Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications
Abstract
:1. Introduction
2. General Characteristics
2.1. Botanical and Ecological Characteristics
2.2. Chemical Characteristics
3. Ethnopharmacology and Potential Uses in Phytotherapy—General Information
4. Biological Activities Confirmed by Scientific Reports and Potential Applications in Cosmetology
4.1. Antibacterial Activity
4.2. Antifungal Activity
4.3. Antioxidant Activity
4.4. Anti-Inflammatory Activity
5. Uses Based on the CosIng Database
6. Safety of Use
7. Trans-Anethole as the Main Active Component of I. verum Essential Oil —Chemical Characteristics, Importance in Cosmetology, and Safety of Use
7.1. General Characteristics
7.2. Biological Activity and Potential Cosmetological Applications
7.2.1. Antibacterial Activity
7.2.2. Antifungal Activity
7.2.3. Antioxidant Activity
7.2.4. Anti-Inflammatory Activity
7.2.5. Activity against Obesity
7.3. Safety of Use
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, G.W.; Hu, W.T.; Huang, B.K.; Qin, L.P. Illicium verum: A review on its botany, traditional use, chemistry and pharmacology. J. Ethnopharmacol. 2011, 136, 10–20. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Chemical Industry Press: Beijing, China, 2005.
- European Directorate for the Quality of Medicines & Health Care. European Pharmacopoeia, 4th ed.; EDQM: Strasbourg, France, 2002. [Google Scholar]
- Patra, J.K.; Das, G.; Bose, S.; Banerjee, S.; Vishnuprasad, C.N.; del Pilar Rodriguez-Torres, M.; Shin, H.S. Star anise (Illicium verum): Chemical compounds, antiviral properties, and clinical relevance. Phyther. Res. 2020, 34, 1248–1267. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Costache, I.I.; Miron, A. Anethole and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 247–267. [Google Scholar] [PubMed]
- Reveal, J.L.; Chase, M.W. APG III: Bibliographical information and synonymy of Magnoliidae. Phytotaxa 2011, 19, 71–134. [Google Scholar] [CrossRef] [Green Version]
- Simpson, M.G. Diversity and classification of flowering plants. In Plants Systematics, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2005; pp. 195–196. [Google Scholar]
- Saunders, R.M.K.; Okuyama, E. Plant Sources of the Genus Illicium. Therapeutic and Pharmacological Properties of Illicium. In Illicium, Pimpinella and Foeniculum; Jodral, M.M., Ed.; CRC Press: Boca Raton, FL, USA, 2004; Volume 40, pp. 22–33, 155–156. [Google Scholar]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chinese star anise and anise, magic herbs in traditional Chinese medicine and modern pharmaceutical science. Asian J. Med. Biol. Res. 2019, 5, 162–179. [Google Scholar] [CrossRef] [Green Version]
- Lim, T.K. Illicium verum. In Edible Medicinal and Non-Medicinal Plants; Springer: Dordrecht, The Netherlands, 2013; Volume 6, pp. 151–160. [Google Scholar]
- Charles, D.J. Anise star. In Antioxidant Properties of Spices, Herbs and Other Sources; Springer: New York, NY, USA, 2013; pp. 165–169. ISBN 9781461443. [Google Scholar]
