trans-Dichloro(triphenylarsino)(N,N-dialkylamino)platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance
Abstract
:1. Introduction
2. Results and Discussion
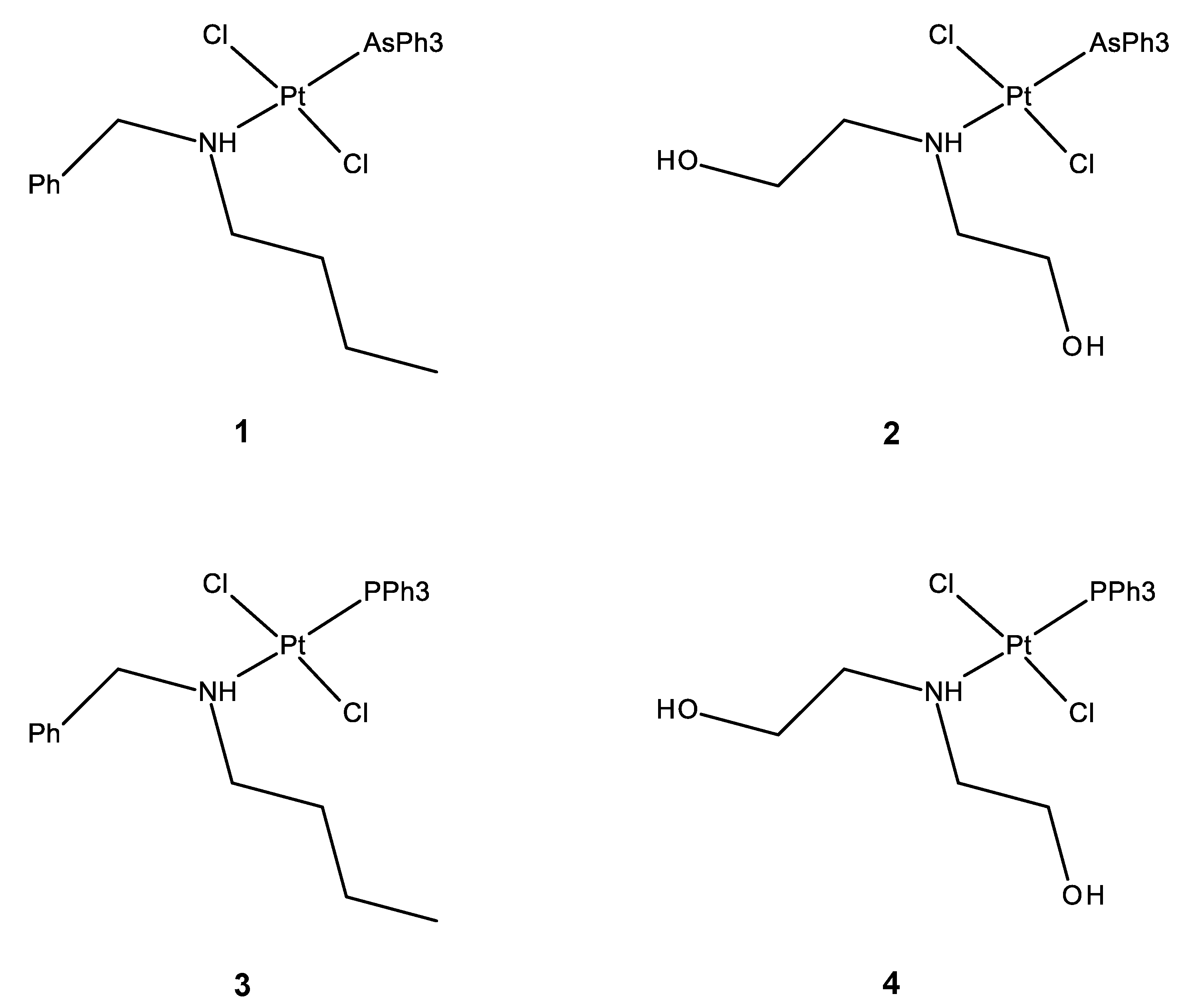
2.1. Synthesis
2.2. Antiproliferative Activity
2.3. Interaction with DNA
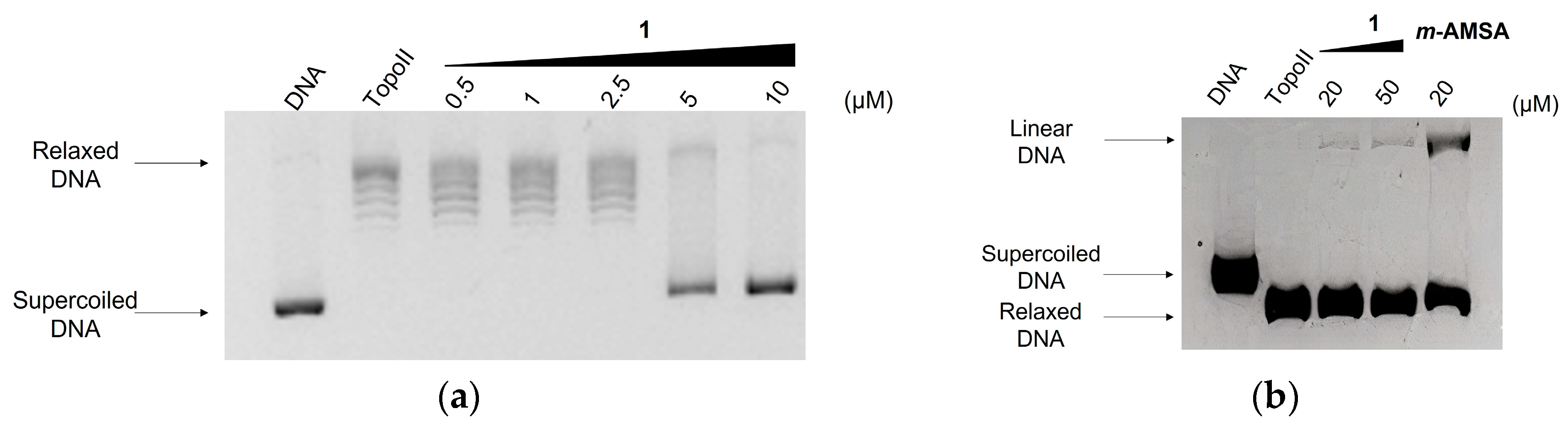
2.4. Effect on Topoisomerase II Catalytic Cycle
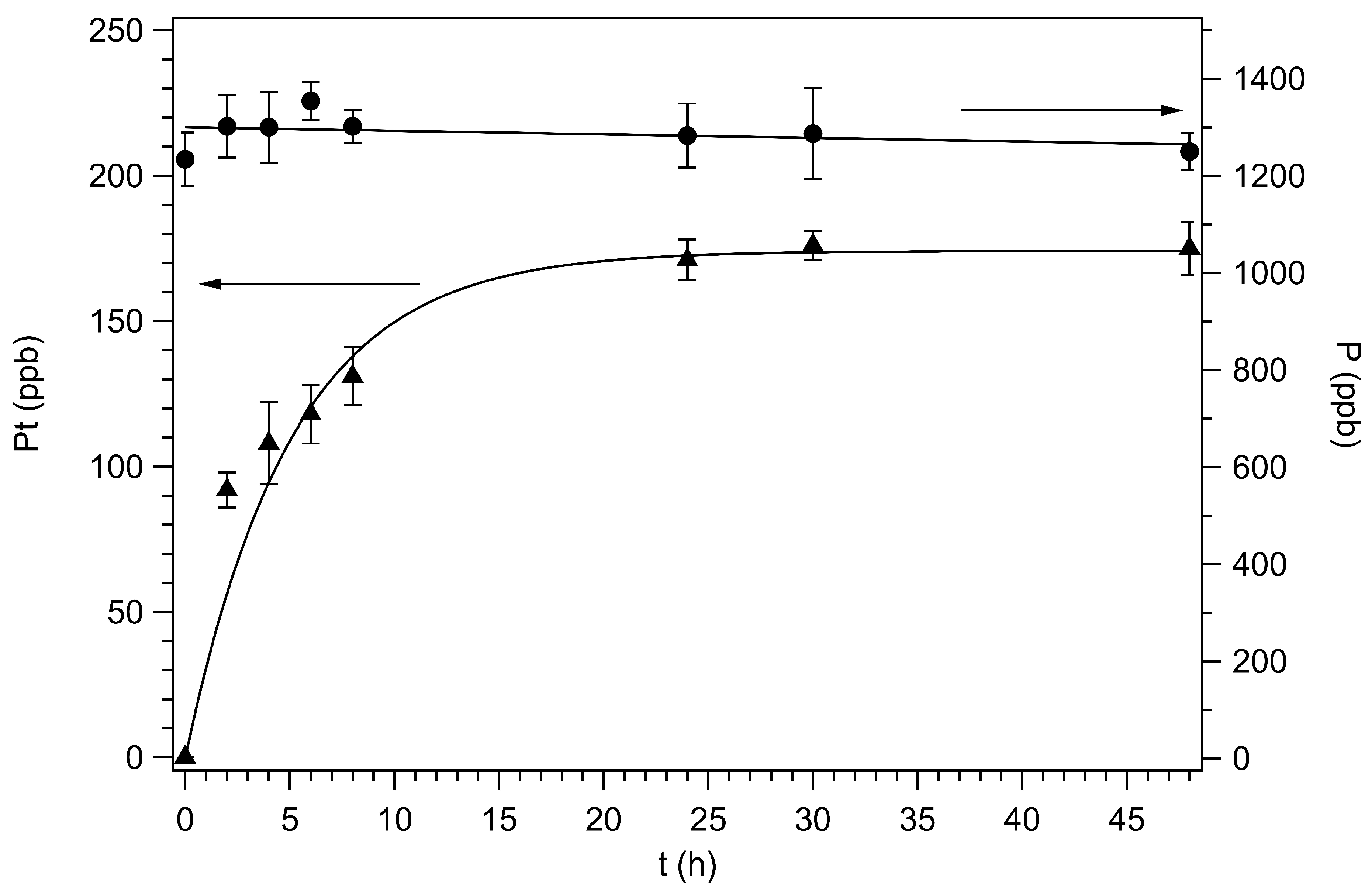
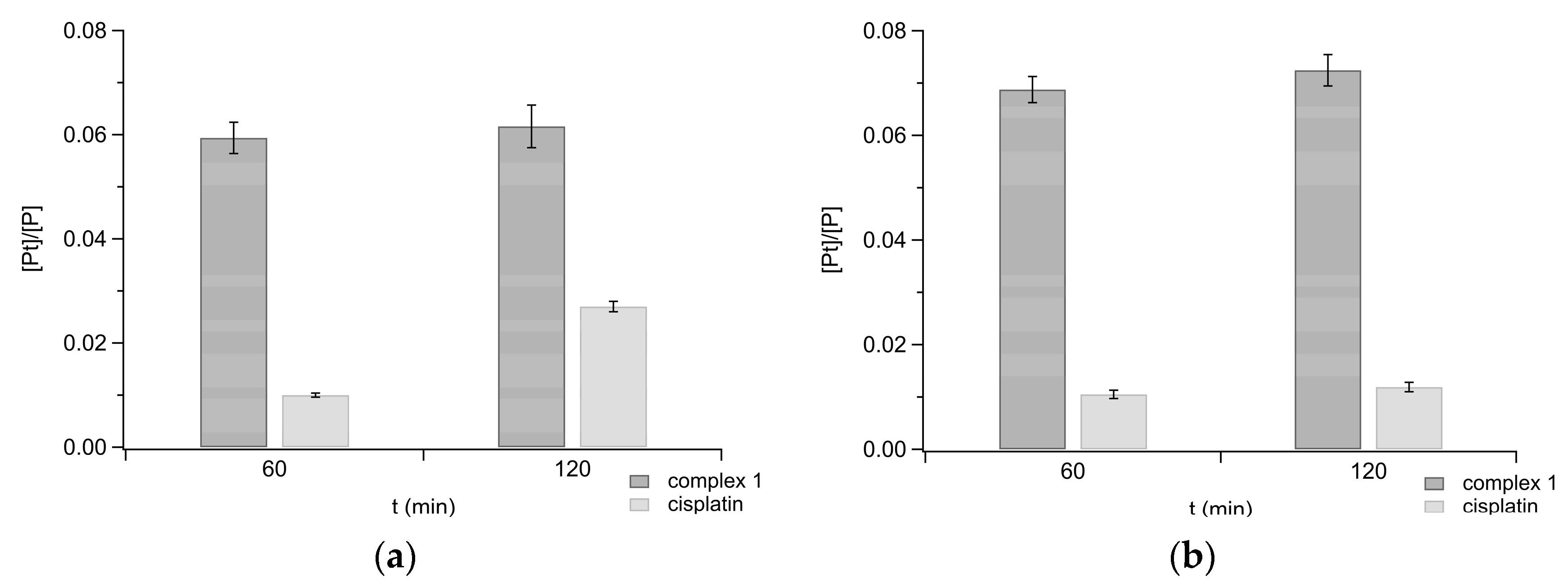
2.5. Uptake in Ovarian Carcinoma Cells
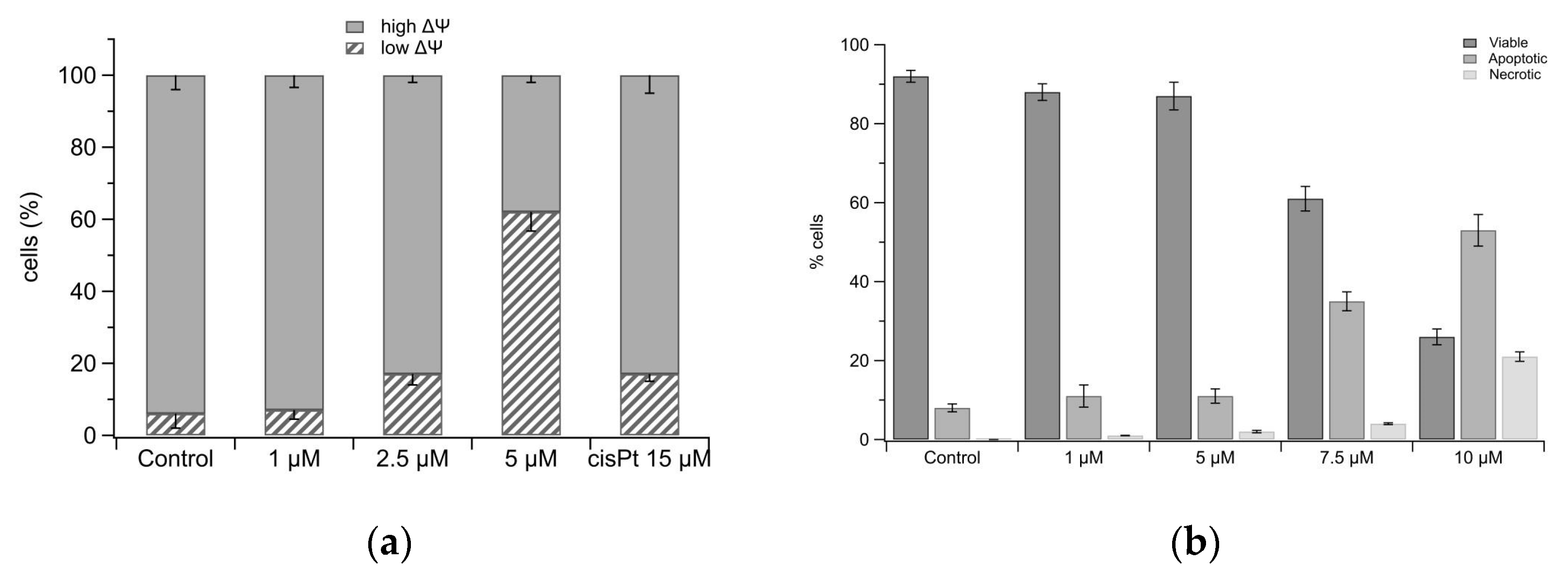
2.6. Mitochondria Damage and Cell Death
3. Materials and Methods
3.1. General Information
3.2. Preparation and Characterization of New Complexes
3.2.1. Synthesis of cis-[PtCl2(AsPh3)(NCMe)]
3.2.2. Synthesis of trans-[Pt(µ-Cl)Cl(AsPh3)]2
3.2.3. Synthesis of trans-[PtCl2(AsPh3)(NHBzBu)] (1)
3.2.4. Synthesis of trans-[PtCl2(AsPh3)(NH(CH2OH)2)] (2)
3.3. Cell Cultures
3.4. Inhibition Growth Assay
3.5. Nucleic Acids
3.6. Binding to DNA
3.7. DNA Topoisomerase Relaxation Assay
3.8. Topoisomerase II-Mediated DNA Cleavage
3.9. Cell Uptake
3.10. Mitochondrial Transmembrane Potential Measurement
3.11. Evaluation of Apoptotic Cell Death by Annexin V-FITC and Propidium Iodide Staining
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [Green Version]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.W.; Tew, K.D.; Townsend, D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar] [CrossRef]
- Santos, N.A.G.D.; Ferreira, R.S.; Santos, A.C.D. Overview of cisplatin-induced neurotoxicity and ototoxicity, and the protective agents. Food Chem. Toxicol. 2019, 136, 111079. [Google Scholar] [CrossRef]
- Tang, Q.; Wang, X.; Jin, H.; Mi, Y.; Liu, L.; Dong, M.; Chen, Y.; Zou, Z. Cisplatin-induced ototoxicity: Updates on molecular mechanisms and otoprotective strategies. Eur. J. Pharm. Biopharm. 2021, 163, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Michels, J.; Brenner, C.; Szabadkai, G.; Harel-Bellan, A.; Castedo, M.; Kroemer, G. Systems biology of cisplatin resistance: Past, present and future. Cell Death Dis. 2014, 5, e1257. [Google Scholar] [CrossRef] [Green Version]
