Production of an Anise- and Woodruff-like Aroma by Monokaryotic Strains of Pleurotus sapidus Grown on Citrus Side Streams
Abstract
1. Introduction
2. Results and Discussion
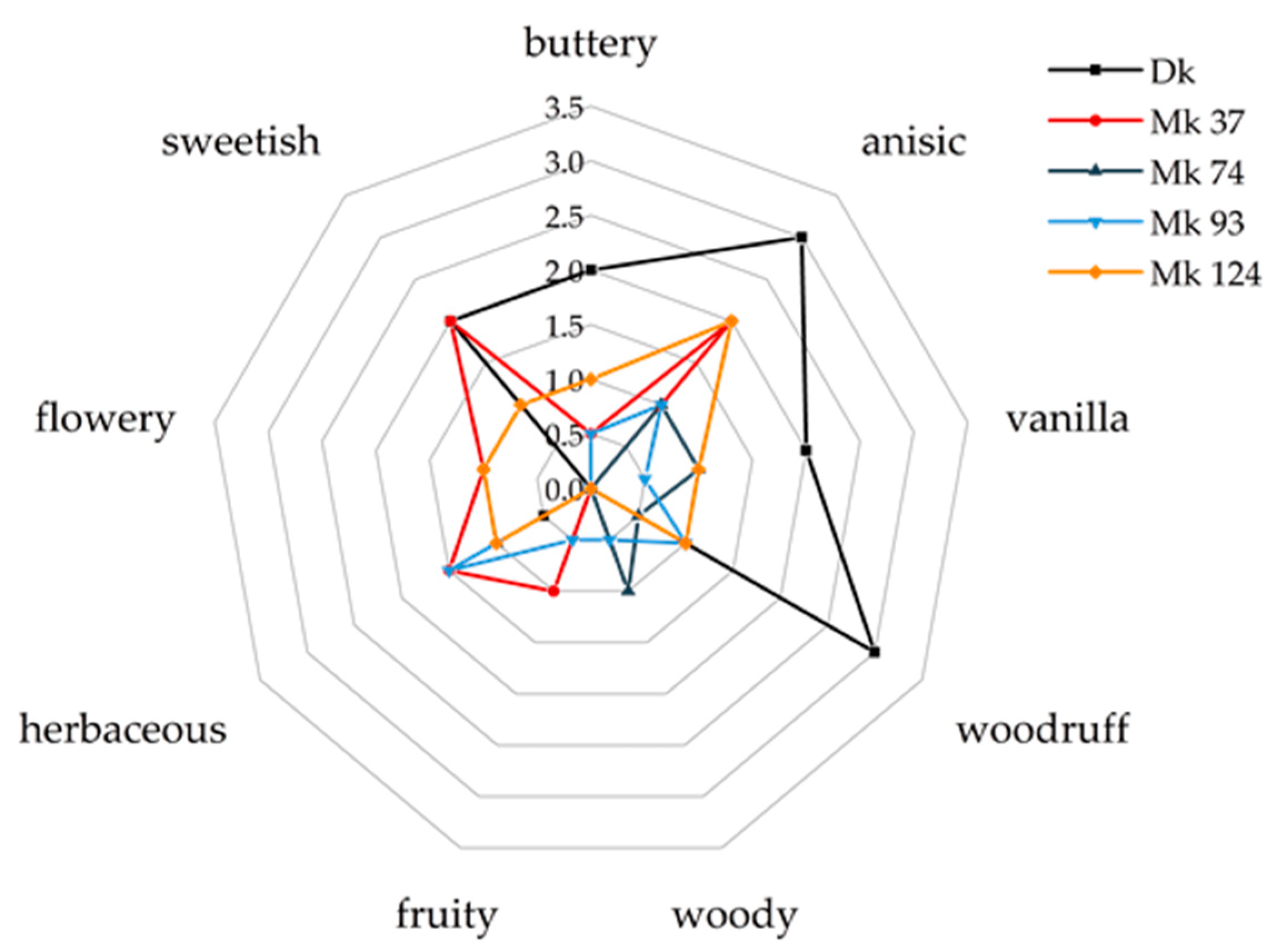
2.1. Screening of Di- and Monokaryotic Strains of Pleurotus sapidus
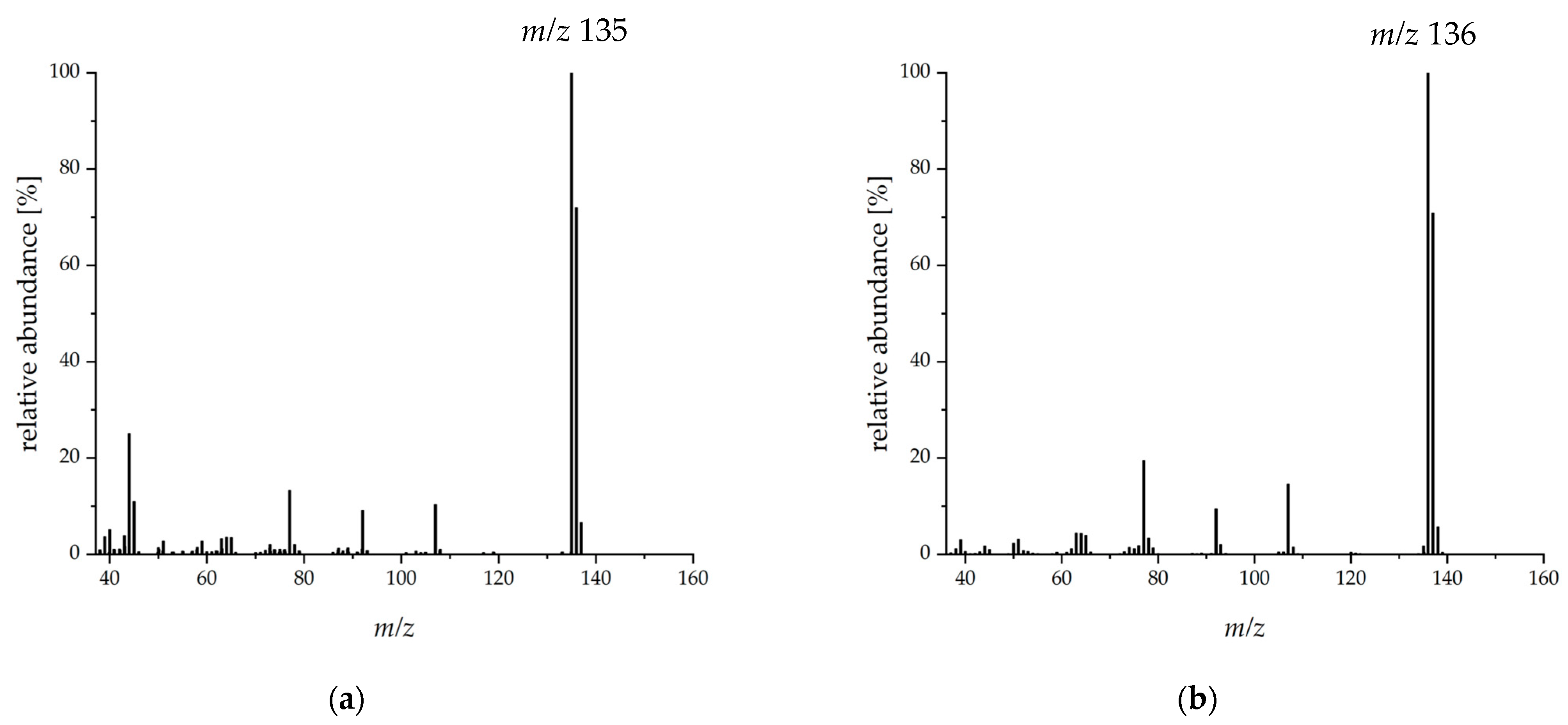
2.2. Aroma Extract Dilution Analysis and Compound Identification
2.3. Stable Isotope Dilution Analysis
2.4. Supplementation with l-Tyrosine and Formation of 2-HPP
2.5. Odor Threshold of 2-HPP
2.6. Proposed Pathways
3. Materials and Methods
3.1. Chemicals
3.2. Citrus Side Stream
3.3. Fungal Strains
3.4. Cultivation of Pleurotus sapidus
3.5. Screening of Surface Cultures
3.6. Liquid/Liquid Extraction, Solvent-Assisted Flavor Evaporation, and Sensory Analysis
3.7. Aroma Extract Dilution Analysis
3.8. Identification and Structure Elucidation
3.9. Stable Isotope Dilution Analysis of p-Anisaldehyde
3.10. Supplementation of l-Tyrosine
3.11. Synthesis of 1-(4-Methoxyphenyl)-1,[2-13C]-Propanedione and [2-13C]-HPP
