Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS
Abstract
:1. Introduction
2. Results
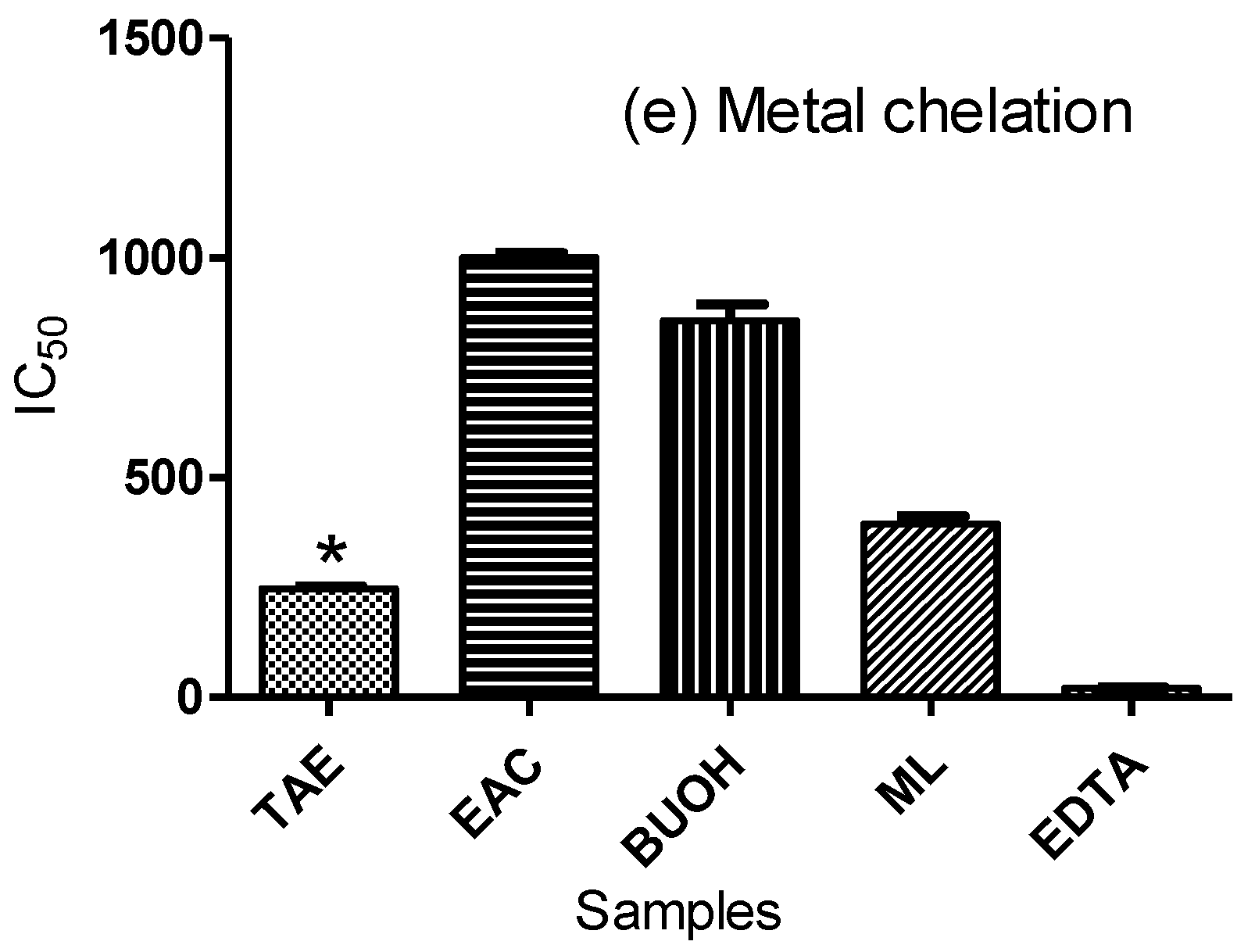
2.1. Evaluation of the Antioxidant Activity In Vitro
2.2. Evaluation of the Antimicrobial Activity
2.2.1. Well Diffusion Assay
2.2.2. Determination of Minimum Inhibitory Concentration (MIC)
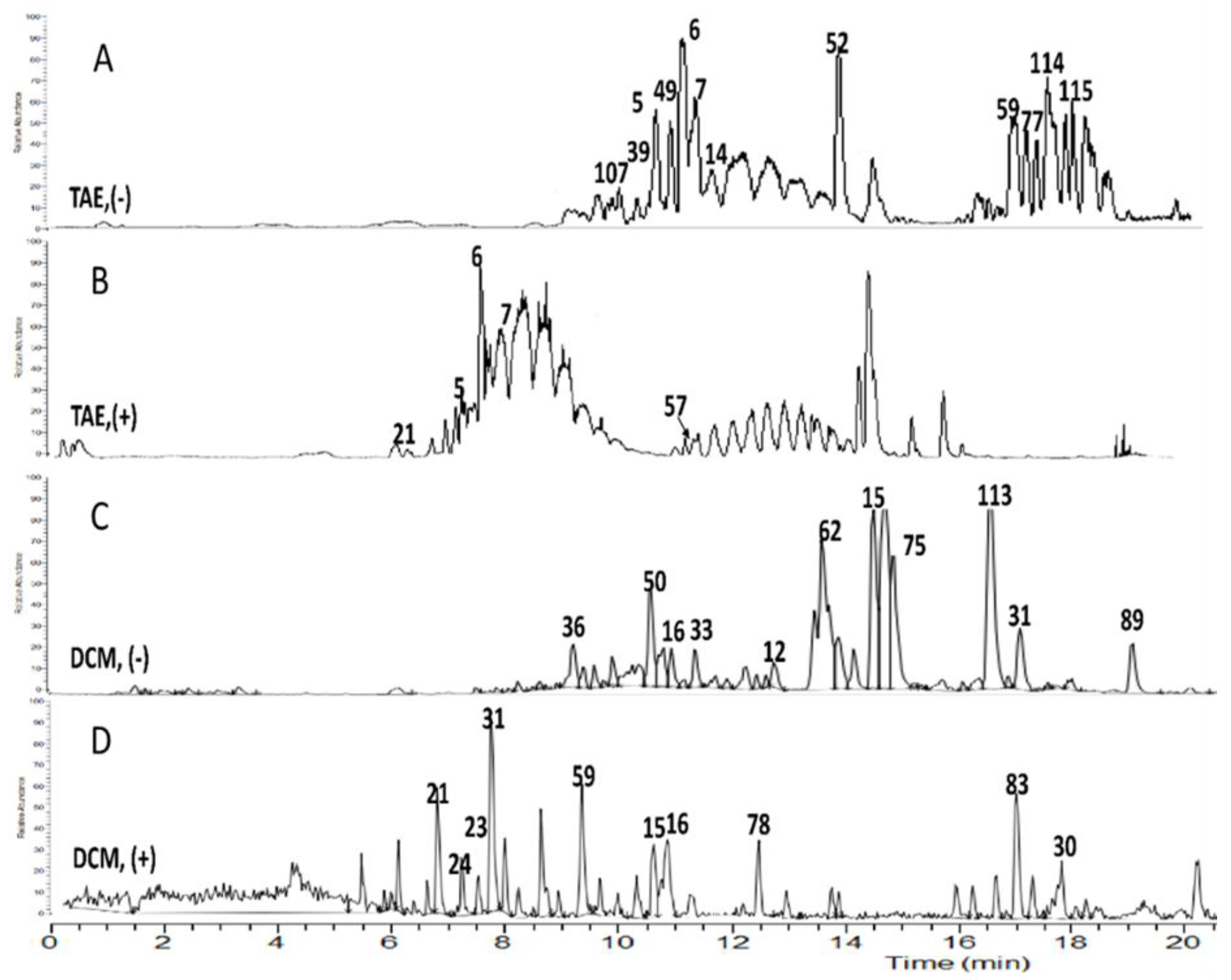
2.3. UPLC/ESI/TOF-MS Metabolic Profiling of (TAE) of Celery Aerial Parts
2.3.1. Flavonoids
2.3.2. Coumarins and Benzofurans
2.3.3. Phenylpropanoids
2.3.4. Terpenes
3. Discussion
3.1. Evaluation of Antioxidant Activity in Correlation to UPLC/ESI/TOF-MS Metabolite Profiling
3.2. Evaluation of Antimicrobial Activity in Correlation to UPLC/ESI/TOF-MS Metabolite Profiling
4. Material and Method
4.1. Chemicals, Reagents, and Microbial Strains
4.2. Plant Material and Sample Preparation
4.3. Evaluation of the Antioxidant Activity
4.3.1. FRAP Assay “Trolox Equivalent”
4.3.2. ORAC Assay “Trolox Equivalent”
4.3.3. ABTS Radical Scavenging Assay
4.3.4. DPPH Radical Scavenging Assay
4.3.5. Metal Chelation Assay
4.4. Evaluation of the Antimicrobial Activity
4.4.1. Well Diffusion Assay
4.4.2. Minimum Inhibitory Concentration (MIC)
4.5. Metabolite Profiling of Celery Extract Using UPLC/ESI/TOF-MS
4.5.1. Celery Extract Preparation
4.5.2. Instrument and Spectral Acquisition
4.5.3. UPLC/ESI/TOF-MS Data Processing
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kelly, F.J. Use of antioxidants in the prevention and treatment of disease. J. Int. Fed. Clin. Chem. 1998, 10, 21–23. [Google Scholar] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant Phytochemicals for the Prevention and Treatment of Chronic Diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Protection against tissue damage in vivo by desferrioxamine: What is its mechanism of action? Free Radic. Biol. Med. 1989, 7, 645–651. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.P.; Leong, L.P.; Koh, J.H.W. Antioxidant activities of aqueous extracts of selected plants. Food Chem. 2006, 99, 775–783. [Google Scholar] [CrossRef]
- Branković, S.; Kitić, D.; Radenković, M.; Veljković, S.; Kostić, M.; Miladinović, B.; Pavlović, D. Hypotensive and cardioinhibotory effects of the aqueous and ethanol extracts of celery (Apium graveolens, Apiaceae). Acta Med. Median. 2010, 49, 13–16. [Google Scholar]
- Sorour, M.A.; Hassanen, N.H.M.; Ahmed, M.H.M. Natural Antioxidant Changes in Fresh and Dried celery (Apium graveolens). Am. J. Energy Eng. 2015, 3, 12. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, S.K.; Ceska, O.; Warrington, P.J.; Ashwood-Smith, M.J. Increased furocoumarin content of celery during storage. J. Agric. Food Chem. 1985, 33, 1153–1157. [Google Scholar] [CrossRef]
- Khairullah, A.R.; Solikhah, T.I.; Ansori, A.N.M.; Hidayatullah, A.R.; Hartadi, E.B.; Ram, S.C.; Fadholly, A. Review on the Pharmacological and Health Aspects of Apium Graveolens or Celery: An Update. Syst. Rev. Pharm. 2021, 12, 606–612. [Google Scholar]
- Brahmachari, G. Spectroscopic Properties of Natural Flavonoids; World Scientific: Singapore, 2018. [Google Scholar]