- Pokhriyal, R.; Hariprasad, R.; Kumar, L.; Hariprasad, G. Chemotherapy Resistance in Advanced Ovarian Cancer Patients. Biomark Cancer 2019, 11, 1–19. [Google Scholar] [CrossRef]
- Vousden, K.H.; Lane, D.P. p53 in health and disease. Nat. Rev. Mol. Cell Biol. 2007, 8, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kelland, L. The resurgence of platinum-based cancer chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Duan, M.; Ulibarri, J.; Liu, K.J.; Mao, P. Role of Nucleotide Excision Repair in Cisplatin Resistance. Int. J. Mol. Sci. 2020, 21, 9248. [Google Scholar] [CrossRef]
- Yarbro, C.H. Carboplatin: A clinical review. Semin. Oncol. Nurs. 1989, 5, 63–69. [Google Scholar] [CrossRef]
- Graham, J.; Mushin, M.; Kirkpatrick, P. Oxaliplatin. Nat. Rev. Drug Discov. 2004, 3, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Rottenberg, S.; Disler, C.; Perego, P. The rediscovery of platinum-based cancer therapy. Nat. Rev. Cancer 2021, 21, 37–50. [Google Scholar] [CrossRef] [PubMed]
- McMullen, M.; Madariaga, A.; Lheureux, S. New approaches for targeting platinum-resistant ovarian cancer. Semin. Cancer Biol. 2021, 77, 167–181. [Google Scholar] [CrossRef]
- Ramos-Lima, F.J.; Quiroga, A.G.; Perez, J.M.; Font-Bardia, M.; Solans, X.; Navarro-Ranninger, C. Synthesis and characterization of new transplatinum complexes containing phosphane groups−cytotoxic studies in cisplatin-resistant cells. Eur. J. Inorg. Chem. 2003, 8, 1591–1598. [Google Scholar] [CrossRef]
- Ramos-Lima, F.J.; Quiroga, A.G.; Garcia-Serrelde, B.; Blanco, F.; Carnero, A.; Navarro-Ranninger, C. New trans-platinum drugs with phosphines and amines as carrier ligands induce apoptosis in tumor cells resistant to cisplatin. J. Med. Chem. 2007, 50, 2194–2199. [Google Scholar] [CrossRef] [PubMed]