3.12. Approximation of the Odor Threshold of 2-HPP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| AEDA | Aroma extract dilution analysis |
| CSS(M) | Citrus side stream (medium) |
| Dk | Dikaryon/dikaryotic strain |
| DyP | Dye-decolorizing peroxidase |
| FD | Flavor dilution |
| 1-HPP | 1-Hydroxy-1-(4-methoxyphenyl)-2-propanone |
| 2-HPP | 2-Hydroxy-1-(4-methoxyphenyl)-1-propanone |
| IS | Internal standard |
| LLE | Liquid/liquid extraction/liquid/liquid extract |
| MEP | Malt extract peptone |
| Mk | Monokaryon/monokaryotic strain |
| ni | Not identified |
| O | Olfactometry |
| ODP | Olfactory detection port |
| P/D | Pentane/diethylether |
| PSA | Pleurotus sapidus |
| RI | Retention index |
| SAFE | Solvent-assisted flavor evaporation |
| SIDA | Stable isotope dilution analysis |
| SNS | Standard nutrition solution |
| STD | Standard |
| ThDP | Thiamine diphosphate |
References
- Food and Agriculture Organization of the United Nations (FAO). FAOSTAT. Available online: http://www.fao.org/faostat/en/#data/QCL (accessed on 8 October 2021).
