Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages
Abstract
:1. Introduction
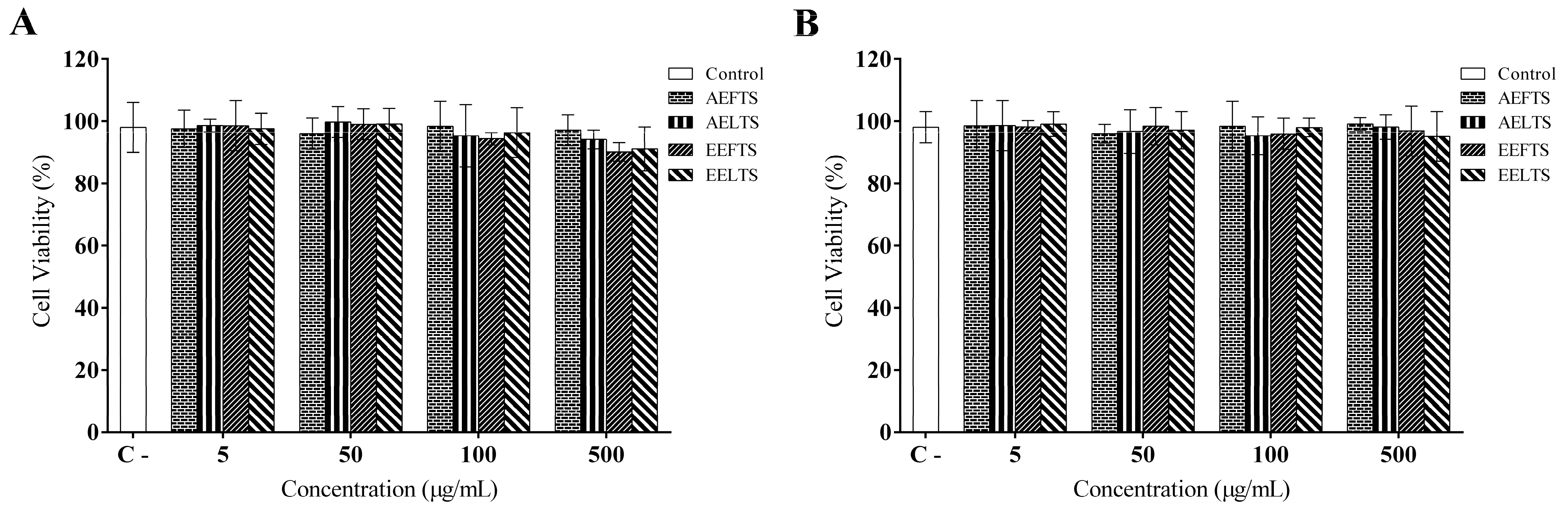
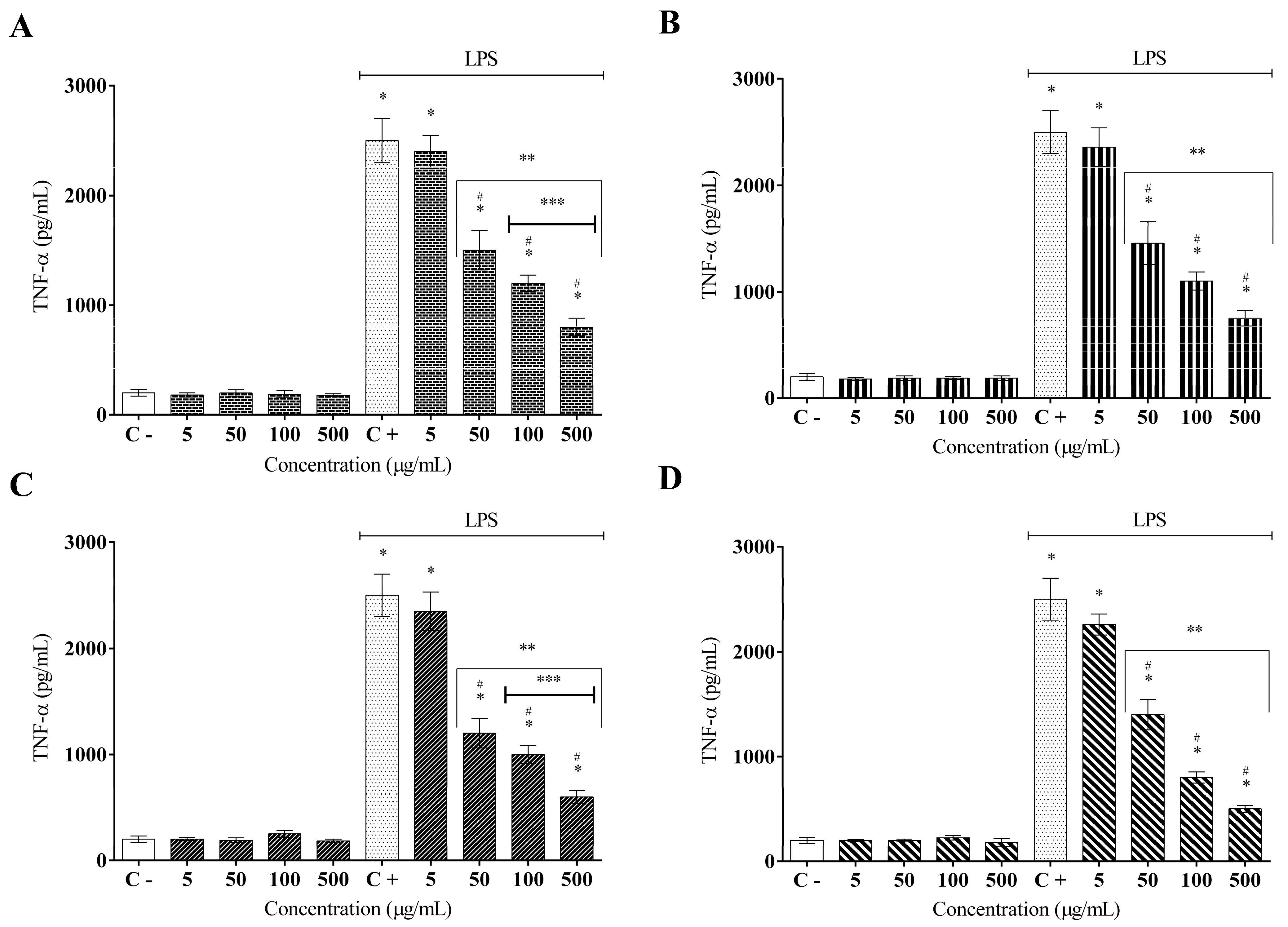
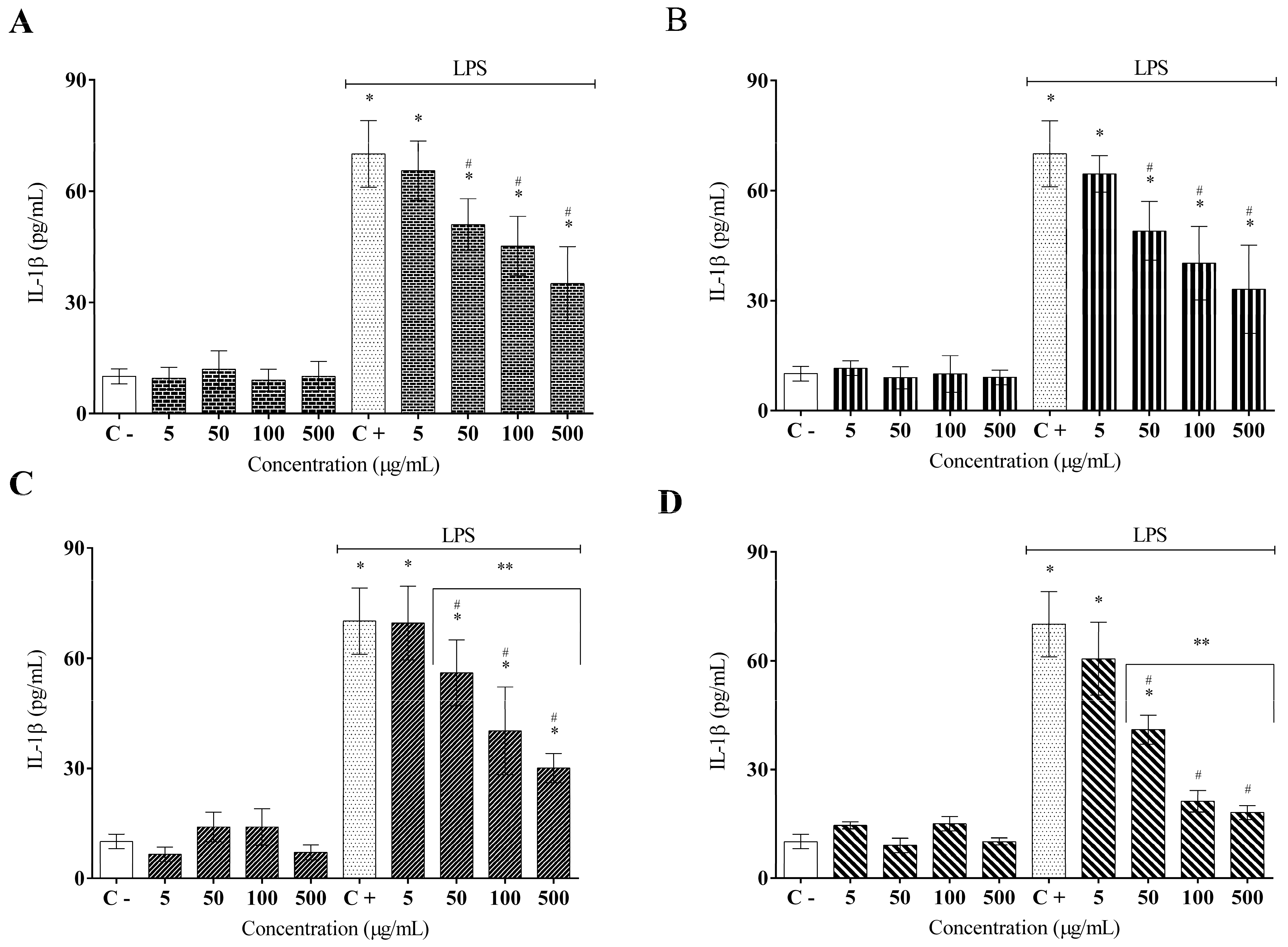
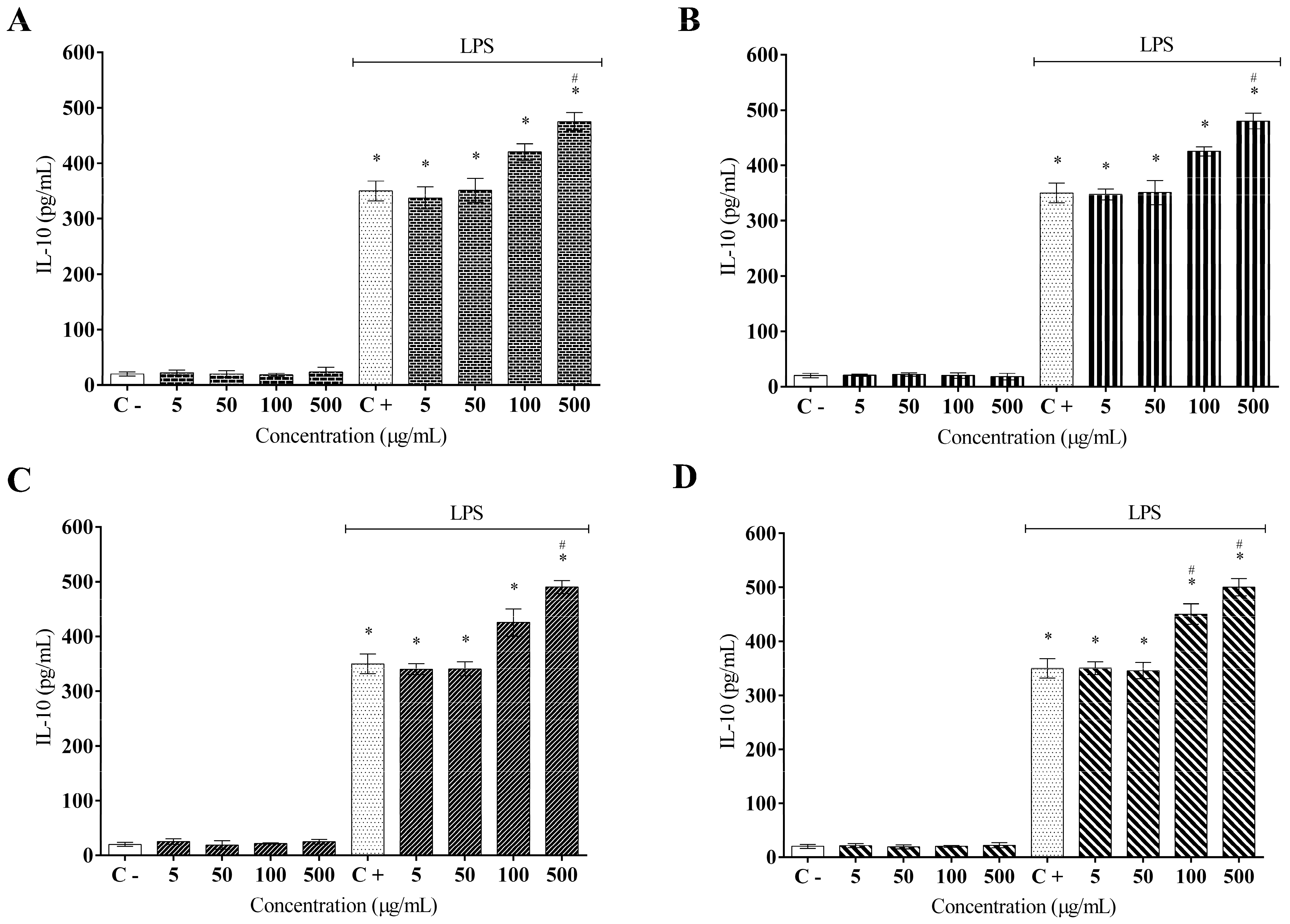
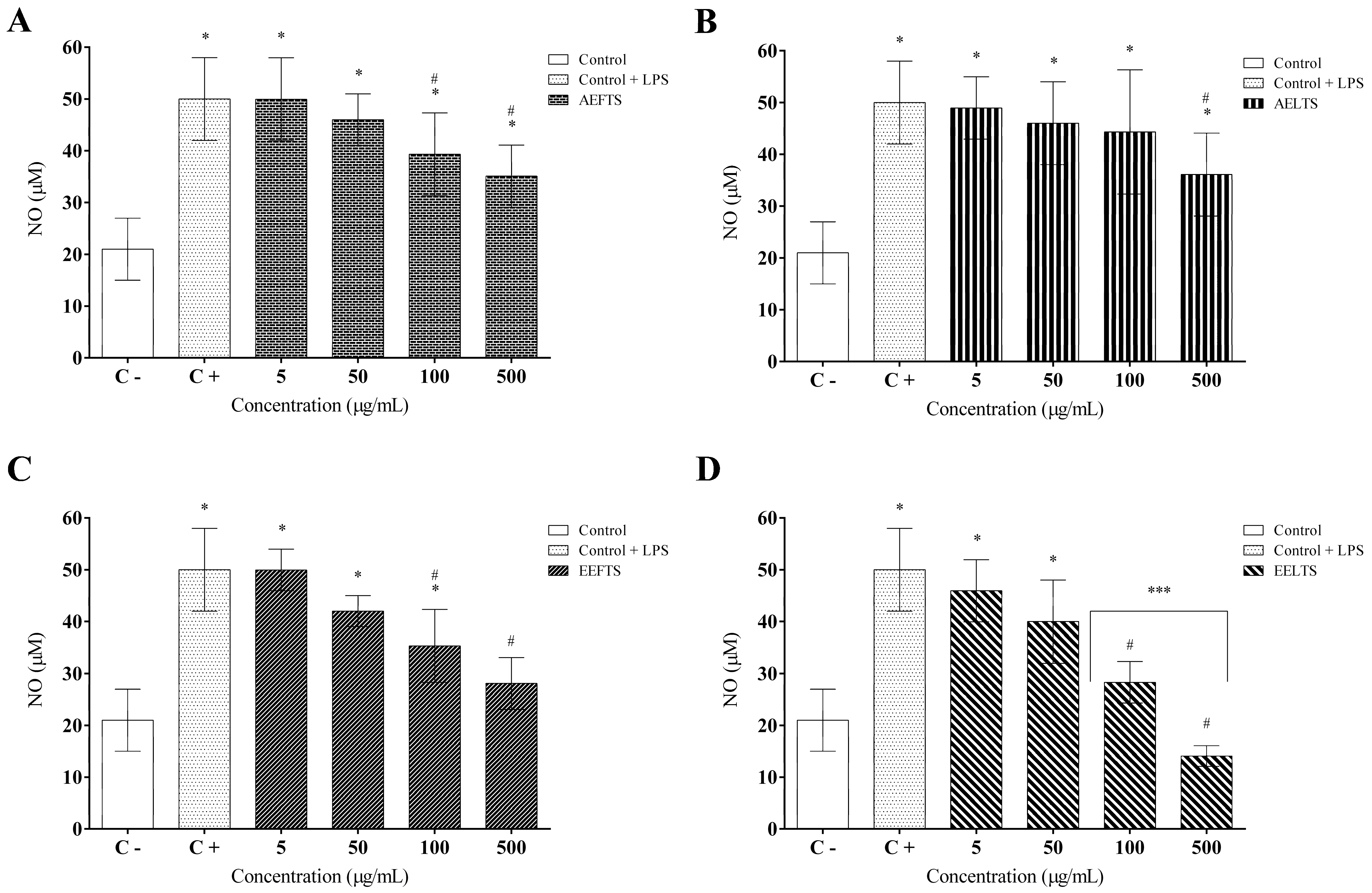
2. Results
3. Discussion
4. Material and Methods
4.1. Plant Material and Extract Preparation
4.2. Chemical Characterization by Ultrafast Liquid Chromatography Coupled to Mass Spectrometry (LC–MS/MS)
4.3. Cell Culture
4.4. MTT Viability Assay
4.5. Alamar Blue® Viability Assay
4.6. Cytokine Measurement of (TNF-α, IL1-β, IL-6, and IL10)
4.7. Measurement of Prostaglandin E2 (PGE2) and Nitric Oxide (NO) Production
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kulkarni, O.P.; Lichtnekert, J.; Anders, H.J.; Mulay, S.R. The immune system in tissue environments regaining homeostasis after injury: Is “inflammation” always inflammation? Mediators. Inflamm. 2016, 1, 2856213. [Google Scholar] [CrossRef] [Green Version]
- Villanueva, J.R.; Esteban, J.M.; Villanueva, L.R. Solving the puzzle: What is behind our forefathers’ anti-inflammatory remedies? J. Intercult. Ethnopharmacol. 2016, 6, 128–143. [Google Scholar] [CrossRef]
- Parisi, L.; Bassani, B.; Tremolati, M.; Gini, E.; Farronato, G.; Bruno, A. Natural killer cells in the orchestration of chronic inflammatory diseases. J. Immunol. Res. 2017, 1, 4218254. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Alexander, M.; Misharin, A.V.; Budinger, G.R.S. The role of macrophages in the resolution of inflammation. J. Clin. Investig. 2019, 129, 2619–2628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.Y.; Moudgil, K.D. Immunomodulation of autoimmune arthritis by pro-inflammatory cytokines. Cytokine 2017, 98, 87–96. [Google Scholar] [CrossRef]
- Ungprasert, P.; Srivali, N.; Thongprayoon, C. Nonsteroidal anti-inflammatory drugs and risk of incident heart failure: A systematic review and meta-analysis of observational studies. Clin. Cardiol. 2016, 39, 111–188. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, D.I.; Koko, W.S.; Taha, M.M.E.; Mohan, S.; Achoui, M.; Abdulla, M.A.; Mustafa, M.R.; Ahmad, S.; Noordin, M.I.; Yong, C.L.; et al. In vitro and in vivo anti-inflammatory activities of columbin through the inhibition of cycloxygenase-2 and nitric oxide but not the suppression of NF-κB translocation. Eur. J. Pharmacol. 2012, 678, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fokunang, C.N.; Fokunang, E.T.; Frederick, K.; Ngameni, B.; Ngadjui, B. Overview of non-steroidal anti-inflammatory drugs (nsaids) in resource limited countries. MOJ Toxicol 2018, 4, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Alessandri, A.L.; Sousa, L.P.; Lucas, C.D.; Rossi, A.G.; Pinho, V.; Teixeira, M.M. Resolution of inflammation: Mechanisms and opportunity for drug development. Pharmacol. Ther. 2013, 139, 189–212. [Google Scholar] [CrossRef] [Green Version]