- Chempakan, B.; Balaji, S. Star anise. In Chemistry of Spices; Chempakam, B., Parthasarathy, V.A., Zachariach, T.J., Eds.; CABI Biddles Ltd.: King’s Lynn, UK, 2008; pp. 607–610. [Google Scholar]
- Wang, Q.; Jiang, L.; Wen, Q. Effect of three extraction methods on the volatile component of Illicium verum Hook. f. analyzed by GC-MS. Wuhan Univ. J. Nat. Sci. 2007, 12, 529–534. [Google Scholar] [CrossRef]
- Sabry, B.A.; Farouk, A.; Badr, A.N. Bioactivity evaluation for volatiles and water extract of commercialized star anise. Heliyon 2021, 7, e07721. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Hua, R.; Li, M.; Huang, Y.; Li, S.; He, Y.; Shen, Z. Chemical composition and biological activity of star anise Illicium verum extracts against maize weevil, Sitophilus zeamais adults. J. Insect. Sci. 2014, 14, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luís, Â.; Sousa, S.; Wackerlig, J.; Dobusch, D.; Duarte, A.P.; Pereira, L.; Domingues, F. Star anise (Illicium verum Hook. f.) essential oil: Antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour Fragr. J. 2019, 34, 260–270. [Google Scholar] [CrossRef]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal activity of the essential oil of Illicium verum fruit and its main component trans-anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Scopel, R.; da Silva, C.F.; Lucas, A.M.; Garcez, J.J.; do Espirito Santo, A.T.; Almeida, R.N.; Cassel, E.; Vargas, R.M.F. Fluid phase equilibria and mass transfer studies applied to supercritical fluid extraction of Illicium verum volatile oil. Fluid Phase Equilib. 2016, 417, 203–211. [Google Scholar] [CrossRef]
- Hasegawa, T.; Seimiya, H.; Fujihara, T.; Fujiwara, N.; Yamada, H. Aroma profile of star anise and the structure-odor relationship of anethole. Nat. Prod. Commun. 2014, 9, 251–256. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Zhang, Y.; Yuan, X.; Sun, E. Determination of volatile compounds of Illicium verum Hook. F. Using simultaneous distillation-extraction and solid phase microextraction coupled with gas chromatography-mass spectrometry. Trop. J. Pharm. Res. 2015, 14, 1879–1884. [Google Scholar] [CrossRef] [Green Version]
- Li, S.N.; Sun, J.F.; Wang, J.M.; Jin, L.; Zong, T.Q.; Zhou, W.; Li, G. Two new phenolic glycosides from the fruits of Illicium verum. J. Asian Nat. Prod. Res. 2021, 24, 31–38. [Google Scholar] [CrossRef]
- Song, W.Y.; Ma, Y.B.; Bai, X.; Zhang, X.M.; Gu, Q.; Zheng, Y.T.; Zhou, J.; Chen, J.J. Two new compounds and anti-HIV active constituents from Illicium verum. Planta Med. 2007, 73, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.N.; Su, X.H.; Huo, C.H.; Zhang, X.P.; Shi, Q.W.; Gu, Y.C. Chemical constituents of plants from the genus Illicium. Chem. Biodivers. 2009, 6, 963–989. [Google Scholar] [CrossRef]
- Sayyar, H.T. Exotic Spice Illicium Verum Hook. F. J. Bahria Univ. Med. Dent. Coll. 2018, 8, 189–193. [Google Scholar] [CrossRef]
- Mauseth, J.D. Plants as sources of medicines, drugs, and psychoactive compounds. In Plants and People; Jones & Bartlett Learning: Burlington, MA, USA, 2012; pp. 353–354. [Google Scholar]