- Rasheed, D.M.; Emad, A.M.; Ali, S.F.; Ali, S.S.; Farag, M.A.; Meselhy, M.R.; Sattar, E.A. UPLC-PDA-ESI/MS metabolic profiling of dill shoots bioactive fraction; evidence of its antioxidant and hepatoprotective effects in vitro and in vivo. J. Food Biochem. 2021, 45, e13741. [Google Scholar] [CrossRef]
- Zhou, K.; Zhao, F.; Liu, Z.; Zhuang, Y.; Chen, L.; Qiu, F. Triterpenoids and Flavonoids from Celery (Apium graveolens). J. Nat. Prod. 2009, 72, 1563–1567. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.R.; Wu, Q.; Simon, J.E. Recent Advances in Anthocyanin Analysis and Characterization. Curr. Anal. Chem. 2008, 4, 75–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farag, M.A.; Khattab, A.R.; Maamoun, A.A.; Kropf, M.; Heiss, A.G. UPLC-MS metabolome-based classification of Lupinus and Lens seeds: A prospect for phyto-equivalency of its different accessions. Food Res. Int. 2018, 115, 379–392. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.A.; Molina, E.; Yordi, E.G. Coumarins—An important class of phytochemicals. Phytochem.-Isol. Characterisation Role Hum. Health 2015, 25, 533–538. [Google Scholar]
- Dercks, W.; Trumble, J.; Winter, C. Impact of atmospheric pollution on linear furanocoumarin content in celery. J. Chem. Ecol. 1990, 16, 443–454. [Google Scholar] [CrossRef]
- Beltagy, A.M.; Beltagy, D.M. Chemical composition of Ammi visnaga L. and new cytotoxic activity of its constituents Khellin and Visnagin. J. Pharm. Sci. Res. 2015, 7, 285. [Google Scholar]
- Zengin, G.; Sinan, K.I.; Ak, G.; Mahomoodally, M.F.; Paksoy, M.Y.; Picot-Allain, C.; Glamočlija, J.; Sokovic, M.; Jekő, J.; Cziáky, Z.; et al. Chemical profile, antioxidant, antimicrobial, enzyme inhibitory, and cytotoxicity of seven Apiaceae species from Turkey: A comparative study. Ind. Crop. Prod. 2020, 153, 112572. [Google Scholar] [CrossRef]
- Fiorito, S.; Preziuso, F.; Sharifi-Rad, M.; Marchetti, L.; Epifano, F.; Genovese, S. Auraptene and umbelliprenin: A review on their latest literature acquisitions. Phytochem. Rev. 2020, 1–10. [Google Scholar] [CrossRef]
- Emad, A.M.; Ali, S.F.; Abdel-Rahman, E.A.; Meselhy, M.R.; Farag, M.A.; Ali, S.S.; Abdel-Sattar, E.A. Anti-inflammatory and antioxidant effects of Apium graveolens L. extracts mitigate against fatal acetaminophen-induced acute liver toxicity. J. Food Biochem. 2020, 44, e13399. [Google Scholar] [CrossRef]
- Kolarovic, J.; Popovic, M.; Zlinská, J.; Trivic, S.; Vojnovic, M. Antioxidant Activities of Celery and Parsley Juices in Rats Treated with Doxorubicin. Molecules 2010, 15, 6193–6204. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Jia, J.; Zhang, D.; Xie, J.; Xu, X.; Wei, D. In vitro and in vivo antioxidant activities of a flavonoid isolated from celery (Apium graveolens L. var. dulce). Food Funct. 2013, 5, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Uddin, Z.; Shad, A.A.; Bakht, J.; Ullah, I.; Jan, S. In vitro antimicrobial, antioxidant activity and phytochemical screening of Apium graveolens. Pak. J. Pharm. Sci. 2015, 28, 1699–1704. [Google Scholar] [PubMed]