- Winand, L.; Sester, A.; Nett, M. Bioengineering of anti-inflammatory natural products. ChemMedChem 2021, 16, 767–776. [Google Scholar] [CrossRef]
- Kisiriko, M.; Anastasiadi, M.; Terry, L.A.; Yasri, A.; Beale, M.H.; Ward, J.L. Phenolics from medicinal and aromatic plants: Characterisation and potential as biostimulants and bioprotectants. Molecules 2021, 26, 6343. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.S.; Ahmad, W.A.N.W.; Budin, S.B.; Zainalabidin, S. Implication of dietary phenolic acids on inflammation in cardiovascular disease. Rev. Cardiovasc. Med. 2020, 21, 225–240. [Google Scholar] [CrossRef]
- Puangpraphant, S.; Cuevas-Rodríguez, E.O.; Oseguera-Toledo, M. Anti-inflammatory and antioxidant phenolic compounds. In Current Advances for Development of Functional Foods Modulating Inflammation and Oxidative Stress; Levy, N., Ed.; Academic Press: Cambridge, MA, USA, 2022; pp. 165–180. [Google Scholar]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal plant analysis: A historical and regional discussion of emergent complex techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Taneja, R.; Sharma, A. The genus Turnera: A review update. Pharm. Biol. 2005, 43, 383–391. [Google Scholar] [CrossRef]
- Rocha, L.; Nogueira, J.W.A.; Figueiredo, M.F.; Loiola, M.I.B. Flora of Ceará: Turneraceae. Rodriguésia 2018, 69, 1673–1700. [Google Scholar] [CrossRef]
- Antonio, M.A.; Brito, A.R.M.S. Oral anti-inflammatory and antiulcerogenic activities of a hydroalcoholic extract and partitioned fractions of Turnera ulmifolia (Turneraceae). J. Ethnopharmacol. 1998, 61, 215–228. [Google Scholar] [CrossRef]
- Galvez, J.; Gracioso, J.S.; Camuesco, D.; Vilegas, W.; Brito, A.R.M.S.; Zarzuelo, A. Intestinal antiinflammatory activity of a lyophilized infusion of Turnera ulmifolia in TNBS rat colitis. Fitoterapia 2006, 77, 515–520. [Google Scholar] [CrossRef]
- Nascimento, M.A.; Silva, A.K.; França, L.C.; Quignard, E.L.J.; López, J.A.; Almeida, M.G. Turnera ulmifolia L. (Turneraceae): Preliminary study of its antioxidant activity. Bioresour. Technol. 2006, 97, 1387–1391. [Google Scholar] [CrossRef]