- González-Molina, E.; Domínguez-Perles, R.; Moreno, D.A.; García-Viguera, C. Natural Bioactive Compounds of Citrus limon for Food and Health. J. Pharm. Biomed. Anal. 2010, 51, 327–345. [Google Scholar] [CrossRef]
- Omarini, A.; Dambolena, J.S.; Lucini, E.; Jaramillo Mejía, S.; Albertó, E.; Zygadlo, J.A. Biotransformation of 1,8-Cineole by Solid-State Fermentation of Eucalyptus waste from the essential oil industry using Pleurotus ostreatus and Favolus tenuiculus. Folia Microbiol. 2016, 61, 149–157. [Google Scholar] [CrossRef]
- Trapp, T.; Zajul, M.; Ahlborn, J.; Stephan, A.; Zorn, H.; Fraatz, M.A. Submerged Cultivation of Pleurotus sapidus with Molasses: Aroma Dilution Analyses by Means of Solid Phase Microextraction and Stir Bar Sorptive Extraction. J. Agric. Food Chem. 2018, 66, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Bosse, A.K.; Fraatz, M.A.; Zorn, H. Formation of Complex Natural Flavours by Biotransformation of Apple Pomace with Basidiomycetes. Food Chem. 2013, 141, 2952–2959. [Google Scholar] [CrossRef]
- Bilal, M.; Asgher, M. Biodegradation of Agrowastes by Lignocellulolytic Activity of an Oyster Mushroom, Pleurotus sapidus. J. Natl. Sci. Found. Sri Lanka 2016, 44, 399. [Google Scholar] [CrossRef]
- Galperin, I.; Javeed, A.; Luig, H.; Lochnit, G.; Rühl, M. An Aryl-Alcohol Oxidase of Pleurotus sapidus: Heterologous Expression, Characterization, and Application in a 2-Enzyme System. Appl. Microbiol. Biotechnol. 2016, 100, 8021–8030. [Google Scholar] [CrossRef] [PubMed]
- Lauber, C.; Schwarz, T.; Nguyen, Q.K.; Lorenz, P.; Lochnit, G.; Zorn, H. Identification, Heterologous Expression and Characterization of a Dye-Decolorizing Peroxidase of Pleurotus sapidus. AMB Express 2017, 7, 164. [Google Scholar] [CrossRef]
- Linke, D.; Bouws, H.; Peters, T.; Nimtz, M.; Berger, R.G.; Zorn, H. Laccases of Pleurotus sapidus: Characterization and Cloning. J. Agric. Food Chem. 2005, 53, 9498–9505. [Google Scholar] [CrossRef]
- Zorn, H.; Peters, T.; Nimtz, M.; Berger, R.G. The Secretome of Pleurotus sapidus. Proteomics 2005, 5, 4832–4838. [Google Scholar] [CrossRef]
- Krahe, N.-K.; Berger, R.G.; Witt, M.; Zorn, H.; Omarini, A.B.; Ersoy, F. Monokaryotic Pleurotus sapidus Strains with Intraspecific Variability of an Alkene Cleaving DyP-Type Peroxidase Activity as a Result of Gene Mutation and Differential Gene Expression. Int. J. Mol. Sci. 2021, 22, 1363. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, A.; Caramelo, L.; Prieto, A.; Martínez, M.J.; Martínez, A.T. Anisaldehyde Production and Aryl-Alcohol Oxidase and Dehydrogenase Activities in Ligninolytic Fungi of the Genus Pleurotus. Appl. Environ. Microbiol. 1994, 60, 1783–1788. [Google Scholar] [CrossRef] [PubMed]
- Krings, U.; Hapetta, D.; Berger, R.G. Bioconversion of β-Myrcene to Perillene by Pleurotus ostreatus. Biocatal. Biotransform. 2008, 26, 288–295. [Google Scholar] [CrossRef]
- Trapp, T.; Kirchner, T.; Birk, F.; Fraatz, M.A.; Zorn, H. Biosynthesis of Stereoisomers of Dill Ether and Wine Lactone by Pleurotus sapidus. J. Agric. Food Chem. 2019, 67, 13400–13411. [Google Scholar] [CrossRef] [PubMed]
- Abraham, B.G.; Berger, R.G. Higher Fungi for Generating Aroma Components through Novel Biotechnologies. J. Agric. Food Chem. 1994, 42, 2344–2348. [Google Scholar] [CrossRef]
- Jensen, K.A.; Evans, K.M.C.; Kirk, T.K.; Hammel, K.E. Biosynthetic Pathway for Veratryl Alcohol in the Ligninolytic Fungus Phanerochaet chrysosporium. Appl. Environ. Microbiol. 1994, 60, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Krings, U.; Hinz, M.; Berger, R.G. Degradation of [2H]Phenylalanine by the Basidiomycete Ischnoderma benzoinum. J. Biotechnol. 1996, 51, 123–129. [Google Scholar] [CrossRef]