- Masuoka, N.; Matsuda, M.; Kubo, I. Characterisation of the antioxidant activity of flavonoids. Food Chem. 2012, 131, 541–545. [Google Scholar] [CrossRef]
- Xiao, J. Dietary flavonoid aglycones and their glycosides: Which show better biological significance? Crit. Rev. Food Sci. Nutr. 2017, 57, 1874–1905. [Google Scholar] [CrossRef]
- Al-Majedy, Y.; Al-Amiery, A.; Kadhum, A.A.; BakarMohamad, A. Antioxidant activity of coumarins. Syst. Rev. Pharm. 2017, 8, 24. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Arsenie, L.V.; Lacatusu, I.; Oprea, O.; Bordei, N.; Bacalum, M.; Badea, N. Azelaic acid-willow bark extract-panthenol—Loaded lipid nanocarriers improve the hydration effect and antioxidant action of cosmetic formulations. Ind. Crop. Prod. 2020, 154, 112658. [Google Scholar] [CrossRef]
- Baldioli, M.; Servili, M.; Perretti, G.; Montedoro, G. Antioxidant activity of tocopherols and phenolic compounds of virgin olive oil. J. Am. Oil Chem. Soc. 1996, 73, 1589–1593. [Google Scholar] [CrossRef]
- Khotimah, H.; Diyantoro, D.W.I.; Sundari, A.S. Screening In Vitro Antimicrobial Activity of Celery (Apium graveolens) against Staphylococcus sp. Malays. J. Med. Health Sci. 2020, 16, 72–77. [Google Scholar]
- Prakoso, Y.A.; Rini, C.S.; Rahayu, A.; Sigit, M.; Widhowati, D. Celery (Apium graveolens) as a potential antibacterial agent and its effect on cytokeratin-17 and other healing promoters in skin wounds infected with methicillin-resistant Staphylococcus aureus. Vet. World 2020, 13, 865–871. [Google Scholar] [CrossRef]
- Mickymaray, S. Efficacy and Mechanism of Traditional Medicinal Plants and Bioactive Compounds against Clinically Important Pathogens. Antibiotics 2019, 8, 257. [Google Scholar] [CrossRef] [Green Version]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, S.S.; Selvaraj, A.; Ng, Z.Y.; Palanisamy, M.; Mickmaray, S.; Cheong, P.C.H.; Lim, R.L.H. Isolation of actinomycetes with antibacterial activity against multi-drug resistant bacteria. Malays. J. Microbiol. 2018, 14, 293–305. [Google Scholar] [CrossRef]
- Shelef, L. Antimicrobial effects of spices. J. Food Saf. 1984, 6, 29–44. [Google Scholar] [CrossRef]
- Farbood, M.I.; MacNeil, J.H.; Ostovar, K. Effect of Rosemary Spice Extractive on Growth of Microorganisms in Meats. J. Milk Food Technol. 1976, 39, 675–679. [Google Scholar] [CrossRef]
- Nikaido, H.; Vaara, M. Molecular basis of bacterial outer membrane permeability. Microbiol. Rev. 1985, 49, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Christova-Bagdassarian, V.; Bagdassarian, K.S.; Atanassova, M. Phenolic profile: Antioxidant and antibacterial activities from the Apiaceae family (dry seeds). Mintage J. Pharm. Med. Sci. 2013, 2, 26–31. [Google Scholar]
- Suganya, S.; Bharathidasan, R.; Senthilkumar, G.; Madhanraj, P.; Panneerselvam, A. Antibacterial activity of essential oil extracted from Coriandrum sativam (L.) and GC-MS analysis. Chem. Pharm. Res. 2012, 4, 1846–1850. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Cheng, L.; Zhong, G.-Y.; Liu, R.H. Antioxidant and Antiproliferative Activities of Twenty-Four Vitis vinifera Grapes. PLoS ONE 2014, 9, e105146. [Google Scholar] [CrossRef]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Boly, R.; Lamkami, T.; Lompo, M.; Dubois, J.; Guissou, I. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) leaves. Int. J. Toxicol. Pharmacol. Res. 2016, 8, 29–34. [Google Scholar]