- Min, H.J.; Kim, C.S.; Hyun, H.J.; Bae, Y.S. Essential oil analysis of Illicium anistum L. extracts. J. Korean Wood Sci Technol. 2017, 45, 682–688. [Google Scholar]
- Yang, J.F.; Yang, C.H.; Chang, H.W.; Yang, C.S.; Wang, S.M.; Hsieh, M.C.; Chuang, L.Y. Chemical composition and antibacterial activities of Illicium verum against antibiotic-resistant pathogens. J. Med. Food. 2010, 13, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Benmalek, Y.; Yahia, O.A.; Belkebir, A.; Fardeau, M.L. Anti-microbial and anti-oxidant activities of Illicium verum, Crataegus oxyacantha ssp. monogyna and Allium cepa red and white varieties. Bioengineered 2013, 4, 244–248. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.C.; Hsieh, Y.Y.; Chuang, L.Y. Comparison of the phytochemical composition and antibacterial activities of the various extracts from leaves and twigs of Illicium verum. Molecules 2021, 26, 3909. [Google Scholar] [CrossRef]
- Li, H.; Wu, X.; Li, X.; Cao, X.; Li, Y.; Cao, H.; Men, Y. Multistage Extraction of Star Anise and Black Pepper Derivatives for Antibacterial, Antioxidant, and Anticancer Activity. Front. Chem. 2021, 9, 660138. [Google Scholar] [CrossRef]
- Dzamic, A.; Sokovic, M.; Ristic, M.S.; Grijic-Jovanovic, S.; Vukojevic, J.; Marin, P.D. Chemical composition and antifungal activity of Illicium verum and Eugenia caryophyllata essential oils. Chem. Nat. Compd. 2009, 45, 259–261. [Google Scholar] [CrossRef]
- Yazdani, D.; Rezazadeh, S.; Amin, G.; Zainal Abidin, M.A.; Shahnazi, S.; Jamalifar, H. Antifungal activity of dried extracts of anise (Pimpinella anisum L.) and star anise (Illicium verum Hook, f.) against dermatophyte and saprophyte fungi. J. Med. Plants 2009, 8, 24–29. [Google Scholar]
- Aly, S.E.; Sabry, B.A.; Shaheen, M.S.; Hathout, A.S. Assessment of antimycotoxigenic and antioxidant activity of star anise (Illicium verum) in vitro. J. Saudi Soc. Agric. Sci. 2016, 15, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Dinesha, R.; Thammannagowda, S.S.; Prabhu, M.S.L.; Madhu, C.S.; Srinivas, L. The antioxidant and DNA protectant activities of Star Anise (Illicium verum) aqueous extracts. J. Pharmacogn. Phytochem. 2014, 2, 98–103. [Google Scholar]
- Yang, C.H.; Chang, F.R.; Chang, H.V.; Wang, S.M.; Hsieh, M.C.; Chuang, L.Y. Investigation of the antioxidant activity of Illicium verum extracts. J. Med. Plants Res. 2012, 6, 314–324. [Google Scholar]
- Sung, Y.Y.; Kim, Y.S.; Kim, H.K. Illicium verum extract inhibits TNF-α- and IFN-γ-induced expression of chemokines and cytokines in human keratinocytes. J. Ethnopharmacol. 2012, 144, 182–189. [Google Scholar] [CrossRef]
- Cosmetic Ingredient Database (CosIng). Available online: www.ec.europa.eu (accessed on 1 September 2021).
- Newberne, P.; Smith, R.L.; Doull, J.; Goodman, J.I.; Munro, I.C.; Portoghese, P.S.; Wagner, B.M.; Weil, C.S.; Woods, L.A.; Adams, T.B.; et al. The FEMA GRAS assessment of trans-anethole used as a flavouring substance. Food Chem. Toxicol. 1999, 37, 789–811. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA). Anisi Stellati Fructus. In The European Agency for the Evaluation of Medical Products Veterinary Medicines and Informaion Technology Unit; European Medicines Agency: London, UK, 2000. [Google Scholar]