- Lapadatescu, C.; Giniès, C.; Le Quéré, J.-L.; Bonnarme, P. Novel Scheme for Biosynthesis of Aryl Metabolites from L-Phenylalanine in the Fungus Bjerkandera adusta. Appl. Environ. Microbiol. 2000, 66, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Omarini, A.B.; Plagemann, I.; Schimanski, S.; Krings, U.; Berger, R.G. Crosses Between Monokaryons of Pleurotus sapidus or Pleurotus florida Show an Improved Biotransformation of (+)-Valencene to (+)-Nootkatone. Bioresour. Technol. 2014, 171, 113–119. [Google Scholar] [CrossRef]
- Zhong, S.; Ren, J.; Chen, D.; Pan, S.; Wang, K.; Yang, S.; Fan, G. Free and Bound Volatile Compounds in Juice and Peel of Eureka Lemon. FSTR 2014, 20, 167–174. [Google Scholar] [CrossRef]
- Guneser, B.A.; Yilmaz, E. Bioactives, Aromatics and Sensory Properties of Cold-Pressed and Hexane-Extracted Lemon (Citrus limon L.) Seed Oils. J. Am. Oil Chem. Soc. 2017, 94, 723–731. [Google Scholar] [CrossRef]
- Cannon, R.J.; Kazimierski, A.; Curto, N.L.; Li, J.; Trinnaman, L.; Jańczuk, A.J.; Agyemang, D.; Da Costa, N.C.; Chen, M.Z. Identification, Synthesis, and Characterization of Novel Sulfur-Containing Volatile Compounds from the In-Depth Analysis of Lisbon Lemon Peels (Citrus limon L. Burm. f. cv. Lisbon). J. Agric. Food Chem. 2015, 63, 1915–1931. [Google Scholar] [CrossRef]
- Choi, H.S.; Kondo, Y.; Sawamura, M. Characterization of the Odor-Active Volatiles in Citrus Hyuganatsu (Citrus tamurana Hort. ex Tanaka). J. Agric. Food Chem. 2001, 49, 2404–2408. [Google Scholar] [CrossRef] [PubMed]
- Moshonas, M.G.; Shaw, P.E.; Veldhuis, M.K. Analysis of Volatile Constituents from Meyer Lemon Oil. J. Agric. Food Chem. 1972, 20, 751–752. [Google Scholar] [CrossRef]
- Busmann, D.; Berger, R.G. Conversion of Myrcene by Submerged Cultured Basidiomycetes. J. Biotechnol. 1994, 37, 39–43. [Google Scholar] [CrossRef]
- Gallois, A.; Gross, B.; Langlois, D.; Spinnler, H.-E.; Brunerie, P. Influence of culture conditions on production of flavour compounds by 29 ligninolytic Basidiomycetes. Mycol. Res. 1990, 94, 494–504. [Google Scholar] [CrossRef]
- Drawert, F.; Berger, R.G.; Neuhäuser, K. Biosynthesis of Flavor Compounds by Microorganisms 4. Characterization of the Major Principles of the Odor of Pleurotus euosmus. Eur. J. Appl. Microbiol. Biotechnol. 1983, 18, 124–127. [Google Scholar] [CrossRef]
- Blanch, G.P.; Nicholson, G.J. Determination of the Enantiomeric Composition of Limonene and Limonene-1,2-epoxide in Lemon Peel by Multidimensional Gas Chromatography with Flame-Ionization Detection and Selected Ion Monitoring Mass Spectrometry. J. Chromatogr. Sci. 1998, 36, 37–43. [Google Scholar] [CrossRef]
- Belletti, N.; Ndagijimana, M.; Sisto, C.; Guerzoni, M.E.; Lanciotti, R.; Gardini, F. Evaluation of the Antimicrobial Activity of Citrus Essences on Saccharomyces cerevisiae. J. Agric. Food Chem. 2004, 52, 6932–6938. [Google Scholar] [CrossRef]
- Chamblee, T.S.; Clark, B.C., Jr.; Brewster, G.B.; Radford, T.; Iacobucci, G.A. Quantitative Analysis of the Volatile Constituents of Lemon Peel Oil. Effects of Silica Gel Chromatography on the Composition of its Hydrocarbon and Oxygenated Fractions. J. Agric. Food Chem. 1991, 39, 162–169. [Google Scholar] [CrossRef]