- Santos, J.S.; Brizola, V.R.A.; Granato, D. High-throughput assay comparison and standardization for metal chelating capacity screening: A proposal and application. Food Chem. 2017, 214, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Bhalodia, N.R.; Shukla, V. Antibacterial and antifungal activities from leaf extracts of Cassia fistula l.: An ethnomedicinal plant. J. Adv. Pharm. Technol. Res. 2011, 2, 104–109. [Google Scholar] [CrossRef]
- Nisha, M.; Subramanian, M.; Prathyusha, P.; Santhanakrishnan, R. Comparative studies on antimicrobial activity of Artemisia sieversiana Ehrhart. Ex. Willd. and Origanum vulgare L. Int. J. PharmTech Res. 2010, 2, 1124–1127. [Google Scholar]
- Daoud, A.; Malika, D.; Bakari, S.; Hfaiedh, N.; Mnafgui, K.; Kadri, A.; Gharsallah, N. Assessment of polyphenol composition, antioxidant and antimicrobial properties of various extracts of Date Palm Pollen (DPP) from two Tunisian cultivars. Arab. J. Chem. 2019, 12, 3075–3086. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, H.A.; Khan, R.A.; Abdel-Hafez, A.A.; Abdel-Aziz, M.; Ahmed, E.; Enany, S.; Mahgoub, S.; Al-Rugaie, O.; Alsharidah, M.; Aly, M.S.A.; et al. Phytochemical Profiling, In Vitro and In Silico Anti-Microbial and Anti-Cancer Activity Evaluations and Staph GyraseB and h-TOP-IIβ Receptor-Docking Studies of Major Constituents of Zygophyllum coccineum L. Aqueous-Ethanolic Extract and Its Subsequent Fractions: An Approach to Validate Traditional Phytomedicinal Knowledge. Molecules 2021, 26, 577. [Google Scholar] [PubMed]


| Antioxidant Assay | TAE (Average ± SD) | DCM (Average ± SD) | EAC (Average ± SD) | BUOH (Average ± SD) | ML (Average ± SD) |
|---|---|---|---|---|---|
| FRAP μM TE/mg sample | 45.57 ± 4.46 | 233.47 ± 15.14 | 76.80 ± 9.68 | 19.75 ± 19.76 | 53.47 ± 5.02 |
| ORAC μM TE/mg sample | 558.74 ± 45.90 | 1076 ± 25.73 | 888.83 ± 70.50 | 890.18 ± 53 | 402.02 ± 23.18 |
| ABTS “IC50” µg/ml | 34.52 ± 3.25 | 37.78 ± 1.24 | 103.7 ± 4.47 | 32.56 ± 0.09 | 100.0 ± 0.58 |
| DPPH “IC50” µg/ml | 930.8 ± 42.50 | 474.4 ± 19.80 | 591.4 ± 27.05 | 1119 ± 62.30 | 973.4 ± 50.21 |
| Metal chelation “IC50” µg/ml | 246.6 ± 5.78 | nd | 1000 ± 10.40 | 856.7 ± 37.13 | 394.1 ± 17.81 |
| TAE Average (mm) ± SD | DCM Average (mm) ± SD | EAC Average (mm) ± SD | BUOH Average (mm) ± SD | ML Average (mm) ± SD | |
|---|---|---|---|---|---|
| Zones of inhibition | |||||
| Staphylococcus aureus | 13.4 ± 1.5 | 12.0 ±1.0 | nd | nd | nd |
| Escherichia coli | 11.0 ± 1.2 | 10.0 ± 1.3 | nd | nd | nd |
| Candida albicans | nd | nd | nd | nd | nd |
| Peak # | Rt (min.) | Metabolite Name | Mol. Ion m/z (−)/(+) | Elemental Composition | Δ Mass (ppm) | MS2 Ions m/z (−)/(+) | DCM Fraction |
|---|---|---|---|---|---|---|---|
| Flavonoids | |||||||
| 1 | 8.00 | Noidesol A | 479.11841 | C22H23O12− | 0.02 | 451.12241, 317.06561 | |
