2022. "The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide" Molecules 27, no. 3: 965. https://doi.org/10.3390/molecules27030965
APA StyleKopańska, M., Batoryna, M., Banaś-Ząbczyk, A., Błajda, J., & Lis, M. W. (2022). The Effect of α-Tocopherol on the Reduction of Inflammatory Processes and the Negative Effect of Acrylamide. Molecules, 27(3), 965. https://doi.org/10.3390/molecules27030965