- Brescia, F.F.; Pitelas, W.; Yalman, S.; Popa, F.; Hausmann, H.G.; Wende, R.C.; Fraatz, M.A.; Zorn, H. Formation of Diastereomeric Dihydromenthofurolactones by Cystostereum murrayi and Aroma Dilution Analysis Based on Dynamic Headspace Extraction. J. Agric. Food Chem. 2021, 69, 5997–6004. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Maroto, M.C.; Díaz-Maroto Hidalgo, I.J.; Sánchez-Palomo, E.; Pérez-Coello, M.S. Volatile Components and Key Odorants of Fennel (Foeniculum vulgare Mill.) and Thyme (Thymus vulgaris L.) Oil Extracts Obtained by Simultaneous Distillation−Extraction and Supercritical Fluid Extraction. J. Agric. Food Chem. 2005, 53, 5385–5389. [Google Scholar] [CrossRef] [PubMed]
- Zeller, A.; Rychlik, M. Character Impact Odorants of Fennel Fruits and Fennel Tea. J. Agric. Food Chem. 2006, 54, 3686–3692. [Google Scholar] [CrossRef] [PubMed]
- Afifi, S.M.; El-Mahis, A.; Heiss, A.G.; Farag, M.A. Gas Chromatography-Mass Spectrometry-Based Classification of 12 Fennel (Foeniculum vulgare Miller) Varieties Based on Their Aroma Profiles and Estragole Levels as Analyzed Using Chemometric Tools. ACS Omega 2021, 6, 5775–5785. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Y.; Yuan, X.; Sun, E. Determination of Volatile Compounds of Illicium verum Hook. f. Using Simultaneous Distillation-Extraction and Solid Phase Microextraction Coupled with Gas Chromatography-Mass Spectrometry. Trop. J. Pharm. Res. 2015, 14, 1879. [Google Scholar] [CrossRef]
- Brunschwig, C.; Senger-Emonnot, P.; Aubanel, M.-L.; Pierrat, A.; George, G.; Rochard, S.; Raharivelomanana, P. Odor-Active Compounds of Tahitian Vanilla Flavor. Food Res. Int. 2012, 46, 148–157. [Google Scholar] [CrossRef]
- Zhang, S.; Mueller, C. Comparative Analysis of Volatiles in Traditionally Cured Bourbon and Ugandan vanilla bean (Vanilla planifolia) Extracts. J. Agric. Food Chem. 2012, 60, 10433–10444. [Google Scholar] [CrossRef]
- Takahashi, M.; Inai, Y.; Miyazawa, N.; Kurobayashi, Y.; Fujita, A. Identification of the Key Odorants in Tahitian Cured Vanilla Beans (Vanilla tahitensis) by GC-MS and an Aroma Extract Dilution Analysis. Biosci. Biotechnol. Biochem. 2013, 77, 601–605. [Google Scholar] [CrossRef]
- Shigeto, A.; Hachisuka, S.; Kumazawa, K. Characterization of Potent Odorants in Three Different Cultivars (Madagascar, Comoro and Tahiti) of Vanilla Bean by Aroma Extract Dilution Analysis (AEDA). FSTR 2016, 22, 811–816. [Google Scholar] [CrossRef]
- Honda, Y.; Ori, A.; Tsuchihashi, G.-I. A General Synthetic Method of Chiral 2-Arykalkanoic Esters via Thermal 1,2-Rearrangement. Bull. Chem. Soc. Jpn. 1987, 60, 1027–1036. [Google Scholar] [CrossRef]
- Liu, W.; Chen, C.; Zhou, P. N,N-Dimethylformamide (DMF) as a Source of Oxygen To Access α-Hydroxy Arones via the α-Hydroxylation of Arones. J. Org. Chem. 2017, 82, 2219–2222. [Google Scholar] [CrossRef]
- Koprowski, M.; Łuczak, J.; Krawczyk, E. Asymmetric Oxidation of Enol Phosphates to α-Hydroxy Ketones by (Salen)manganese(III) complex. Effects of the Substitution Pattern of Enol Phosphates on the Stereochemistry of Oxygen Transfer. Tetrahedron 2006, 62, 12363–12374. [Google Scholar] [CrossRef]
- Stadler, M.; Mayer, A.; Anke, H.; Sterner, O. Fatty Acids and Other Compounds with Nematicidal Activity from Cultures of Basidiomycetes. Planta Med. 1994, 60, 128–132. [Google Scholar] [CrossRef]
- Guth, H. Determination of the Configuration of Wine Lactone. Helv. Chim. Acta 1996, 79, 1559–1571. [Google Scholar] [CrossRef]
- Steinhaus, M.; Fritsch, H.T.; Schieberle, P. Quantitation of (R)- and (S)-Linalool in Beer Using Solid Phase Microextraction (SPME) in Combination with a Stable Isotope Dilution Assay (SIDA). J. Agric. Food Chem. 2003, 51, 7100–7105. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Gold, M.H. Direct Cleavage of the Vicinal Diol Linkage of the Lignin Model Compound Dihydroanisoin by the Basidiomycete Phanerochaete chrysosporium. Arch. Microbiol. 1983, 134, 299–302. [Google Scholar] [CrossRef]