- Ulbricht, C.E. Star anise. In Natural Standard Herb And Supplement Guide: An Evidence-Based Reference; Mosby Elsevier: Amsterdam, The Netherlands, 2010; pp. 677–678. [Google Scholar]
- Nakamura, T.; Okuyama, E.; Yamazaki, M. Neurotropic Components from Star Anise (Illicium verum Hook. fil.). Chem. Pharm. Bull. 1977, 57, 364–370. [Google Scholar] [CrossRef] [Green Version]
- Akçan, R.; Lale, A.; Tümer, A.R. Trans-Anethole: A Key Compound in Bogma Raki. Acta Med. Cordoba 2018, 49, 26–31. [Google Scholar]
- De, M.; De, A.K.; Sen, P.; Banerjee, A.B. Antimicrobial properties of Star anise (Illicium verum Hook f). Phyther. Res. 2002, 16, 94–95. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, P.; Pruss, A.; Masiuk, H.; Mnichowska-Polanowska, M.; Kaczmarek, M.; Giedrys-Kalemba, S.; Dołęgowska, B.; Zielińska-Bliźniewska, H.; Olszewski, J. The effect of fennel essential oil and trans-anethole on antibacterial activity of mupirocin against Staphylococcus aureus isolated from asymptomatic carriers. Postep Dermatol. Alergol. 2019, 36, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hançer Aydemir, D.; Çifci, G.; Aviyente, V.; Boşgelmez-Tinaz, G. Quorum-sensing inhibitor potential of trans-anethole aganist Pseudomonas aeruginosa. J. Appl. Microbiol. 2018, 125, 731–739. [Google Scholar] [CrossRef]
- Kim, K.Y.; Lee, H.S.; Seol, G.H. Anti-inflammatory effects of trans-anethole in a mouse model of chronic obstructive pulmonary disease. Biomed. Pharmacother. 2017, 91, 925–930. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Kim, S.H.; Kim, D.S.; Lee, J.E.; Kim, H.K. Illicium verum Extract and Trans-Anethole Attenuate Ovalbumin-Induced Airway Inflammation via Enhancement of Foxp3+ Regulatory T Cells and Inhibition of Th2 Cytokines in Mice. Mediat. Inflamm. 2017, 2017, 7506808. [Google Scholar] [CrossRef] [Green Version]
- Moradi, J.; Abbasipour, F.; Zaringhalam, J.; Maleki, B.; Ziaee, N.; Khodadoustan, A.; Janahmadi, M. Anethole, a Medicinal Plant Compound, Decreases the Production of Pro-Inflammatory TNF-α and IL-1β in a Rat Model of LPS-Induced Periodontitis. Iran J. Pharm Res. 2014, 13, 1319–1325. [Google Scholar] [PubMed]
- Kang, N.H.; Mukherjee, S.; Min, T.; Kang, S.C.; Yun, J.W. Trans-anethole ameliorates obesity via induction of browning in white adipocytes and activation of brown adipocytes. Biochimie 2018, 151, 1–13. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the safety and efficacy of anthranilate derivatives (chemical group 18) when used as flavourings for all animal species. EFSA J. 2011, 9, 2441. [Google Scholar]
- European Chemical Agency (ECHA). Available online: www.echa.europa.eu (accessed on 15 September 2021).
- Poon, T.S.C.; Freeman, S. Cheilitis caused by contact allergy to anethole in spearmint flavoured toothpaste. Australas. J. Dermatol. 2006, 47, 300–301. [Google Scholar] [CrossRef]