- Quiroga, A.G.; Ramos-Lima, F.J.; Alvarez-Valdes, A.; Font-Bardia, M.; Bergamo, A.; Sava, G.; Navarro-Ranninger, C. Synthesis, characterization and tumor cell growth inhibition of new trans platinum complexes with phosphane derivatives. Polyhedron 2011, 30, 1646–1650. [Google Scholar] [CrossRef]
- Dalla Via, L.; Garcia-Argaez, A.N.; Agostinelli, E.; Belli Dell’Amico, D.; Labella, L.; Samaritani, S. New trans dichloro (triphenylphosphine)platinum(II) complexes containing N-(butyl),N-(arylmethyl)amino ligands: Synthesis, cytotoxicity and mechanism of action. Bioorg. Med. Chem. 2016, 24, 2929–2937. [Google Scholar] [CrossRef] [Green Version]
- Belli Dell’Amico, D.; Colalillo, M.; Dalla Via, L.; Dell’Acqua, M.; García-Argáez, A.N.; Hyeraci, M.; Labella, L.; Marchetti, F.; Samaritani, S. Synthesis and reactivity of cytotoxic platinum(II) complexes of bidentate oximes—A step towards the functionalization of bioactive complexes. Eur. J. Inorg. Chem. 2018, 1589–1594. [Google Scholar] [CrossRef]
- Hyeraci, M.; Colalillo, M.; Labella, L.; Marchetti, F.; Samaritani, S.; Scalcon, V.; Rigobello, M.P.; Dalla Via, L. Platinum(II) complexes bearing triphenylphosphine and chelating oximes: Antiproliferative effect and biological profile in resistant cells. ChemMedChem 2020, 15, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Hyeraci, M.; Scalcon, V.; Folda, A.; Labella, L.; Marchetti, F.; Samaritani, S.; Rigobello, M.P.; Dalla Via, L. New platinum(II) complexes affecting different biomolecular targets in resistant ovarian carcinoma cells. ChemMedChem 2021, 16, 1956–1966. [Google Scholar] [CrossRef]
- Dalla Via, L.; García-Argáez, A.N.; Adami, A.; Grancara, S.; Martinis, P.; Toninello, A.; Belli Dell’Amico, D.; Labella, L.; Samaritani, S. Synthesis, antiproliferative and mitochondrial impairment activities of bis-alkyl-amino transplatinum complexes. Bioorg. Med. Chem. 2013, 21, 6965–6972. [Google Scholar] [CrossRef] [PubMed]