- Brito, N.J.N.; López, J.A.; Nascimento, M.A.; Macêdo, J.B.M.; Silva, G.A.; Oliveira, C.N.; Rezende, A.U.; Brandão-Neto, J.; Schwarz, A.; Almeida, M.G. Antioxidant activity and protective effect of Turnera ulmifolia Linn. var. elegans against carbon tetrachloride-induced oxidative damage in rats. Food Chem. Toxicol. 2012, 50, 4340–4347. [Google Scholar] [CrossRef]
- Szewczyk, K.; Zidorn, C. Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana–Turnera diffusa. J. Ethnopharmacol. 2014, 152, 424–443. [Google Scholar] [CrossRef] [PubMed]
- Montanher, A.B.; Zucolotto, S.M.; Schenkel, E.P.; Fröde, T.S. Evidence of anti-inflammatory effects of Passiflora edulis in an inflammation model. J. Ethnopharmacol. 2007, 109, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Kwon, O.K.; Ryu, H.W.; Paik, J.H.; Paryanto, I.; Yuniato, P.; Choi, S.; Oh, S.R.; Ahn, K.S. Anti-inflammatory effects of Passiflora foetida L. in LPS-stimulated RAW264.7 macrophages. Int. J. Mol. Med. 2018, 41, 3709–3716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, N.C.; Oliveira, J.M.; Morrone, M.D.S.; Albanus, R.D.; Amarante, M.D.S.M.; Camillo, C.D.S.; Langassner, S.M.Z.; Gelain, D.P.; Moreira, J.C.F.; Dalmolin, R.J.S.; et al. Turnera subulata: Anti-inflammatory properties in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Med. Food 2016, 19, 922–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luz, J.R.D.; Nascimento, T.E.S.; Morais, L.V.F.; Cruz, A.K.M.; Rezende, A.A.; Brandão-Neto, J.; Ururahy, M.A.G.; Luchessi, A.D.; López, J.A.; Rocha, H.A.O.; et al. Thrombin inhibition: Preliminary assessment of the anticoagulant potential of Turnera subulata (Passifloraceae). J. Med. Food 2019, 22, 384–392. [Google Scholar] [CrossRef]
- Neuenschwander, P.F. Coagulation cascade: Overview. In Encyclopedia of Respiratory Medicine, 2nd ed.; Janes, S.M., Ed.; Elsevier: Oxford, UK, 2022; pp. 479–488. [Google Scholar]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Lee, M.W.; Katz, P.O. Nonsteroidal antiinflammatory drugs, anticoagulation, and upper gastrointestinal bleeding. Clin. Geriatr. Med. 2021, 37, 31–42. [Google Scholar] [CrossRef]
- Nunes, C.D.R.; Barreto, M.B.; Pereira, S.M.F.; Cruz, L.L.; Passos, M.S.; Moraes, L.P.; Vieira, I.J.C.; Oliveira, D.B. Plants as sources of anti-inflammatory agentes. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Shin, S.A.; Joo, B.J.; Lee, J.S.; Ryu, G.; Han, M.; Kim, W.Y.; Park, H.H.; Lee, J.H.; Lee, C.S. Phytochemicals as anti-inflammatory agents in animal models of prevalent inflammatory diseases. Molecules 2020, 25, 5932. [Google Scholar] [CrossRef]