- Rudzki, E.; Grzywa, Z. Sensitizing and irritating properties of star anise oil. Contact Dermat. 1976, 2, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bravo, B.; Bernal, A.P.; Garcia-Hernández, M.J.; Camacho, F. Occupational contact dermatitis from anethole in food handlers. Contact Dermat. 1997, 37, 38. [Google Scholar] [CrossRef] [PubMed]

| Group of Compounds | Raw Material | Compound Name | References |
|---|---|---|---|
| Phenolic compounds | Essential oil | Trans-anethole, cis-anethole, estragole | [16] |
| Fruit | Shikimic acid | [4] | |
| Root | Illiverin A, 4-allyl-2-(3-methylbut-2-enyl)-1,6-methylenedioxybenzene-3-ol, illicinole, 3-hydroxy-4,5-me-thylenedioxyallyl-benzene, (−)-illicinone-A, 4-allyl-4-(3-methylbut-2-enyl)-1,2-methylenedioxycyclohexa-2,6-dien-5-one, 3,4-seco-(24Z)-cycloart-4(28),24-diene-3,26-dioic acid, 26-methyl ester | [22] | |
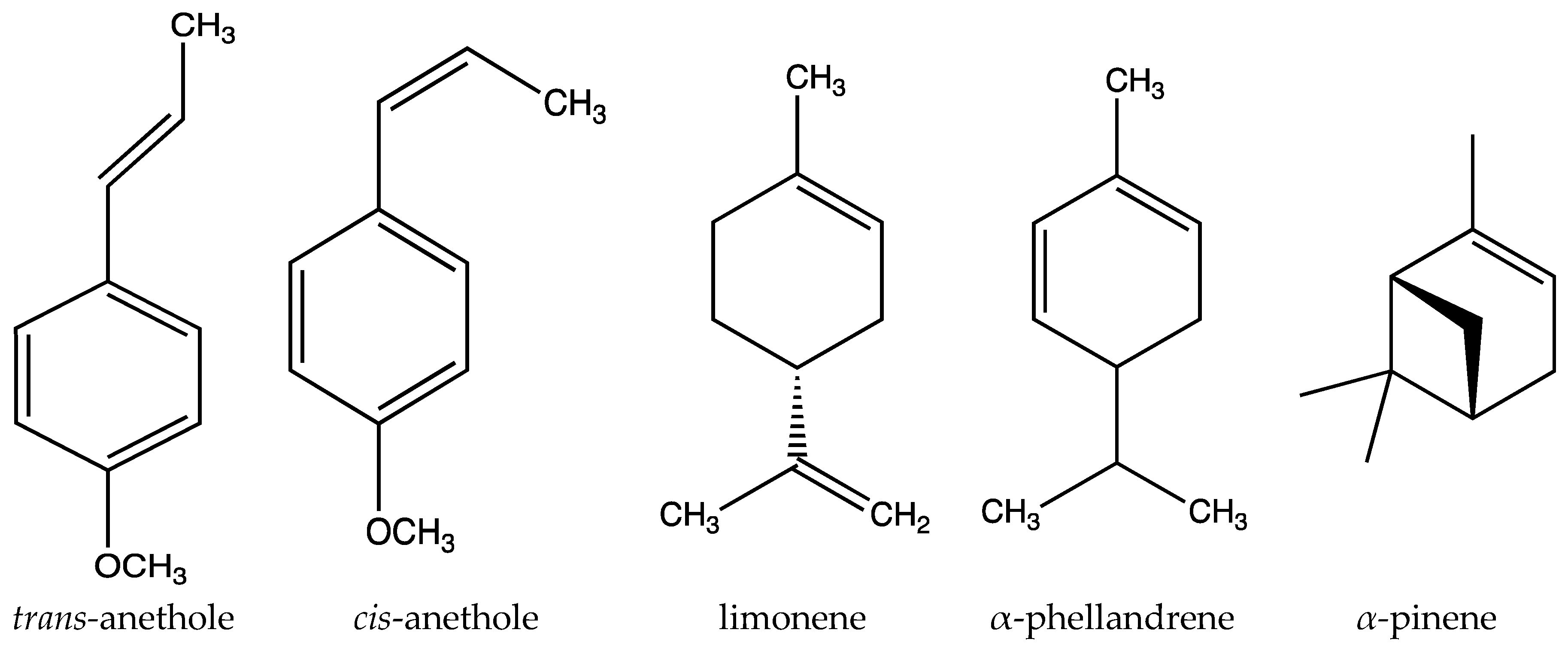
| Monoterpenoids | Essential oil | α-Pinene, p-cymene, limonene, linalool, terpinen-4-ol, α-terpineol, eugenol, γ -terpineol, ơ-3-carene, camphene, β-myrcene, trans-ocymene, terpinolene, γ -terpinene | [4,17] |
| Sesquiterpenoids | Essential oil | α-Phellandrene, α-muurolene, β-caryophyllene, α-copaene, trans-α-bergamotene, foeniculin, β-elemene, cyperene, α-caryophyllene, (+)-9-epiledene, cubebene | [4,17] |
| Root | Tashironin, tashironin A, 11-O-debenzoyl-11α-O-2-methylcyclopent-1-enecarboxyltashironin, veranisatins A–C | [1,23] | |
| Flavonoids | Essential oil | Trans-chalcone | [18] |
| Fruit | Kaempferol and glucosides, quercetin and glucosides | [1] | |
| Fatty acids | Fruit | Linoleic acid, stearic acid, myristic acid | [4] |
| Alkylglucosides | Fruit | R-sec-butyl-d-glucopyranoside | [4] |
| Biphenyl-type neolignans | Leaf | Verimol G and verimol H, 4,4′-dihydroxy-3,3′-dimethoxy-9,9′-epoxylignan | [23] |
| Aldehydes | Essential oil | p-Anisaldehyde | [4,18] |
| Other | Essential oil | Anisoxide, 2-(1-cyclopentenyl)-furan, isobornyl thiocyanoacetate | [8,17] |
| Form | Function |
|---|---|
| Illicium verum fruit extract | Perfuming, skin conditioning |
| Illicium verum fruit water | Fragrance, perfuming |
| Illicium verum fruit powder | Exfoliating |
| Illicium verum fruit oil | Perfuming |
| Illicium verum seed oil | Fragrance, oral care, tonic |
| Illicium verum leaf oil | Flavoring, fragrance, skin conditioning |
| Manufacturer | Country | Trade Name | Form | Form of I. verum in a Composition of the Cosmetic (INCI) According to the Manufacturer | Function |
|---|---|---|---|---|---|
| MUGLER www.mugler.com | France | Cuir Impertinent | Perfumed water | Star Anise—top note | Perfuming |
| Tonymoly www.tonymoly.us | Korea | I’M POMEGRANATE Mask Sheet | Mask sheet | Illicium verum (Anise) Fruit Extract | Moisturizing, elasticizing |
| I’M REAL Makgeolli Mask Sheet | Mask sheet | Illicium verum (Anise) Extract | Smoothing, moisturizing | ||
| I’M AVOCADO Nutrition Beauty Mask Sheet | Mask sheet | Illicium verum (Anise) Fruit Extract | Nourishing, revitalizing | ||