- Kuźnik, N.; Wendt, O.F. The Trans Effect and Trans Influence of Triphenyl Arsine in Platinum(II) Complexes. A Comparative Mechanistic and Structural Study. J. Chem. Soc. Dalton Trans. 2002, 3074–3078. [Google Scholar] [CrossRef]
- Cheeseman, P.; Odell, A.L.; Raethel, A. Trans-Effect Order for Alkene, Alkyne, Phosphine, Arsine, Stibine, and Sulphide Ligands from Studies of Diethylamine Exchange Reactions of L,PtC12,[14C]NHEt, in Various Solvents. Chem. Commun. 1968, 1496–1498. [Google Scholar] [CrossRef]
- Ayyannan, G.; Veerasamy, P.; Mohanraj, M.; Raja, G.; Manimaran, A.; Velusamy, M.; Bhuvanesh, N.; Nandhakumar, R.; Jayabalakrishnan, C. Biological evaluation of organometallic palladium(II) complexes containing 4-hydroxybenzoic acid (3-ethoxy-2-hydroxybenzylidene)hydrazide: Synthesis, structure, DNA/protein binding, antioxidant activity and cytotoxicity. Appl. Organometal. Chem. 2017, 31, e3599. [Google Scholar] [CrossRef]
- Prakash, G.; Manikandan, R.; Viswanathamurthy, P.; Velmurugan, K.; Nandhakumar, R. Ruthenium(III) S-methylisothiosemicarbazone Schiff base complexes bearing PPh3/AsPh3 coligand: Synthesis, structure and biological investigations, including antioxidant, DNA and protein interaction, and in-vitro anticancer activities. J. Photochem. Photobiol. B Biol. 2014, 138, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Sampath, C.; Jayabalakrishnan, C. Biomolecule interaction and cytotoxicity of ruthenium(III) benzothiazole substituted ferrocenyl thiosemicarcazone complexes. Arab. J. Chem. 2017, 10 (Suppl. 2), S3207–S3215. [Google Scholar] [CrossRef] [Green Version]
- Kamatchi, T.S.; Kalaivani, P.; Poornima, P.; Padma, V.V.; Fronczek, F.R.; Natarajan, K. New organometallic ruthenium(II) complexes containing chelidonic acid (4-oxo-4H-pyran-2,6-dicarboxylic acid): Synthesis, structure and in vitro biological activity. RSC Adv. 2014, 4, 2004–2022. [Google Scholar] [CrossRef]