- Okamoto, K.; Narayama, S.; Katsuo, A.; Shigematsu, I.; Yanase, H. Biosynthesis of p-Anisaldehyde by the White-Rot Basidiomycete Pleurotus ostreatus. J. Biosci. Bioeng. 2002, 93, 207–210. [Google Scholar] [CrossRef]
- Silk, P.J.; Macaulay, J.B. Stereoselective Biosynthesis of Chloroarylpropane Diols by the Basidiomycete Bjerkandera adusta. Chemosphere 2003, 52, 503–512. [Google Scholar] [CrossRef]
- Swarts, H.J.; Verhagen, F.J.M.; Field, J.A.; Wijnberg, J.B.P.A. Identification and Synthesis of Novel Chlorinated p-Anisylpropanoid Metabolites from Bjerkandera Species. J. Nat. Prod. 1998, 61, 1110–1114. [Google Scholar] [CrossRef]
- Silk, P.J.; Aubry, C.; Lonergan, G.C.; Macaulay, J.B. Chlorometabolite Production by the Ecologically Important White Rot Fungus Bjerkandera adusta. Chemosphere 2001, 44, 1603–1616. [Google Scholar] [CrossRef]
- Brambilla, U.; Nasini, G.; Pava, O.V.D. Secondary Mold Metabolites, Part 49. Isolation, Structural Elucidation, and Biomimetic Synthesis of Trametol, a New 1-Arylpropane-1,2-diol Produced by the Fungus Trametes sp. J. Nat. Prod. 1995, 58, 1251–1253. [Google Scholar] [CrossRef]
- Buswell, J.A.; Odier, E.; Kirk, T.K. Lignin Biodegradation. Crit. Rev. Biotechnol. 1987, 6, 1–60. [Google Scholar] [CrossRef]
- Brauns, F.E. The Chemistry of Lignin; Academic Press Inc.: New York, NY, USA, 1952. [Google Scholar]
- Pepper, J.M.; Saha, M. The Synthesis of Aroyl Methylketones as Lignin Model Substances. Can. J. Chem. 1964, 42, 113–120. [Google Scholar] [CrossRef]
- Nakamura, K.; Kondo, S.-I.; Kawai, Y.; Hida, K.; Kitano, K.; Ohno, A. Enantio- and Regioselective Reduction of α-Diketones by Baker’s Yeast. Tetrahedron Asymmetry 1996, 7, 409–412. [Google Scholar] [CrossRef]
- Quin, M.B.; Flynn, C.M.; Wawrzyn, G.T.; Choudhary, S.; Schmidt-Dannert, C. Mushroom Hunting by Using Bioinformatics: Application of a Predictive Framework Facilitates the Selective Identification of Sesquiterpene Synthases in Basidiomycota. Chembiochem 2013, 14, 2480–2491. [Google Scholar] [CrossRef]
- Neuser, F.; Zorn, H.; Berger, R.G. Generation of Odorous Acyloins by Yeast Pyruvate Decarboxylases and their Occurrence in Sherry and Soy Sauce. J. Agric. Food Chem. 2000, 48, 6191–6195. [Google Scholar] [CrossRef]
- Zorn, H.; Schüler, M.; Berger, R.G. Pyruvate Decarboxylase Catalysed Formation of Terpenoid α-Hydroxy Ketones. Biocatal. Biotransform. 2003, 21, 341–347. [Google Scholar] [CrossRef]
- Beck, H.C. Biosynthetic Pathway for Halogenated Methoxybenzaldehydes in the White Rot Fungus Bjerkandera adusta. FEMS Microbiol. Lett. 1997, 149, 233–238. [Google Scholar] [CrossRef]
- Mester, T.; Swarts, H.J.; Romero i Solé, S.; de Bont, J.A.M.; Field, J.A. Stimulation of Aryl Metabolite Production in the Basidiomycete Bjerkandera sp. Strain BOS55 with Biosynthetic Precursors and Lignin Degradation Products. Appl. Environ. Microbiol. 1997, 63, 1987–1994. [Google Scholar] [CrossRef]
- Demir, A.S.; Şeşenoglu, Ö.; Eren, E.; Hosrik, B.; Pohl, M.; Janzen, E.; Kolter, D.; Feldmann, R.; Dünkelmann, P.; Müller, M. Enantioselective Synthesis of ɑ-Hydroxy Ketones via Benzaldehyde Lyase-Catalyzed C-C Bond Formation Reaction. Adv. Synth. Catal. 2002, 344, 96–103. [Google Scholar] [CrossRef]
- Dünnwald, T.; Demir, A.S.; Siegert, P.; Pohl, M.; Müller, M. Enantioselective Synthesis of (S)-2-Hydroxypropanone Derivatives by Benzoylformate Decarboxylase Catalyzed C-C Bond Formation. Eur. J. Org. Chem. 2000, 11, 2161–2170. [Google Scholar] [CrossRef]