| I’M RED WINE Pore Care Beauty Mask Sheet | Mask sheet | Illicium verum (Anise) Fruit Extract | Cleansing, tightening | ||
| Elizavecca www.elizavecca.com | Korea | Pore Clean Up AHA Fruit Toner | Cleansing toner | Illicium verum (Anise) Fruit Extract | Exfoliating, cleansing, moisturizing |
| Gold CF-NEST Collagen Jella Pack Beauty Mask | Face mask | Illicium verum (Anise) Fruit Extract | Elasticizing, firming, exfoliating | ||
| Gold CF-NEST, White Bomb Eye Cream | Eye cream | Illicium verum (Anise) Fruit Extract | Brightening, smoothing | ||
| Skin Liar Primer | Face primer | Illicium verum (Anise) Fruit Extract | Brightening, smoothing | ||
| Perfect Sparking Peeling Pad | Peeling pad | Illicium verum (Anise) Fruit Extract | Cleansing, exfoliating | ||
| Klairs www.klairscosmetics.com | Korea | Rich Moist Soothing Serum | Serum | Illicium verum (Anise) Fruit Extract | Moisturizing, soothing |
| Freshly Juiced Vitamin Drop Serum | Serum | Illicium verum (Anise) Fruit Extract | Brightening, smoothing, improving the skin condition | ||
| Rich Moist Foaming Cleanser | Foaming cleanser | Illicium verum (Anise) Fruit Extract | Moisturizing, soothing, refreshing, cleansing | ||
| Supple Preparation All Over Lotion | Lotion | Illicium verum (Anise) Fruit Extract | Moisturizing, protective, soothing | ||
| Son & Park www.en.sonandpark.com | Korea | Beauty Water | Cleansing water/ toner | Illicium verum (Anise) Fruit/Seed Oil | Moisturizing, refreshing, exfoliating, cleansing |
| COSRX www.cosrx.com | Korea | Low pH Barrier Mist | Face mist | Illicium verum (Anise) Fruit Extract | Moisturizing, refreshing, elasticizing, restoring pH balance |
| D’Alba www.dalbaglobal.com | Korea | White Truffle Whitening | Cream | Illicium verum (Anise) Fruit Extract | Brightening, protective, elasticizing |
| Missha www.missha.com | Korea | Real Solution Tencel Sheet Mask | Sheet mask | Illicium verum (Anise) Fruit Extract | Moisturizing, soothing, strengthening the skin natural barrier |
| Aromatica www.thearomatica.com | Korea | Rosemary Scalp Scaling Shampoo | Shampoo | Illicium verum (Anise) Fruit Extract | Nourishing, exfoliating |
| Doctor Babor www.babor.com | Germany | 3D Hydro Gel Face Mask | Face mask | Illicium verum (Anise) Fruit Extract | Moisturizing, elasticizing, refreshing, toning |
| Oceanic www.oceanic.com.pl | Poland | Facial Sheet Mask Rose + Phytocollagen | Sheet mask | Illicium verum (Anise) Fruit Extract | Regenerating, smoothing, improving the skin condition |
| Facial Sheet Mask Lemon + Vitamin C | Sheet mask | Illicium verum (Anise) Fruit Extract | Brightening, smoothing, rejuvenating, revitalizing | ||
| EO Laboratorie www.ec-l.ru/en | Russia | Smoothness& Tonus Scrub | Scrub | Illicium verum Oil | Elasticizing, moisturizing, softening, exfoliating |
| PIXI www.pixibeauty.com | United States | Rose Glow Mist | Mist | Illicium verum (Anise) Fruit Extract | Moisturizing, refreshing, elasticizing, protective against free radicals |