| 2 | 9.53 | Apigenin-C-dihexoside | 593.14972/ 595.16516 | C27H29O15−/ C27H31O15+ | 0.64/ −0.98 | 473.10767, 353.06583/577.15472, 457.11240, 325.07031 | |
| 3 | 10.12 | Apigenin-O-dihexosyl pentoside | 727.20697 | C32H39O19+ | −1.42 | 595.16425,433.11163, 271.05960 | |
| 4 | 10.35 | Chrysoeriol-O-dihexosyl pentoside | 757.21661 | C33H41O20+ | −1.36 | 625.17542,463.12268, 301.07040, 286.04706 | |
| 5 | 10.85/10.95 | Luteolin-O-hexosyl pentoside * | 579.13342/ 581.14941 | C26H27O15−/ C26H29O15+ | 1.77/ −1.18 | 447.09232,285.03989/449.10730, 287.05487 | |
| 6 | 11.40/14.02 | Apiin * | 563.13843/ 565.15326 | C26H27O14−/ C26H29O14+ | 1.96/ −3.401 | 431.09698,269.04495/433.11264, 271.06009 | |
| 7 | 11.50/12.13 | Chrysoeriol-O-hexosyl pentoside * | 593.14832/ 595.16650 | C27H29O15−/ C27H31O15+ | 2.96/ 1.26 | 461.10800,299.05548, 284.03226/463.12268, 301.07031 | |
| 8 | 12.10 | Chrysoeriol malonyl-O- hexosyl pentoside | 681.16431 | C30H33O18+ | 0.12 | 549.12354, 301.07050 | |
| 9 | 12.15 | apigenin-O-dihexosyl deoxy hexoside | 739.1839/ 741.20148 | C36H35O17−/ C36H37O17+ | −1.41 | 545.12793, 269.04468/579.14844, 433.11194, 271.05972 | |
| 10 | 12.37 | Chrysoeriol-O-hexoside deoxyhexoside hexoside | 771.21106 | C37H39O18+ | −1.53 | 463.12256, 301.07043 | |
| 11 | 12.56 | Apigenin-O-acyl hexosyl pentoside | 607.16412 | C28H31O15+ | −2.68 | 475.12262, 271.05981 | |
| 12 | 12.68 | Luteolin | 287.05487 | C15H11O6+ | −0.50 | 270.14902, 258.05222 | + |
| 13 | 12.75 | Chrysoeriol acyl-O-hexosyl pentoside | 637.17535 | C29H33O16+ | −1.51 | 505.13290, 301.07010 | |
| 14 | 13.31 | Apigenin diacyl-O-deoxyhexosyl pentoside * | 633.17981 | C30H33O15+ | −0.86 | 501.13834, 271.05984 | |
| 15 | 13.39/13.43 | Apigenin | 269.04465/271.05981 | C15H9O5−/ C15H11O5+ | 0.74/ −1.07 | 225.05508,149.02399/214.09019, 153.01828 | + |
| 16 | 13.73 | Chrysoeriol | 301.07059 | C16H13O6+ | −0.25 | 286.04684, 181.04985 | + |
| 17 | 14.03 | Apigenin-O-hexosyl dimethyl caffoeyl-C-pentoside | 761.2627 | C37H45O17+ | 7.79/ 4.52 | 629.22205, 271.06000 | |
| 18 | 19.93 | Apigenin-O-acyl (trimethyl ether) methoxy galloyl quinoyl (acyl pentosyl) pentoside | 803.38306 | C42H59O15+ | −2.23 | 671.34082, 271.05969 | |
| Coumarins and Benzofurans | |||||||
| 19 | 0.95 | Methylumbelliferyl acetate | 217.048 | C12H9O4− | −6.94 | 181.07140 | |
| 20 | 2.30 | Aesculin | 339.07104 | C15H15O9− | −0.05 | 177.01891 | |
| 21 | 6.78 | Isofraxidin * | 223.0602 | C11H11O5+ | 0.45 | 208.03674, 163.03891 | |
| 22 | 7.41 | Isofraxidin-O-hexoside | 383.0943 | C17H19O10− | −3.76 | 221.04509, 193.05090 | |
| 23 | 9.09 | Umbellifolide | 265.14282 | C15H21O4+ | −2.32 | 247.13225,203.10645, 175.07507 | |
| 24 | 9.90 | Dihydroxybiscoumarin | 323.05542 | C18H11O6+ | 1.26 | 295.05978, 267.06494, 149.02306 | |
| 25 | 10.66 | Isopimpinellin-O-hexoside | 409.11224 | C19H21O10+ | −1.67 | 247.06020 | |
| 26 | 12.92 | Cleomiscosin A | 387.10712 | C20H19O8+ | −0.84 | 369.09616, 337.07004, 161.05956 | |
| 27 | 13.05 | Licocoumarone | 341.1376 | C20H21O5+ | −2.20 | 323.12717, 271.09604, 137.05949 | |
| 28 | 13.53 | Khellin | 261.07565 | C14H13O5+ | −0.38 | 246.05215 | |