- Yang, Y.; Saand, M.A.; Abdelaal, W.B.; Zhang, J.; Wu, Y.; Li, J.; Fan, H.; Wang, F. iTRAQ-based comparative proteomic analysis of two coconut varieties reveals aromatic coconut cold-sensitive in response to low temperature. J. Proteom. 2020, 30, 103766. [Google Scholar] [CrossRef]
- Góral, I.; Wojciechowski, K. Surface activity and foaming properties of saponin-rich plants extracts. Adv. Colloid. Interface Sci. 2020, 279, 102145. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.W.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Freitas, C.L.A.; Santos, F.F.P.; Dantas-Junior, O.M.; Inácio, V.V.; Matias, W.F.F.; Quintans-Júnior, L.J.; Aguiar, J.J.S.; Coutinho, H.D.M. Enhancement of antibiotic activity by phytocompounds of Turnera subulata. Nat. Prod. Res. 2020, 34, 2384–2388. [Google Scholar] [CrossRef]
- Salvador, M.J.; Ferreira, E.O.; Mertens-Talcott, S.U.; De Castro, W.V.; Butterweck, V.; Derendorf, H.; Dias, D.A. Isolation and HPLC quantitative analysis of antioxidant flavonoids from Alternanthera tenella Colla. Z. Nat. C. 2006, 61, 19–25. [Google Scholar] [CrossRef]
- Ninfali, P.; Bacchiocca, M.; Antonelli, A.; Biagiotti, E.; Di Gioacchino, A.M.; Piccoli, G.; Stocchi, V.; Brandi, G. Characterization and biological activity of the main flavonoids from Swiss Chard (Beta vulgaris subspecies cycla). Phytomedicine 2007, 14, 216–221. [Google Scholar] [CrossRef]
- Gennari, L.; Felletti, M.; Blasa, M.; Angelino, D.; Celeghini, C.; Corallini, A.; Ninfali, P. Total extract of Beta vulgaris var. cicla seeds versus its purified phenolic components: Antioxidant activities and antiproliferative effects against colon cancer cells. Phytochem. Anal. 2011, 22, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Pisklak, M.A.; Kaliszewska, D.; Stolarczyk, M.; Kiss, A.K. Activity-guided isolation, identification and quantification of biologically active isomeric compounds from folk medicinal plant Desmodium adscendens using high performance liquid chromatography with diode array detector, mass spectrometry and multidimentional nuclear magnetic resonance spectroscopy. J. Pharm. Biomed. Anal. 2015, 102, 54–63. [Google Scholar] [CrossRef]
- Marincaş, O.; Feher, I.; Magdas, D.A.; Puşcaş, R. Optimized and validated method for simultaneous extraction, identification and quantification of flavonoids and capsaicin, along with isotopic composition, in hot peppers from different regions. Food Chem. 2018, 267, 255–262. [Google Scholar] [CrossRef]