- Kamatchi, T.S.; Chitrapriya, N.; Lee, H.; Fronczek, C.F.; Fronczek, F.R.; Natarajan, K. Ruthenium(II)/(III) complexes of 4-hydroxy-pyridine-2,6-dicarboxylic acid with PPh3/AsPh3 as co-ligand: Impact of oxidation state and co-ligands on anticancer activity in vitro. Dalton Trans. 2012, 41, 2066–2077. [Google Scholar] [CrossRef] [PubMed]
- Fisher-Fodor, E.; Moldovan, N.; Virag, P.; Soritau, O.; Brie, I.; Lonnecke, P.; Hey-Hawkins, E.; Silaghi-Dumitrescu, L. The CellScan technology for in vitro studies on novel platinum complexes with organoarsenic ligands. Dalton Trans. 2008, 6393–6400. [Google Scholar] [CrossRef]
- Belli Dell’Amico, D.; Labella, L.; Marchetti, F.; Samaritani, S. A Convenient Route to Dinuclear Chloro-Bridged Platinum(II) Derivatives via Nitrile Complexes. Dalton Trans. 2012, 41, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 2009, 9, 327–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, J.; Huang, H.Y.; Lee, S.W.; Chen, T.J.; Tai, H.C.; Hsu, H.P.; Chang, K.Y.; Li, C.F. TOP2A overexpression as a poor prognostic factor in patients with nasopharyngeal carcinoma. Tumour. Biol. 2014, 35, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Ozaki, S.; Yasui, D.; Yamamoto, H.; Zaitsu, J.; Taniyama, D.; Saitou, A.; Kuraoka, K.; Hirata, T.; Taniyama, K. Overexpression of topoisomerase II alpha protein is a factor for poor prognosis in patients with luminal B breast cancer. Oncotarget 2018, 9, 26701–26710. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Shao, B.; Zhou, Y.; Chen, Z. High expression of TOP2A in hepatocellular carcinoma is associated with disease progression and poor prognosis. Oncol. Lett. 2020, 20, 232. [Google Scholar] [CrossRef]