| 29 | 13.54 | Visnagin/Desmethoxykhellin | 231.065 | C13H11O4+ | −0.80 | 216.04149, 175.03882 | + |
| 30 | 13.60 | Encecalin/methyleupatoriochromene | 233.11469 | C14H17O3+ | −10.86 | 217.04517 | + |
| 31 | 14.35 | Senkyunolide F | 205.08675 | C12H13O3− | −4.04 | 161.09692, 148.01640, 132.05791 | + |
| 32 | 14.70 | Isopimpinellin (Dimethoxypsoralen) | 247.06464 | C13H11O5+ | −1.34 | 232.03647 | + |
| 33 | 16.48 | Marmesin | 245.08145 | C14H13O4− | −2.51 | 203.03471, 161.02415 | + |
| Phenylpropanoids | |||||||
| 34 | 4.17/3.70 | Caffeoylquinic acid | 353.08698/355.10165 | C16H17O9−/C16H19O9+ | 0.77/ −1.99 | 233.04478, 191.05551, 179.03462/337.09155, 289.07062, 193.04955, 163.03885 | |
| 35 | 5.09 | Caffeic acid | 179.03452 | C9H7O4− | −3.55 | 135.04529 | + |
| 36 | 8.73/8.86 | Coumaroylquinic acid | 337.09204/339.10663 | C16H17O8−/ C16H19O8+ | 0.73/ −2.40 | 191.05577, 163.03981/147.04385 | |
| 37 | 9.84 | Feruloylquinic acid | 369.11801 | C17H21O9+ | 0.00 | 177.05449, 145.02818 | |
| 38 | 10.79 | Dillapional-O-dihexoside | 559.16492 | C24H31O15− | −1.48 | 397.11212, 235.06065 | |
| 39 | 11.07 | Dimethoxyfuranohydrocoumaroyl-O-dihexoside * | 589.17474 | C25H33O16− | 1.12 | 427.12338, 265.07101 | |
| 40 | 15.12 | Octyl methoxycinnamate | 291.19534 | C18H27O3+ | −0.45 | 273.18475 | + |
| Aliphatic acids and Phenolic acids/glycosides | |||||||
| 41 | 2.48 | Elenolic acid | 241.07 | C11H13O6− | 0.72 | 153.01907, 109.02943 | + |
| 42 | 3.09 | Hydroxyisophthalic acid | 181.01372 | C8H5O5− | −3.15 | 137.02428 | |
| 43 | 3.68 | Monotropeoside | 445.1333 | C19H25O12− | −1.62 | 427.1212,385.1129, 269.1021 | |
| 44 | 3.93 | Isopropylmalic acid | 175.06 | C7H11O5− | 3.31 | 157.05049, 115.04002, 113.06070 | + |
| 45 | 4.38 | 2-Hydroxyisocaproic acid hexoside | 293.12308 | C12H21O8− | −0.05 | 131.07114 | + |
| 46 | 5.31 | Vanilloloside | 317.12027 | C14H21O8+ | −8.91 | 299.10376, 203.05258, 185.04211, | |
| 47 | 9.70 | Syringoylquinic acid | 371.09723 | C16H19O10− | −0.12 | 249.06111 | |
| 48 | 11.09 | Hydroxypropofol O-glucuronide | 369.15372 | C18H25O8− | −1.83 | 351.14371, 311.11267 | |
| 49 | 11.10 | Ptelatoside A * | 413.1438 | C19H25O10− | 0.23 | 351.14352, 311.11240, 269.10214 | |
| 50 | 11.78 | Azelaic acid | 187.09712 | C9H15O4− | 3.39 | 125.09694 | + |
| 51 | 11.82 | Ptelatoside B | 427.15964 | C20H27O10− | −0.55 | 325.12830, 161.04520 | |
| 52 | 12.73 | Dihydrogalloyl-O-hexoside * | 329.19583 | C17H29O6− | −0.11 | 161.04507 | + |
| Iridoids | |||||||
| 53 | 1.34 | Adenosmoside | 363.16559 | C16H27O9− | −1.74 | 241.00191 | + |
| 54 | 2.61 | Geniposidic acid | 375.12534 | C16H23O10+ | −8.62 | 313.12543, 231.08340 | |
| 55 | 3.08 | Acetylloganic acid | 419.15076 | C18H27O11+ | −9.61 | 357.15198, 275.11020 | |
| 56 | 3.83 | Tudoside | 417.10211 | C17H21O12− | −1.54 | 285.06100, 241.07098, 152.01129 | |
| 57 | 6.36 | Geniposide * | 389.14102 | C17H25O10+ | −8.23 | 327.14093, 245.09958, | |
| 58 | 8.50 | Sweroside | 357.11835 | C16H21O9− | 0.96 | 193.05029 | |
| 59 | 16.01 | Valdiate * | 311.18552 | C17H27O5+ | 0.71 | 293.17484, 255.12274, 237.11218 | + |