- Bezerra, A.G.; Negri, G.; Duarte-Almeida, J.M.; Smaili, S.S.; Carlini, E.A. Phytochemical analysis of hydroethanolic extract of Turnera diffusa Willd and evaluation of its effects on astrocyte cell death. Einstein 2016, 14, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Willer, J.; Jöhrer, K.; Greil, R.; Zidorn, C.; Çiçek, S.S. Cytotoxic properties of damiana (Turnera diffusa) extracts and constituents and a validated quantitative UHPLC-DAD assay. Molecules 2019, 24, 855. [Google Scholar] [CrossRef] [Green Version]
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; et al. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food. Chem. 2018, 66, 10369–10379. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I.; Raguzzini, A. Flavonoids as anti-inflammatory agents. Proc. Nutr. Soc. 2010, 69, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects–A comprehensive review. Crit. Rev. Food. Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Yang, H.; Huang, J.; Mao, Y.; Wang, L.; Li, R.; Ha, C. Vitexin alleviates interleukin-1β-induced inflammatory responses in chondrocytes from osteoarthritis patients: Involvement of HIF-1α pathway. Scand. J. Immunol. 2019, 90, e12773. [Google Scholar] [CrossRef]
- Cao, H.; Wang, X.; Zhang, B.; Ren, M. The protective effect of vitexin in septic encephalopathy by reducing leukocyte-endothelial adhesion and inflammatory response. Ann. Palliat. Med. 2020, 9, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Du, X.; Chen, S.; Liang, J.; Huang, S.; Hou, S.; Gao, J.; Ding, P. Effect of vitexin on alleviating liver inflammation in a dextran sulfate sodium (DSS)-induced colitis model. Biomed. Pharmacother. 2020, 121, 109683. [Google Scholar] [CrossRef]
- Tu, Y.; Xiao, T.; Gong, G.; Bian, Y.; Li, Y. A new isoflavone with anti-inflammatory effect from the seeds of Millettia pachycarpa. Nat. Prod. Res. 2019, 34, 981–987. [Google Scholar] [CrossRef]
- Xie, C.; Park, K.H.; Kong, S.S.; Cho, K.M.; Lee, D.H. Isoflavone-enriched soyben leaves attenuate ovariectomy-induced osteoporosis in rats by anti-inflammatory activity. J. Sci. Food. Agric. 2020, 111, 1499–1506. [Google Scholar] [CrossRef]
- Yin, P.; Zhang, Z.; Li, J.; Shi, Y.; Jin, N.; Zou, W.; Gao, G.; Wang, W.; Liu, F. Ferulic acid inhibits bovine endometrial epithelial cells against LPS-induced inflammation via suppressing NF-κB and MAPK pathway. Res. Vet. Sci. 2020, 126, 164–169. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Hussein, O.E.; Hezayen, W.G.; Bin-Jumah, M.; El-Twab, S.M.A. Ferulic acid prevents oxidative stress, inflammation, and liver injury via upregulation of Nrf2/HO-1 signaling in methotrexate-induced rats. Environ. Sci. Pollut. Res. Int. 2020, 27, 7910–7921. [Google Scholar] [CrossRef]