- Ogino, M.; Fujii, T.; Nakazawa, Y.; Higuchi, T.; Koibuchi, Y.; Oyama, T.; Horiguchi, J.; Shirabe, K. Implications of Topoisomerase (TOP1 and TOP2α) expression in patients with breast cancer. In Vivo 2020, 34, 3483–3487. [Google Scholar] [CrossRef]
- Ferrandina, G.; Petrillo, M.; Carbone, A.; Zannoni, G.; Martinelli, E.; Prisco, M.; Pignata, S.; Breda, E.; Savarese, A.; Scambia, G. Prognostic role of topoisomerase-IIalpha in advanced ovarian cancer patients. Br. J. Cancer 2008, 98, 1910–1915. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Zhao, H.; Ren, M.; Chen, Q.; Li, J.; Li, Z.; Yin, C.; Yue, W. TOP2A Promotes Tumorigenesis of high-grade serous ovarian cancer by regulating the TGF-β/Smad pathway. J. Cancer 2020, 11, 4181–4192. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, D.W.; Pouliot, L.M.; Hall, M.D.; Gottesman, M.M. Cisplatin resistance: A cellular self-defense mechanism resulting from multiple epigenetic and genetic changes. Pharmacol. Rev. 2012, 64, 706–721. [Google Scholar] [CrossRef] [Green Version]
- Bondi, R.; Dalla Via, L.; Hyeraci, M.; Pagot, G.; Labella, L.; Marchetti, F.; Samaritani, S. Cytotoxicity and DNA interaction in a series of aryl terminated iminopyridine Pt(II) complexes. J. Inorg. Biochem. 2021, 21, 111335. [Google Scholar] [CrossRef]
- Harrach, S.; Ciarimboli, G. Role of transporters in the distribution of platinum-based drugs. Front. Pharmacol. 2015, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osellame, L.D.; Blacker, T.S.; Duchen, M.R. Cellular and molecular mechanisms of mitochondrial function. Best Pract. Res. Clin. Endocrinol. Metab. 2012, 26, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Armarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996. [Google Scholar]
- Reichmann, E.; Rice, S.A.; Thomas, C.A.; Doty, P. A further examination of the molecular weight and size of desoxypentose nucleic acid. J. Am. Chem. Soc. 1954, 76, 3047. [Google Scholar] [CrossRef]