- Postemsky, P.D.; Bidegain, M.A.; Lluberas, G.; Lopretti, M.I.; Bonifacino, S.; Inés Landache, M.; Zygadlo, J.A.; Fernández-Lahore, M.; Omarini, A.B. Biorefining via Solid-State Fermentation of Rice and Sunflower By-Products Employing Novel Monosporic Strains from Pleurotus sapidus. Bioresour. Technol. 2019, 289, 121692. [Google Scholar] [CrossRef] [PubMed]
- Fraatz, M.A.; Naeve, S.; Hausherr, V.; Zorn, H.; Blank, L.M. A Minimal Growth Medium for the Basidiomycete Pleurotus sapidus for Metabolic Flux Analysis. Fungal Biol. Biotechnol. 2014, 1, 9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van den Dool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Fraatz, M.A.; Riemer, S.J.L.; Stöber, R.; Kaspera, R.; Nimtz, M.; Berger, R.G.; Zorn, H. A Novel Oxygenase from Pleurotus sapidus Transforms Valencene to Nootkatone. J. Mol. Catal. B Enzym. 2009, 61, 202–207. [Google Scholar] [CrossRef]
- Neuser, F.; Zorn, H.; Berger, R.G. Formation of Aliphatic and Aromatic a-Hydroxy Ketones by Zygosaccharomyces bisporus. Z. Nat. 2000, 55, 560–568. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. Readily Accessible 12-I-5 Oxidant for the Conversion of Primary and Secondary Alcohols to Aldehydes and Ketones. J. Org. Chem. 1983, 48, 4155–4156. [Google Scholar] [CrossRef]
- Speicher, A.; Bomm, V.; Eicher, T. Dess-Martin-Periodinan (DMP). J. Prakt. Chem. 1996, 338, 588–590. [Google Scholar] [CrossRef]
- Ullrich, F.; Grosch, W. Identification of the Most Intense Volatile Flavour Compounds Formed During Autoxidation of Linoleic Acid. Z. Lebensm.-Unters.-Forsch. 1987, 184, 277–282. [Google Scholar] [CrossRef]
- Teranishi, R.; Buttery, R.G.; Guadagni, D.G. Odor Quality and Chemical Structure in Fruit and Vegetable Flavors. Ann. N. Y. Acad. Sci. 1974, 237, 209–216. [Google Scholar] [CrossRef]
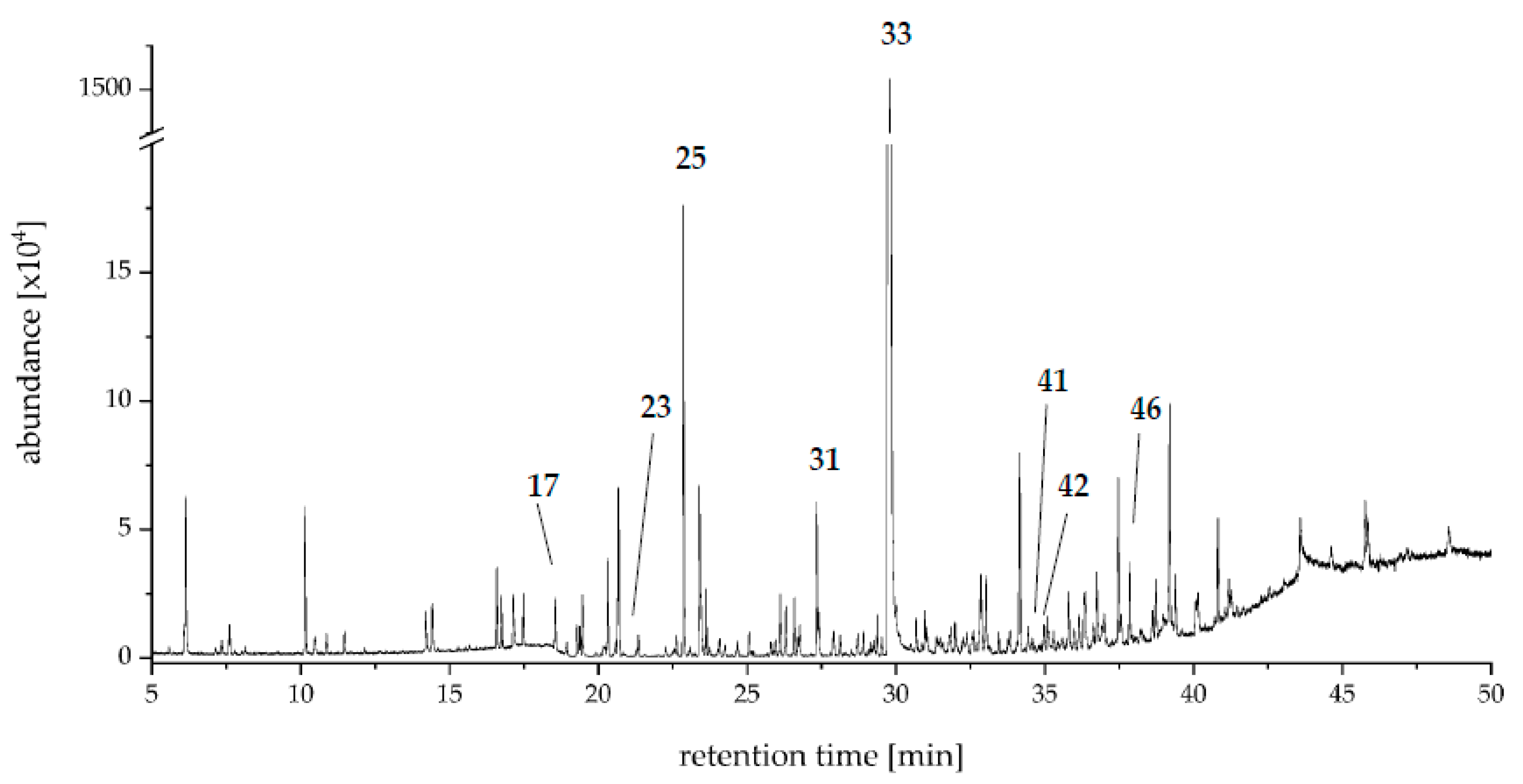

| No. | RI | Compound | Perceived Odor | FD Factor | Identification 1 | |
|---|---|---|---|---|---|---|
| VF-WAXms | DB-5 ms | |||||
| 1 | 1016 | 930 | α-pinene | herbaceous, sweetish, terpenic | 128 | MS, RI, STD, O |
| 2 | 1036 | <800 | 2-methylbut-3-en-2-ol | herbaceaous | 2048 | MS, RI, STD, O |
| 3 | 1062 | Ni 4 | sweetish, fruity | 128 | ||
| 4 | 1102 | 974 | β-pinene | herbaceous, green, sourish | 512 | MS, RI, STD, O |
| 5 | 1120 | 970 | sabinene | terpenic, herbaceous, musty | 2048 | MS, RI, STD, O |
| 6 | 1197 | 1027 | (R)-limonene | citrus, fresh | 256 | MS, RI, STD, O |
| 7 | 1209 | 2-methyl-n-butanol | green, sweetish | 512 | MS, RI, STD, O | |
| 8 | 1268 | 1023 | p-cymene | citrus, fresh, terpenic | 8192 | MS, RI, STD, O |
| 9 2 | 1296 | ni | green, herbaceous | 512 | ||
| 10 2 | 1340 | ni | minty, sweetish | 512 | ||
| 11 | 1345 | 913 | anisole | flowery, sweetish | 1024 | MS, RI, STD, O |
| 12 | 1355 | 878 | hept-1-en-3-ol (IS) | green, spicy | 256 | |
| 13 2,3 | 1375 | ni | citrus, herbaceous | 16,384 | ||
| 14 2,3 | 1424 | ni | musty, terpenic | 256 | ||
| 15 3 | 1448 | 1131 | (Z)-limonene oxide | flowery, sweetish | 1024 | MS, RI, STD, O |
| 16 | 1469 | 1085 | (Z)-linalool oxide (furanoid) | citrus, herbaceous, sweetish | 64 | MS, RI, STD, O |
| 17 | 1510 | 1184 | 3,6-dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran (dill ether)-isomer | green, citrus, sweetish | 32,768 | MS, RI, STD 5, O |
| 18 | 1530 | 958 | benzaldehyde | sweetish | 1024 | MS, RI, STD, O |
| 19 | 1550 | 1098 | linalool | flowery, fruity | 2048 | MS, RI, STD, O |
| 20 | 1593 | 1229 | 3,6-dimethyl-2,3,3a,4,5,7a-hexahydrobenzofuran (dill ether)-isomer | minty, citrus | 128 | MS, RI, STD 5, O |
| 21 | 1601 | 1179 | terpinen-4-ol | terpenic, green | 1024 | MS, RI, STD, O |
| 22 3 | 1608 | 1196 | dihydrocarvone | |||