- Taniguchi, K.; Karin, M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Gupta, P.; Barthwal, M.K. IL-1 β genesis: The art of regulating the regulator. Cell. Mol. Immunol. 2018, 15, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Alunno, A.; Carubbi, F.; Giacomelli, R.; Gerli, R. Cytokines in the pathogenesis of rheumatoid arthritis: New players and therapeutic targets. BMC Rheumatol. 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Hamidzadeh, K.; Christensen, S.M.; Dalby, E.; Chandrasekaran, P.; Mosser, D.M. Macrophages and the recovery from acute and chronic inflammation. Annu. Rev. Physiol. 2017, 79, 567–592. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Choi, S.I.; Son, S.R.; Han, H.S.; Ahn, H.S.; Shin, Y.K.; Lee, S.H.; Lee, K.T.; Kwon, H.C.; Jang, D.S. Chemical Constituents of the Leaves of Campanula takesimana (Korean Bellflower) and Their Inhibitory Effects on LPS-induced PGE2 Production. Plants 2020, 9, 1232. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2019, 60, 2083–2097. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Padwad, Y. Plant polyphenol-based second-generation synbiotic agents: Emerging concepts, challenges, and opportunities. Nutrition 2020, 77, 110785. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Morales-Tapia, P.; Moncada-Basualto, M.; Pozo-Martínez, J.; Olea-Azar, C.; Nesic, A.; Cabrera-Barjas, G. Polyphenolic composition and antioxidant activity (ORAC, EPR and cellular) of different extracts of Argylia radiata vitroplants and natural roots. Molecules 2022, 27, 610. [Google Scholar] [CrossRef]
- Jiang, J.; Dai, J.; Cui, H. Vitexin reverses the autophagy dysfunction to attenuate MCAO-induced cerebral ischemic stroke via mTOR/Ulk1 pathway. Biomed. Pharmacother. 2018, 99, 583–590. [Google Scholar] [CrossRef]
- Babaei, F.; Moafizad, A.; Darvishvand, Z.; Mirzababaei, M.; Hosseinzadeh, H.; Nassiri-Asl, M. Review of the effects of vitexin in oxidative stress-related diseases. Food Sci. Nutr. 2020, 8, 2569–2580. [Google Scholar] [CrossRef] [Green Version]
- Kelner, M.J.; Uglik, S.F. Mechanism of prostaglandin E2 release and increase in PGH2/PGE2 isomerase activity by PDGF: Involvement of nitric oxide. Arch. Biochem. Biophys. 1994, 312, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Mollace, V.; Muscoli, C.; Masini, E.; Cuzzocrea, S.; Salvemini, D. Modulation of prostaglandin biosynthesis by nitric oxide and nitric oxide donors. Pharmacol. Rev. 2005, 57, 217–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, J.H.; Conway, E.M. Cross talk pathways between coagulation and inflammation. Circ. Res. 2016, 118, 1392–1408. [Google Scholar] [CrossRef] [PubMed]