- Dalla Via, L.; Santi, S.; Di Noto, V.; Venzo, A.; Agostinelli, E.; Calcabrini, A.; Condello, M.; Toninello, A. Platinum(II) chloride indenyl complexes: Electrochemical and biological evaluation. J. Biol. Inorg. Chem. 2011, 16, 695–713. [Google Scholar] [CrossRef] [PubMed]
- Cossarizza, A.; Baccaranicontri, M.; Kalashnikova, G.; Franceschi, C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem. Biophys. Res. Commun. 1993, 197, 40–45. [Google Scholar] [CrossRef] [PubMed]

| Bond Length (Å) | Bond Angle (°) | ||
|---|---|---|---|
| Pt(1)-N(1) | 1.969 (3) | N(1)-Pt(1)-Cl(2) | 177.32 (11) |
| Pt(1)-Cl(2) | 2.2624 (11) | N(1)-Pt(1)-Cl(1) | 88.16 (11) |
| Pt(1)-Cl(1) | 2.3462 (10) | Cl(2)-Pt(1)-Cl(1) | 90.43 (4) |
| Pt(1)-As(1) | 2.3610 (4) | N(1)-Pt(1)-As(1) | 93.42 (10) |
| Cl(2)-Pt(1)-As(1) | 88.14 (3) | ||
| Cl(1)-Pt(1)-As(1) | 175.98 (3) | ||
| GI50 (µM) 1 | ||||||
|---|---|---|---|---|---|---|
| Complex | A2780 | A2780cis | HeLa | HT-29 | A549 | Met-5A |
| 1 | 0.49 ± 0.18 | 1.85 ± 0.16 | 5.75 ± 0.80 | 4.42 ± 0.78 | 4.48 ± 0.48 | 1.66 ± 0.84 |
| 2 | 3.66 ± 1.36 | 6.62 ± 1.50 | 10.9 ± 1.9 | 8.70 ± 1.80 | 17.9 ± 2.0 | 4.24 ± 0.75 |
| 3 2 | 3.55 ± 0.26 | 3.63 ± 0.21 | 5.31 ± 0.65 | nd | nd | |
| 4 3 | nd | nd | 0.42 ± 0.06 | nd | 2.3 ± 0.7 | |
| cisplatin | 1.08 ± 0.14 | 6.64 ± 0.77 | 1.42 ± 0.20 | 3.07 ± 1.05 | 2.45 ± 0.25 | |
| Identification code | Shelx | |
| Empirical formula | C20H18AsCl2NPt | |
| Formula weight | 613.26 | |
| Temperature | 296(2) K | |
| Wavelength | 0.71073 Å | |
| Crystal system | Triclinic | |
| Space group | P-1 | |
| Unit cell dimensions | a = 9.1126(5) Å | α = 62.7160(10)°. |
| b = 10.9018(6) Å | β = 77.1370(10)°. | |
| c = 11.6047(7) Å | γ = 84.6470(10)°. | |
| Volume | 998.83(10) Å3 | |
| Z | 2 | |
| Density (calculated) | 2.039 Mg/m3 | |
| Absorption coefficient | 8.93 mm−1 | |
| F(000) | 580 | |
| Crystal size | 0.342 × 0.259 × 0.212 mm3 | |
| Theta range for data collection | 3.348 to 30.931°. | |