| 23 | 1630 | ni | flowery, fresh | 32,768 | ||
| 24 | 1634 | 1148 | ni | herbaceous | 8192 | |
| 25 | 1697 | 1193 | α-terpineol | green, herbaceous, flowery | 16,384 | MS, RI, STD, O |
| 26 | 1729 | 1255 | piperitone | citrus, flowery, minty | 64 | MS, RI, STD, O |
| 27 3 | 1763 | 1182 | p-methylacetophenone | sweetish | 2048 | MS, RI, STD, O |
| 28 | 1782 | ni | green, fatty | 2048 | ||
| 29 | 1850 | 1185 | p-cymen-8-ol | sweetish, flowery, citrus | 512 | MS, RI, STD, O |
| 30 | 1881 | ni | flowery, sweetish, green | 1024 | ||
| 31 | 1908 | 1196 | m-anisaldehyde (IS) | flowery, sweetish | 512 | |
| 32 | 1988 | ni | green, sweetish, woodruff | 512 | ||
| 33 | 2034 | 1265 | p-anisaldehyde | anisic, sweetish, woodruff | 262,144 | MS, RI, STD, O |
| 34 | 2080 | 1382 | (E)-methyl cinnamate | balsamic, sweetish | 1024 | MS, RI, STD, O |
| 35 | 2095 | 1371 | methyl-p-anisate | sweetish, herbaceous | 4096 | MS, RI, STD, O |
| 36 | 2121 | ni | minty, sweetish, vanilla | 512 | ||
| 37 | 2155 | 1379 | p-methoxyphenylacetone | green, woody, spicy | 512 | MS, RI, STD, O |
| 38 | 2211 | 1447 | p-methoxypropiophenone | sweetish, fruity, green | 1024 | MS, RI, STD, O |
| 39 | 2240 | 1449 | 3,6-dimethyl-3a,4,5,7a-tetrahydro-1-benzofuran-2(3H)-one (wine lactone)-isomer | sweetish, fruity, green | 2048 | MS, RI, STD, O |
| 40 | 2276 | 1281 | p-methoxybenzyl alcohol | sweetish, herbaceous | 512 | MS, RI, STD, O |
| 41 | 2298 | ni | flowery, waxy, sweetish | 16,384 | ||
| 42 | 2350 | 1524 | ni | flowery, sweetish, herbaceous | 65,536 | |
| 43 | 2400 | 1474 | 3,4-dimethoxybenzaldehyde | sweetish, woodruff | 8192 | MS, RI, STD, O |
| 44 | 2427 | ni | sweetish, fruity | 256 | ||
| 45 | 2461 | ni | flowery, vanilla, herbaceous | 128 | ||
| 46 | 2508 | ni | herbaceous, spicy, flowery | 16,384 | ||
| 47 | 2540 | ni | fruity, herbaceous, sweetish | 1024 | ||
| 48 | 2571 | 1549 | 2-HPP | herbaceous, sweetish | 512 | MS, NMR, HRMS |
| Substrate | Culture Day 4 | Culture Day 6 | Culture Day 8 |
|---|---|---|---|
| Concentration [mg L−1] | |||
| CSS 1 | 9.2 ± 1.7 | 147.0 ± 31.8 | 160.3 ± 26.9 |
| Substrate | Culture Day 4 | Culture Day 6 | Culture Day 8 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| I | II | III | I | II | III | I | II | III | |
| Volume [µL] | |||||||||
| CSS 1 | 10 | 10 | 10 | 200 | 200 | 100 | 300 | 150 | 200 |
| l-tyrosine | 20 | 20 | 30 | 80 | 120 | 100 | 100 | 150 | 80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bürger, F.; Koch, M.; Fraatz, M.A.; Omarini, A.B.; Berger, R.G.; Zorn, H. Production of an Anise- and Woodruff-like Aroma by Monokaryotic Strains of Pleurotus sapidus Grown on Citrus Side Streams. Molecules 2022, 27, 651. https://doi.org/10.3390/molecules27030651
Bürger F, Koch M, Fraatz MA, Omarini AB, Berger RG, Zorn H. Production of an Anise- and Woodruff-like Aroma by Monokaryotic Strains of Pleurotus sapidus Grown on Citrus Side Streams. Molecules. 2022; 27(3):651. https://doi.org/10.3390/molecules27030651
Chicago/Turabian StyleBürger, Friederike, Maximilian Koch, Marco A. Fraatz, Alejandra B. Omarini, Ralf G. Berger, and Holger Zorn. 2022. "Production of an Anise- and Woodruff-like Aroma by Monokaryotic Strains of Pleurotus sapidus Grown on Citrus Side Streams" Molecules 27, no. 3: 651. https://doi.org/10.3390/molecules27030651
APA StyleBürger, F., Koch, M., Fraatz, M. A., Omarini, A. B., Berger, R. G., & Zorn, H. (2022). Production of an Anise- and Woodruff-like Aroma by Monokaryotic Strains of Pleurotus sapidus Grown on Citrus Side Streams. Molecules, 27(3), 651. https://doi.org/10.3390/molecules27030651