| Terpenes | |||||||
| 60 | 5.89 | Euonyminol | 365.14465 | C15H25O10− | −1.49 | 303.14508, 263.11276, 221.10072 | |
| 61 | 6.64 | Phyllaemblic acid B | 349.15143 | C15H25O9+ | −6.08 | 331.12054, 193.12245, 127.03888 | |
| 62 | 7.46 | Anisatin | 327.10745 | C15H19O8− | 0.02 | 165.05574, 147.04503 | + |
| 63 | 9.31 | Citroside A | 385.18555 | C19H29O8− | −0.38 | 249.1127, 205.1230, 179.0558 | |
| 64 | 9.75 | Unknown diterpene acetate | 371.22006 | C23H31O4+ | −4.38 | 327.20108, 283.17490, 221.13826, 177.11200, 133.08582 | |
| 65 | 9.98 | Unknown triterpene | 553.37134 | C31H53O8+ | −3.89 | 535.35925, 351.23840, 267.13351 | |
| 66 | 10.02 | Corchoionoside B | 399.16492 | C19H27O9− | −0.10 | 381.15445, 341.12338, 299.11298 | |
| 67 | 11.70 | Diacetoxyl epoxy-apotirucallenetetraol | 589.37207 | C34H53O8+ | −2.42 | 513.32635 | |
| 68 | 12.45 | Fruticoside E | 645.40393 | C37H57O9− | −2.28 | 569.35181, 511.31049 | |
| 69 | 13.22 | Unknown terpene | 469.33075 | C30H45O4+ | −1.04 | 451.31940 | |
| 70 | 13.34 | Tragopogonsaponin A | 647.37665 | C36H55O10− | −3.59 | 485.32437, | |
| 71 | 13.36 | Quillaic acid | 487.34097 | C30H47O5+ | −1.71 | 469.33044, 451.31979 | |
| 72 | 13.83 | Unknown terpene | 427.26733 | C23H39O7+ | −3.98 | 409.25574, 269.13644 | |
| 73 | 15.36 | Amaranthussaponin | 955.48846 | C48H75O19− | −1.30 | 731.43488, 523.37750, 453.33609 | |
| 74 | 16.43 | Momordicinin | 439.35724 | C30H47O2+ | 0.42 | 421.34732, 393.35141 | |
| 75 | 16.72 | Auraptene | 297.15207 | C19H21O3− | −7.82 | 240.08189, 183.01169 | |
| 76 | 17.42 | Bacobitacin B | 599.31854 | C34H47O9+ | −4.87 | 563.29681, 581.30725, 337.27313 | |
| 77 | 17.76 | Unknown terpene * | 573.30267 | C32H45O9− | 1.50 | 409.23471, 391.22437, 317.06357, 243.02704 | |
| 78 | 17.79 | Tschimganin | 305.17484 | C18H25O4+ | 0.34 | 273.14832, 241.12218 | |
| 79 | 17.86 | Unknown terpene | 573.30249 | C32H45O9− | −5.79 | 409.23468, 391.22430, 317.06351 | |
| 80 | 17.91 | Unknown terpene | 555.28174 | C28H43O11− | 3.15 | 299.04340, 225.00691 | + |
| 81 | 18.20 | Acetyloxy torilolone | 293.1786 | C17H25O4− | 3.35 | 96.95988 | |
| 82 | 19.10 | Unknown terpene | 409.23511 | C26H33O4− | −5.44 | 152.99554 | |
| 83 | 23.83 | Yonogenin | 433.32993 | C27H45O4+ | −3.02 | 307.19000, 293.17441, 149.02310 | |
| 84 | 26.62 | Acetoxy hydroxymethoxy-oleanene | 515.41339 | C33H55O4+ | 7.57 | 329.21429 | |
| Fatty acids | |||||||
| 85 | 9.05 | Traumatic acid | 229.14305 | C12H21O4+ | −1.68 | 211.13272, 193.12216 | |
| 86 | 14.11 | Trihydroxyoctadecenoic acid | 329.2326 | C18H33O5− | 1.06 | 311.22211, 229.14413, 171.10242 | |
| 87 | 14.19 | Oxo octadecadienonic acid | 295.22662 | C18H31O3+ | −0.51 | 277.21600 | + |
| 88 | 15.19 | Octadecenedioic acid | 313.23642 | C18H33O4− | −2.92 | 295.22638, 277.21603 | |
| 89 | 17.06 | Octadecatrienoic acid | 311.2218 | C18H31O4− | 0.37 | - | |
| 90 | 17.98 | Hydroxyoctadecadienoic (Coriolic acid) | 295.22693 | C18H31O3− | 0.54 | 277.21640, 195.13849, 179.14368 | + |
| 91 | 18.85 | Stearyl citrate | 443.29956 | C24H43O7− | −1.74 | 279.23203 | |