- Palhares, L.C.G.F.; Brito, A.S.; Lima, M.A.; Nader, H.B.; London, J.A.; Barsukov, I.L.; Andrade, G.P.V.; Yates, E.A.; Chavante, S.F. A further unique chondroitin sulfate from the shrimp Litopenaeus vannamei with antithrombin activity that modulates acute inflammation. Carbohydr. Polym. 2019, 222, 115031. [Google Scholar] [CrossRef]
- Wang, M.; Carver, J.J.; Phelan, V.V.; Sanchez, L.M.; Garg, N.; Peng, Y.; Nguyen, D.D.; Watrous, J.; Kapono, C.A.; Luzzatto-Knaan, T.; et al. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016, 34, 828–837. [Google Scholar] [CrossRef] [Green Version]
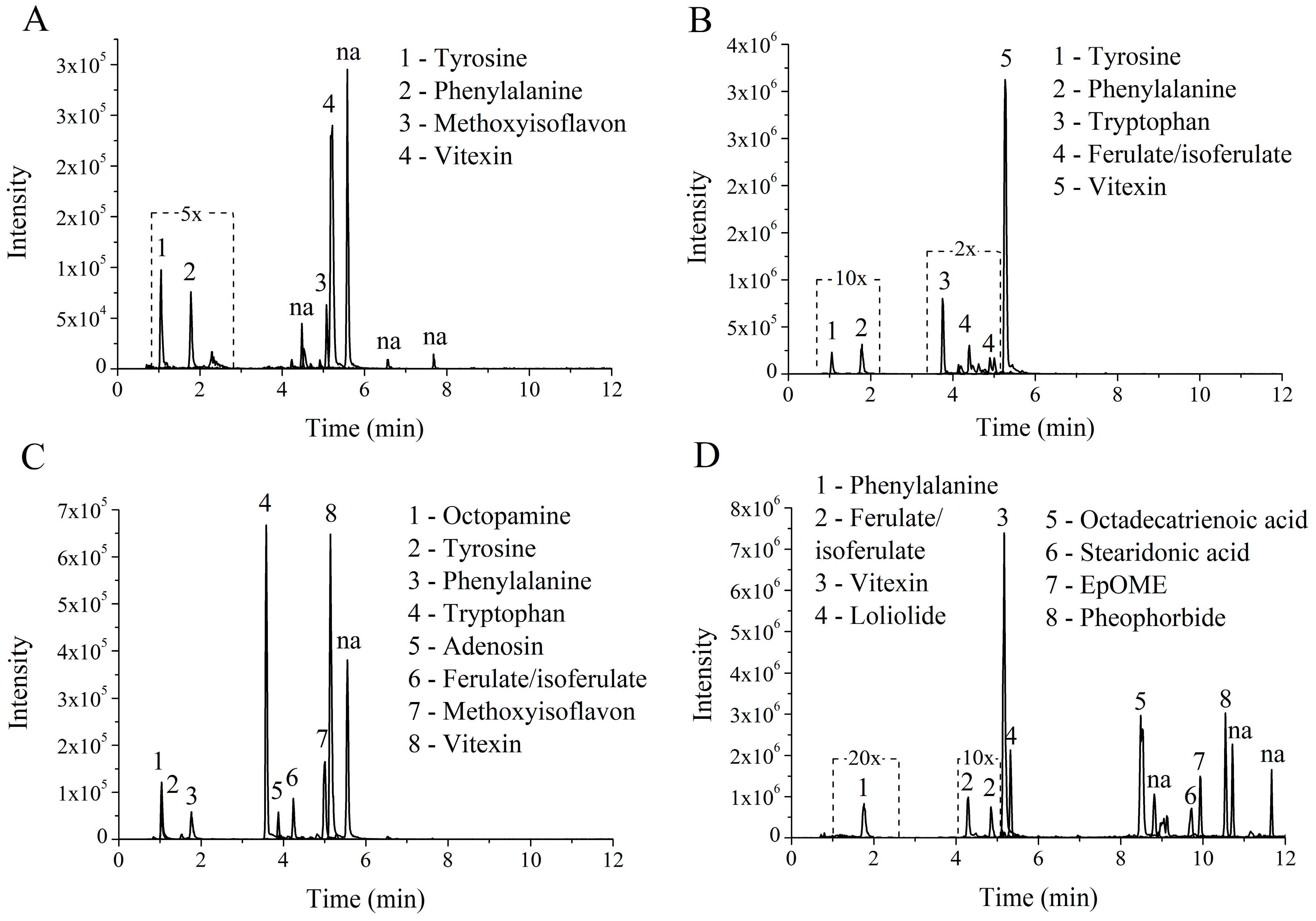


| Peak. | Phytocomponents Matched with GNPS Data Base | Cosine | Mass Diff | Mass | Molecular Formula | Ion Fragments a | Adduct | Extract |
|---|---|---|---|---|---|---|---|---|
| 1 | l-Tyrosine | 0.86 | 0.001 | 165.054 | C9H8O3 | 147.04, 123.05, 119.05, 95.05, 91.06 | [M − NH3 + H]+ | AFETS |
| 2 | Phenylalanine | 0.95 | 0 | 166.086 | C9H11NO2 | 149.06, 131.05, 120.08, 103.05, 53.04 | [M + H]+ | AFETS |
| 3 | 7-O-beta-glucopyranosyl-4′-hydroxy-5-methoxyisoflavone | 0.89 | 0 | 447.129 | C22H22O10 | 285.08, 270.05, 213.05, 152.01 | [M + H]+ | AFETS |
| 4 | Vitexin-2-O-rhamnoside | 0.95 | 0.002 | 579.172 | C27H30O14 | 433.11, 415.10, 397.09, 313.07, 283.06 | [M + H]+ | AFETS |
| 1 | l-Tyrosine | 0.95 | 0.001 | 182.081 | C9H11N1O3 | 165.06, 147.04, 136.07, 123.05, 119.05 | [M + H]+ | ALETS |
| 2 | dl-Phenylalanine | 0.97 | 0 | 166.086 | C9H11NO2 | 149.06, 131.05, 120.08, 103.05, 53.04 | [M + H]+ | ALETS |
| 3 | l-Tryptophan | 0.95 | 0.001 | 205.096 | C11H12N2O2 | 188.07, 159.09, 146.06, 144.08, 118.06 | [M + H]+ | ALETS |
| 4 | Ferulate/isoferulate | 0.93 | 0 | 177.054 | C10H8O3 | 149.06, 145.03, 117.03, 89.04 | M − H2O + H | ALETS |
| 5 | Vitexin-2-O-rhamnoside | 0.94 | 0 | 579.17 | C27H30O14 | 433.11, 415.10, 397.09, 313.07, 283.06 | [M + H]+ | ALETS |
| 1 | dl-Octopamine | 0.95 | 0 | 136.076 | C8H9NO1 | 119.05, 118.06, 107.05, 91.06, 64.04 | [M − H2O + H]+ | HEFTS |
| 2 | l-Tyrosine | 0.94 | 0.001 | 165.054 | C9H8O3 | 147.04, 123.05, 119.05, 95.05, 91.06 | [M − NH3 + H]+ | HEFTS |
| 3 | Phenylalanine | 0.96 | 0 | 166.086 | C9H11NO2 | 149.06, 131.05, 120.08, 103.05, 53.04 | [M + H]+ | HEFTS |
| 4 | l-Tryptophan | 0.94 | 0.001 | 188.07 | C11H9N1O2 | 170.06, 146.06, 144.08, 143.07, 118.07 | [M − NH3 + H]+ | HEFTS |
| 5 | Adenosine, 5_-S-methyl-5_-thio- | 0.91 | 0.004 | 298.097 | C11H15N5O3S | 145.03, 136.06, 97.03, 61.01 | [M + H]+ | HEFTS |
| 6 | Ferulate/isoferulate | 0.89 | 0 | 177.054 | C10H8O3 | 149.06, 145.03, 117.03, 89.04 | [M − H2O + H]+ | HEFTS |