| Index ranges | −13 <= h <= 13, −15 <= k <= 15, −16 <= l <= 16 | |
| Reflections collected | 35,972 | |
| Independent reflections | 6010 [R(int) = 0.0322] | |
| Completeness to theta = 25.242° | 99.8% | |
| Absorption correction | Numerical | |
| Max. and min. transmission | 0.2768 and 0.1532 | |
| Refinement method | Full-matrix least-squares on F2 | |
| Data/restraints/parameters | 6010/0/227 | |
| Goodness-of-fit on F2 | 1.046 | |
| Final R indices [I > 2sigma(I)] | R1 = 0.0327, wR2 = 0.0853 | |
| R indices (all data) | R1 = 0.0363, wR2 = 0.0884 | |
| Extinction coefficient | n/a | |
| Largest diff. peak and hole | 2.063 and −1.809 Å−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyeraci, M.; Agnarelli, L.; Labella, L.; Marchetti, F.; Di Paolo, M.L.; Samaritani, S.; Dalla Via, L. trans-Dichloro(triphenylarsino)(N,N-dialkylamino)platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance. Molecules 2022, 27, 644. https://doi.org/10.3390/molecules27030644
Hyeraci M, Agnarelli L, Labella L, Marchetti F, Di Paolo ML, Samaritani S, Dalla Via L. trans-Dichloro(triphenylarsino)(N,N-dialkylamino)platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance. Molecules. 2022; 27(3):644. https://doi.org/10.3390/molecules27030644
Chicago/Turabian StyleHyeraci, Mariafrancesca, Laura Agnarelli, Luca Labella, Fabio Marchetti, Maria Luisa Di Paolo, Simona Samaritani, and Lisa Dalla Via. 2022. "trans-Dichloro(triphenylarsino)(N,N-dialkylamino)platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance" Molecules 27, no. 3: 644. https://doi.org/10.3390/molecules27030644
APA StyleHyeraci, M., Agnarelli, L., Labella, L., Marchetti, F., Di Paolo, M. L., Samaritani, S., & Dalla Via, L. (2022). trans-Dichloro(triphenylarsino)(N,N-dialkylamino)platinum(II) Complexes: In Search of New Scaffolds to Circumvent Cisplatin Resistance. Molecules, 27(3), 644. https://doi.org/10.3390/molecules27030644