| 92 | 19.46 | Palmitoylhexitol | 419.29932 | C22H43O7− | −2.41 | 255.23193 | |
| 93 | 19.80 | Methyl Linolenate | 293.24759 | C19H33O2+ | 0.28 | 261.22107, 243.21054 | + |
| 94 | 22.71 | Methyl linoleate | 295.26309 | C19H35O2+ | −0.23 | 263.23694, 245.22644 | |
| 95 | 24.95 | Glyceryl ricinolpalmitein | 607.4917 | C37H67O6+ | −2.50 | 589.48108 | |
| Others | |||||||
| 96 | 1.69 | Mannitol | 181.0715 | C6H13O6− | 4.72 | 163.0610, 101.02435 | |
| 97 | 1.77 | Homovanillyl-O-hexoside | 343.10147 | C15H19O9− | 1.32 | 181.05017, 163.03961 | |
| 98 | 2.28 | Uralenneoside | 285.06085 | C12H13O8− | 1.25 | 153.01913 | |
| 99 | 5.39 | Celephthalide derivative | 405.21118 | C19H33O9− | −1.43 | 405.21118 | |
| 100 | 5.70 | Acutilactone | 409.32681 | C25H45O4+ | −10.81 | 276.21655, 160.13304 | |
| 101 | 5.87 | Benzyl-O-hexoside | 269.10275 | C13H17O6− | −2.92 | 171.47818 | |
| 102 | 8.90 | Benzyl-O-hexosyl pentoside | 401.14337 | C18H25O10− | −2.13 | 269.10239, 161.04523 | |
| 103 | 9.00 | Dactylorhin C | 351.12897 | C14H23O10− | −1.13 | 333.11838, 267.07202, 249.06105 | |
| 104 | 9.19 | Dihomo-jasmonic acid | 237.14897 | C14H21O3− | 1.89 | 171.11749 | |
| 105 | 9.67 | Glehlinoside C | 551.17535 | C26H31O13− | −1.03 | 389.12271, 193.05025 | |
| 106 | 9.98 | Falcarindiol | 261.18484 | C17H25O2+ | −0.26 | 219.17432, 205.12210 | |
| 107 | 10.07 | Citrusin A * | 537.19586 | C26H33O12− | −1.48 | 489.17389, 327.12231 | |
| 108 | 10.41 | Hydroxydihydrojasmonic acid-O-hexoside | 435.18478 | C19H31O11− | −3.01 | 389.18011 | |
| 109 | 11.27 | Celephthalide C | 371.1694 | C18H27O8− | −1.74 | 354.15955 | |
| 110 | 12.10 | Hydroxymethyl Celephthalide C | 403.19452 | C19H31O9− | −4.31 | 287.22067, | |
| 111 | 12.90 | Unknown phthalate derivative | 353.19312 | C19H29O6+ | −7.77 | 235.13074, 203.05276 | |
| 112 | 13.84 | Pterosin P | 235.13051 | C14H19O3+ | −10.04 | 217.15845, 179.10645 | |
| 113 | 17.17 | Unknown | 595.28656 | C27H47O14−/ C34H43O9− | −1.5/ 3.9 | 415.22418, 279.23230, 241.01138 | |
| 114 | 17.97 | Unknown * | 325.18344 | C14H29O8− | −6.93 | 183.01180 | |
| 115 | 18.39 | Benthamianone * | 433.23505 | C28H33O4− | −5.28 | 152.99571 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emad, A.M.; Rasheed, D.M.; El-Kased, R.F.; El-Kersh, D.M. Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS. Molecules 2022, 27, 698. https://doi.org/10.3390/molecules27030698
Emad AM, Rasheed DM, El-Kased RF, El-Kersh DM. Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS. Molecules. 2022; 27(3):698. https://doi.org/10.3390/molecules27030698
Chicago/Turabian StyleEmad, Ayat M., Dalia M. Rasheed, Reham F. El-Kased, and Dina M. El-Kersh. 2022. "Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS" Molecules 27, no. 3: 698. https://doi.org/10.3390/molecules27030698
APA StyleEmad, A. M., Rasheed, D. M., El-Kased, R. F., & El-Kersh, D. M. (2022). Antioxidant, Antimicrobial Activities and Characterization of Polyphenol-Enriched Extract of Egyptian Celery (Apium graveolens L., Apiaceae) Aerial Parts via UPLC/ESI/TOF-MS. Molecules, 27(3), 698. https://doi.org/10.3390/molecules27030698