| 7 | 7-O-beta-glucopyranosyl-4′-hydroxy-5-methoxyisoflavone | 0.91 | 0.001 | 447.13 | C22H22O10 | 285.08, 270.05, 213.05, 152.01 | [M + H]+ | HEFTS |
| 8 | Vitexin-2-O-rhamnoside | 0.97 | 0.003 | 579.173 | C27H30O14 | 433.11, 415.10, 397.09, 313.07, 283.06 | [M + H]+ | HEFTS |
| 1 | Phenylalanine | 0.94 | 0 | 166.086 | C9H11NO2 | 149.06, 131.05, 120.08, 103.05, 53.04 | [M + H]+ | HELTS |
| 2 | Ferulate/isoferulate | 0.94 | 0 | 177.054 | C10H8O3 | 149.06, 145.03, 117.03, 89.04 | [M − H2O + H]+ | HELTS |
| 3 | Vitexin-2-O-rhamnoside | 0.94 | 0.001 | 579.171 | C27H30O14 | 433.11, 415.10, 397.09, 313.07, 283.06 | [M + H]+ | HELTS |
| 4 | Loliolide | 0.94 | 0 | 197.117 | C11H16O3 | 179.11, 161.09, 135.12, 133.10, 107.09 | [M + H]+ | HELTS |
| 5 | 9S-Hydroxy-10E,12Z,15Z-octadecatrienoic acid | 0.86 | 0 | 277.216 | C18H28O2 | 259.20, 149.13, 135.12, 121.10, 93.07 | [M − H2O + H]+ | HELTS |
| 6 | 9(10)-EpOME | 0.91 | 0.002 | 279.233 | C18H30O2 | 173.13, 109.10, 95.09, 81.07, 67.06 | [M − H2O + H]+ | HELTS |
| 7 | Stearidonic acid Ethyl ester | 0.86 | 0.001 | 305.248 | C20H32O2 | 259.20, 149.13, 135.12, 121.10, 93.07 | [M + H]+ | HELTS |
| 8 | Pheophorbide A | 0.87 | 0.005 | 593.274 | C35H36N4O5 | 533.25, 460.23, 447.22, 433.24, 431.18 | [M + H]+ | HELTS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luz, J.R.D.d.; Barbosa, E.A.; Nascimento, T.E.S.d.; Rezende, A.A.d.; Ururahy, M.A.G.; Brito, A.d.S.; Araujo-Silva, G.; López, J.A.; Almeida, M.d.G. Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages. Molecules 2022, 27, 1084. https://doi.org/10.3390/molecules27031084
Luz JRDd, Barbosa EA, Nascimento TESd, Rezende AAd, Ururahy MAG, Brito AdS, Araujo-Silva G, López JA, Almeida MdG. Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages. Molecules. 2022; 27(3):1084. https://doi.org/10.3390/molecules27031084
Chicago/Turabian StyleLuz, Jefferson Romáryo Duarte da, Eder A. Barbosa, Thayse Evellyn Silva do Nascimento, Adriana Augusto de Rezende, Marcela Abbott Galvão Ururahy, Adriana da Silva Brito, Gabriel Araujo-Silva, Jorge A. López, and Maria das Graças Almeida. 2022. "Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages" Molecules 27, no. 3: 1084. https://doi.org/10.3390/molecules27031084
APA StyleLuz, J. R. D. d., Barbosa, E. A., Nascimento, T. E. S. d., Rezende, A. A. d., Ururahy, M. A. G., Brito, A. d. S., Araujo-Silva, G., López, J. A., & Almeida, M. d. G. (2022). Chemical Characterization of Flowers and Leaf Extracts Obtained from Turnera subulata and Their Immunomodulatory Effect on LPS-Activated RAW 264.7 Macrophages. Molecules, 27(3), 1084. https://doi.org/10.3390/molecules27031084