| Glow Glycolic Boost | Sheet mask | Illicium verum (Anise) Fruit Extract | Brightening, moisturizing | ||
| Rose Caviar Essense | Flower oil | Illicium verum (Anise) Fruit Extract | Moisturizing, softening, nourishing | ||
| Eco-Dent www.eco-dent.com | Unites States | GentleFloss Mint | Dental floss | Illicium verum Oil | Refreshing, anti-cavity |
| Jason Natural www.jason-personalcare.com | United States | Powersmile, Antiplaque &Whitening Toothpaste | Toothpaste | Illicium verum (Anise) Fruit/Seed Oil | Whitening, reducing unpleasant odor |
| Kerosene www.houseofkerosene.com | Unites States | Black Vines | Perfumed water | Star Anise | Perfuming |
| Dr Bronner’s www.drbronner.com | Great Britain | Peppermint Toothpaste | Toothpaste | Organic Illicium verum (Anise) Seed Oil | Whitening, refreshing, reducing plaque |
| Jo Malone www.jomalone.com | Great Britain | Vanilla & Anise Cologne | Cologne | Star Anise—top note | Perfuming |
| DIESEL www.diesel.com | Italy | Loverdose | Perfumed water | Star Anise—top note | Perfuming |
| OUDFACTORY www.oudfactory.com | United Arab Emirates | Moya Kvitka | Perfumed water | Star Anise | Perfuming |
| Biological Activity | Characteristics | Tested Raw Material/Chemical Compound | References |
|---|---|---|---|
| Antibacterial activity | Inhibitory activity against Staphylococcus aureus | Trans-anethole | [44] |
| Inhibitory activity against: Escherichia coli quorum sensing capacity, lasB expression, and Pseudomonas aeruginosa PAO1 virulence factor production | Trans-anethole | [45] | |
| Inhibitory activity against: Acinetobacter baumannii, Pseudomonas aeruginosa, Staphylococcus aureus | Ethanol extract of the I. verum herb | [27] | |
| Inhibitory activity against Gram-positive bacteria: Bacillus subtilis, B. cereus, B. licheniformis, B. megatarium, Sarcina lutea, Staphylococcus aureus Inhibitory activity against Gram-negative bacteria: Agrobacterium tumefacienes, Bradyrhizobium japonicum, Escherichia coli, Klebsiella pneumoniae, K. aerogenes, Rhizobium leguminosarum | Extract from I. verum fruit | [43] | |
| Inhibitory activity against: Bacillus megatarium, B. subtilis, Rhizobium leguminosarum, Sarcina lutea. | Trans-anetole | [43] | |
| Inhibitory activity against: Acinetobacter baumannii | Essential oil of I. verum | [16] | |
| Antifungal activity | Inhibitory activity against: Alternaria solani, Bipolaris maydis, Botryodiplodia theobromae, Fusarium graminearum, F. oxysporum f. sp. cucumerinum, F. oxysporum f. sp. lycopersici, F. oxysporum f. sp. vasinfectum, Magnaporthe oryzae, Pythium aphanidermatum, Rhizoctonia cerealis and R. solani. | Essential oil from I. verum fruit, and isolated trans-anethole | [17] |
| Inhibitory activity against: Alternaria alternata, Aspergillus niger, A. ochraceus, Aspergillus flavus, A. terreus, A. versicolor, Aureobasidium pullulans, Candida albicans, Cladosporium cladosporioides, C. fulvium, Fusarium tricinctum, F. sporotrichioides, Mucor mucedo, Penicillium funiculosum, P. ochrochloron, Phomopsis helianthi, Phoma magdonaldii, Trichoderma viride, Trichophyton mentagrophytes | Essential oil of I. verum fruit | [31] | |
| Inhibitory activity against: Aspergillus niger, Candida albicans, Epidermophyton floccosum, Microsporum canis and Trichophyton mentagrophytes | Ethanol extract of the I. verum fruit | [32] | |
| Inhibitory activity against aflatoxin B1 and fumonisin B1, 100% antifungal activity in a dose dependent manner (200 ppm) | Essential oil of I. verum fruit | [33] | |
| Antioxidant activity | Strong antioxidant activity in DPPH test (IC50 = 3.46%) | Essential oil of I. verum | [16] |
| Protective activity against DNA damage caused by hydrogen peroxide, inhibitory activity against human peripheral lymphocyte cell death, lipid peroxide inhibitory activity and hydroxyl radicals | Aqueous extract of the I. verum fruit | [34] | |
| Strong antioxidant activity in DPPH test | Ethyl acetate fraction from I. verum fruit | [35] | |
| Anti-inflammatory activity | Inhibitory activity against mRNA expression induced by TNF-a /IFN-γ and protein expression of thymus, regulation of chemokine activation (TARC/CCL17), macrophage-derived chemokine (MDC/CCL22) oral interleukin (IL-6 i IL-1β) | Ethanol extract of the I. verum fruit | [36] |
| Inhibitory activity against nuclear factor (NF-κB) translocation into the nucleus, phosphorylation and IκBα degradation | Trans-anethole isolated from I. verum fruit | [36] | |
| Decreased activity of lactate dehydrogenase, blood pressure regulation, the reduction of level of pro-inflammatory cytokines (IL-4, TNF-α) | Trans-anethole | [46] | |
| Airway hyperresponsiveness suppression, inhibitory activity against immunoglobulin E (IgE) production, reduced production of interleukin 4 (IL-4) in the supernatant of splenocyte cultures | Trans-anethole | [47] | |
| Inhibitory activity against IL-1β i TNF-α expression | Trans-anethole | [48] | |
| Anti-obesity activity | Adipocytes browning induction, lipolysis activation, inhibitory activity against adipogenesis and lipogenesis | Trans-anethole | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharafan, M.; Jafernik, K.; Ekiert, H.; Kubica, P.; Kocjan, R.; Blicharska, E.; Szopa, A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules 2022, 27, 650. https://doi.org/10.3390/molecules27030650
Sharafan M, Jafernik K, Ekiert H, Kubica P, Kocjan R, Blicharska E, Szopa A. Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules. 2022; 27(3):650. https://doi.org/10.3390/molecules27030650
Chicago/Turabian StyleSharafan, Marta, Karolina Jafernik, Halina Ekiert, Paweł Kubica, Ryszard Kocjan, Eliza Blicharska, and Agnieszka Szopa. 2022. "Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications" Molecules 27, no. 3: 650. https://doi.org/10.3390/molecules27030650
APA StyleSharafan, M., Jafernik, K., Ekiert, H., Kubica, P., Kocjan, R., Blicharska, E., & Szopa, A. (2022). Illicium verum (Star Anise) and Trans-Anethole as Valuable Raw Materials for Medicinal and Cosmetic Applications. Molecules, 27(3), 650. https://doi.org/10.3390/molecules27030650








